Abstract
Background:
Squamous cell carcinoma (SCC) is the most common malignancy in oral cavity. Angiogenesis is essential for the development and progression of SCC. Recently, some studies have reported that mast cells may play a role in tumor progression via promoting angiognesis. Since the results of various studies on the role of mast cells in tumor progression is not uniform, the aim of this study was to define the possible role of mast cells in the process of angiogenesis by determining the microvessel density (MVD) and mast cell density (MCD) and the association between them in oral normal mucsoa and oral SCC.
Materials and Methods:
In this retrospective analytical study, paraffinized specimens from 22 cases of normal mucosa and 20 cases of well-differentiated oral SCC were selected. Microvessels were stained by using immunohistochemical technique with anti-CD34 antibody and mast cells with toluidine blue and then were counted at 400× magnification in hot-spot areas under a light microscope. The results were analyzed by using t- test and Pearson's exams. P values less than 0.05 was considered to be significant.
Results:
A significant correlation was noted between MVD and MCD in normal oral mucosa (P<0.001), but in spite of a higher density of mast cells and microvessels observed in oral SCC compared to normal mucosa, there was no significant correlation between them (P=0.731).
Conclusion:
These findings showed that factors other than mast cells may play a role in the upregulation of tumor angiogenesis in oral SCC.
Keywords: Angiogenesis, mast cell, oral squamous cell carcinoma
INTRODUCTION
Based on evidence, tumor microenvironment can affect many cellular vital activities including growth, death, differentiation, gene expression, migration, and invasion.[1,2]
Previous studies have shown that the microvessel density (MVD) plays an important role in the growth and development of tumoral cells, also their metastatic ability.[3] The role of different cells in tumor microenvironment as one of the factors in tumor growth is remarkable. Mast cells play various roles in extracellular matrix degradation, angiogenesis, and innate and acquired immune responses due to their ability to release specific products such as chymase, basic fibroblast growth factor (bFGF), tryptase, heparin, and histamine.[1–3]
Accordingly, it is considered that mast cells have conflicting dual roles from defending against tumors to causing tumor progression. Of course, some of these products induce angiogenesis in tumors.[1,3]
The results of some previous studies[1–4] have shown some controversial results regarding the role of mast cells related to the angiogenesis process. Therefore, we studied the association of mast cells with the angiogenesis process. For this purpose, a CD34 vascular marker was used to identify and count the vessels and toluidine blue staining for mast cells. Finally, their relationship in normal oral mucosa and oral squamous cell carcinoma (OSCC) was compared.
MATERIALS AND METHODS
In this retrospective analytical study, formalin-fixed, paraffin-embedded specimens of 22 clinically normal oral mucosal tissue, free of inflammation and necrosis, and 20 cases of well-differentiated OSCC from the buccal mucosa were obtained from the Department of Oral and Maxillofacial Pathology, School of Dentistry in Isfahan University of Medical Sciences. Three 4 μm thickness sections from each paraffin block were prepared and placed on a slide.
Determination of mast cells
Because sulfated proteoglycans in secretory granules of mast cells have a metachromatic property that can be stained by toluidine blue, the solution used to stain mast cells contained toluindine blue, which was 0.2 g in 100 ml of distilled water and 2 ml of acetic acid. Following deparaffinization by immersing in xylene and descending grades of ethanol, the sections were rinsed in distilled water, stained with toluidine blue for 10 min, and then were rinsed again in distilled water, dehydrated, and mounted.
Determination of microvessels
To determine MVD, we used a specific monoclonal antibody against CD34. Before staining, serial 4-μm-thickness sections were deparaffinized in the same way as above. These sections were immersed in 0.3% H2O2 in methanol for 15 min at room temperature to block the endogenous peroxidase activity. To retrieve the antigens, the slides were heated in a microwave oven and then incubated with monoclonal antibody (Nu-4A1, Nichirei Corp, Tokyo, Japan) for 30 min. After being washed with phosphate-buffered saline (pH 7.0) for 1 h, the slides were incubated with 3,3’-diaminobenzidine (DAKO, Denmark) for 20 min. Finally, the sections were counterstained with Harris hematoxylin and then dehydrated and lamell placed on them.
Quantification of microvascular and mast cell densities
The number of microvessels and mast cells in normal mucosa and oral SCC in four fields under a light microscope (Olympus BX41TF, Tokyo, Japan) at a magnification of 400× on an ocular grid in the area of the most intense vascularization (hot spot) were counted, and the average count in each case recorded. Any endothelial-lined vessel appearing reddish brown and clearly separated from adjacent stromal and tumoral cells was considered to be a single countable microvessel [Figures 1 and 2]. Any cluster of mast cell granules appearing reddish brown and clearly separated from adjacent cell membrane was considered to be a single mast cell [Figures 3 and 4].
Figure 1.
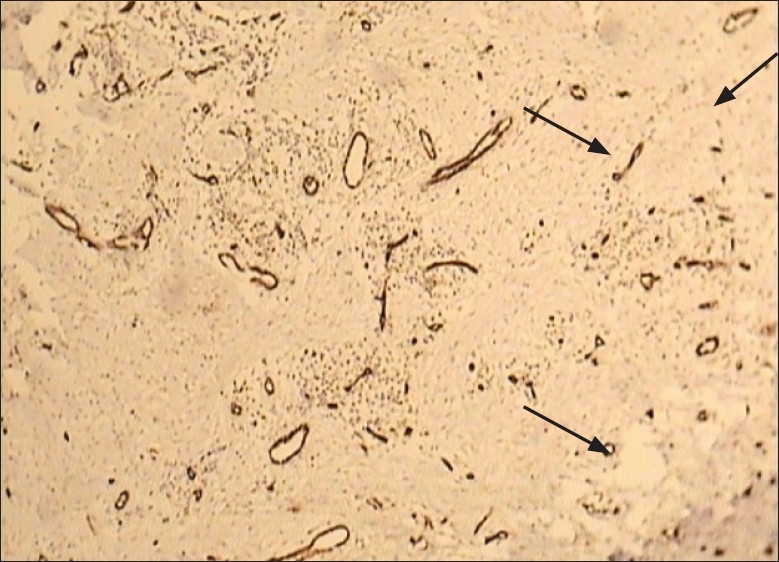
Light microscopic view of microvessels expressed by CD34 marker in normal mucosa
Figure 2.
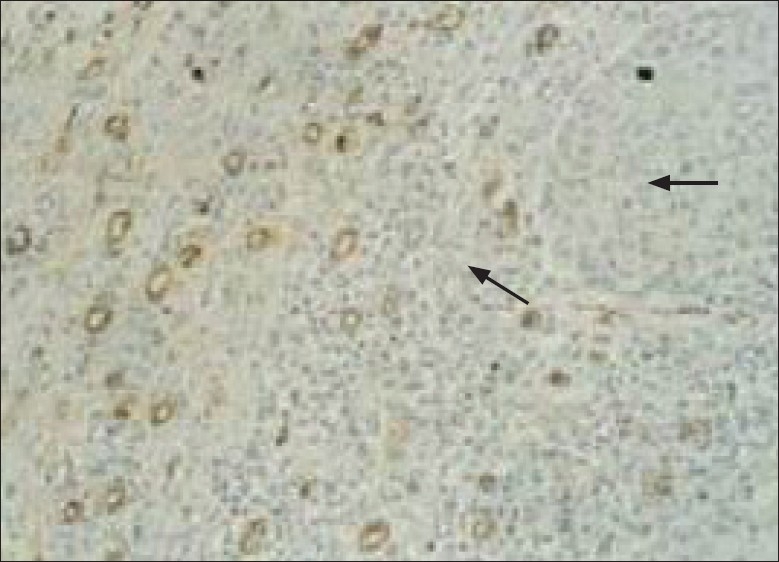
Light microscopic view of microvessels expressed by CD34 marker in OSCC
Figure 3.
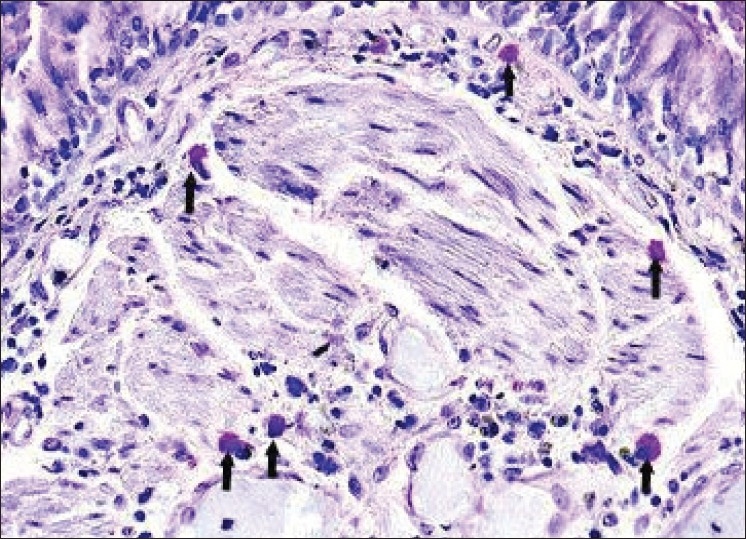
Light microscopic view of mast cells stained by toluidine blue in normal mucosa (arrows)
Figure 4.

Light microscopic view of mast cells stained by toluidine blue in OSCC (arrows)
RESULTS
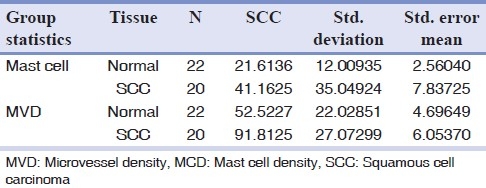
Mean values and standard deviations of MCD (mast cell density) and MVD are shown in Table 1. We used the independent t test and found that the values of MCD (P<0.001) and MVD (P<0.001) were significantly higher in OSCC compared with normal oral mucosa.
Table 1.
Comparison of MVD and MCD between the two groups
Also as shown in Figure 5, the Pearson's correlation showed a significant positive correlation between MCD and MVD in normal oral mucosa (P<0.001), but not in OSCC (P=0.731) [Figure 6].
Figure 5.
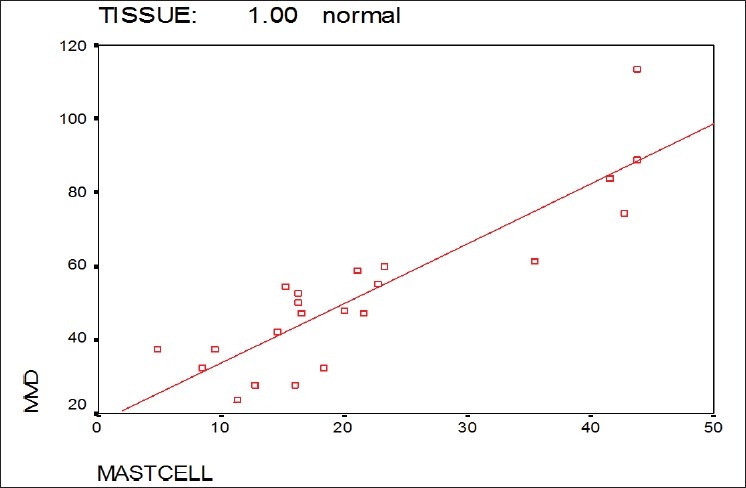
Correlation between MCD and MVD in the normal mucosa group
Figure 6.
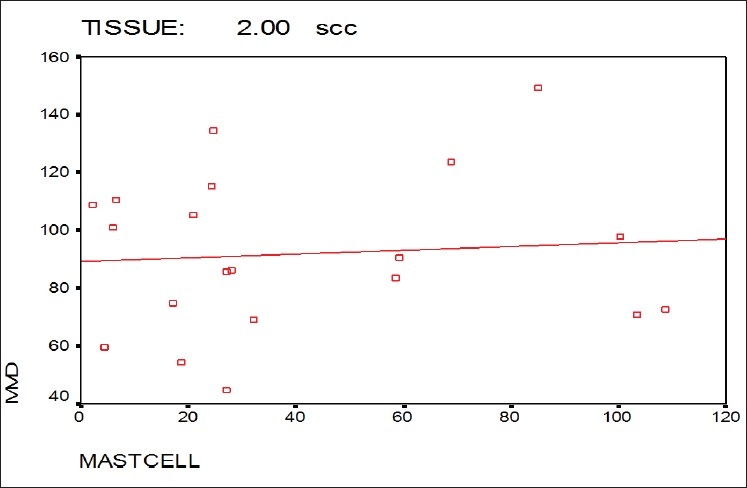
Correlation between MCD and MVD in the OSCC group
DISCUSSION
Sustained tumor growth requires a preference of tumor cell proliferation over cell death or apoptosis. Using an experimental animal model, it has been shown that the initiation of angiogenesis appears to be concomitant with a decrease in tumor cell apoptosis while the levels of tumor cell proliferation remains constant, thus leading to a net tumor growth.[4]
Angiogenesis is the outcome of an imbalance between positive and negative angiogenic factors produced by both tumor and host cells. Among the host cells, which produce and release proangiogenic and angiogenic factors, mast cells are an important source of several factors, such as histamine, heparin, chymase, bFGF, vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β).[5]
In the present study, we used toluidine blue staining to identify and count mast cells. MCD was found to be significantly higher in the OSCC group compared with the normal mucosal group. These findings were similar to those reported by previous studies on various tumors.[3,6,7]
But Oliveira-Neto et al[1] found MCD to be lower in OSCC and premalignant lesions compared to normal controls. They attributed this to the migration failure of mast cells, which possibly reflect a modification in the microenvironment during tumor initiation and progression.[1] On the other hand, certain researchers have shown antitumor functions of mast cells, including natural cytotoxicity and the release of antitumor compounds.[5]
The evaluation of blood vessels has been done by using different vascular markers in several recent studies. The most common utilized markers were VEGF, CD105, CD31, CD34, and von Willebrand factor (VWF).[8]
Some blood vessel antibodies, such as CD105, VWF, and CD34, have the ability to show small, large, and newly formed vessels, while CD31 specifies only the large vessels and the ability to stain tumor cells as well. Furthermore, CD105 does not stain mature vessels.[9] In addition, VWF identifies not only blood vessels but also lymph vessels.
Thus, considering the above points and the frequency of using CD34 in pathology laboratories and the ease of procedure, we used this marker (CD34) to evaluate blood vessels.
In this study, MVD was higher in the OSCC group compared with the normal mucosal group [Figure 1]. Similar results have been reported by earlier studies on OSCC.[4,7,10]
Iamaroon et al[7] and Pazouki et al[11] demonstrated a significant increase in vascularity during transition from normal tissue to early and late carcinoma through different degrees of dysplasia.[7,11]
An increase in the number of microvessels in the OSCC group can prove the role of angiogenesis in the creation and development of tumor.
In this study, a significant positive correlation between MCD and MVD was found in normal individuals while no statistically significant correlation between them was found in the OSCC group, and even in some cases reversed correlation was observed. It means that by increasing the number of microvessels, the less number of mast cells was observed. This can be attributed to other factors that may play a role in tumor angiogenesis.
It is also shown that the interplay between tumor cells and various constituents of the surrounding stroma may be critically important in various aspects of tumor biology, including the indirect induction of angiogenesis. Another factor that has been considered is the role of macrophages in tumor angiogenesis.[12,13]
Macrophages, constituents of tumor stroma, are members of the mononuclear phagocyte system of inflammatory cells. Although very heterogeneous, they perform a wide variety of functions depending on the physiologic or pathophysiologic condition to which they are recruited. The presence of macrophages in tumor stroma is a poor prognostic indicator in a number of neoplasms, including melanomas,[14] gliomas,[15] endometrial cancer,[16] and breast cancer.[17] It is thought that peripheral blood monocytes are recruited to the tumor microenvironment as a result of the secretion of various chemotactic cytokines such as colony-stimulating factor, granulocyte macrophage colony-stimulating factor, VEGF, TGFβ-1, and a number of angiogenic factors including bFGF, VEGF, tumor necrosis factor alpha, and interleukin-8 (IL- 8).[17–19] In head and neck SCCs, tumor cells attract monocytes and activate them to secrete angiogenic factors.[20] In addition, macrophages produce cytokines that act on tumor cells and stimulate them to produce increased levels of IL-8 and VEGF.[20]
Other factors produced by tumor cells that may be related to tumor angiogenesis are VEGF and bFGF. It is shown that tumors can produce stimulating factors in all stages of the formation of new capillaries. Tumor angiogenesis can be initiated by gathering endothelial precursor cells or available budding capillaries such as physiologic angiogensis.[21]
CONCLUSION
Overall, although our results showed a significant difference between the mean values of MCD and MVD in normal mucosa and OSCC, no positive correlation was observed between the values of MCD and MVD in the OSCC group.
We believe that due to the complexity of tumor angiogensis, in order to obtain a reliable relationship between MCD and MVD more studies are needed on different grades of OSCC and a large number of samples to confirm the results of this study; in addition, a study on other factors causing angiogenesis, aside mast cells, is required.
Footnotes
Source of Support: This report is based on a thesis and a research project which was submitted to School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSC degree in Oral and Maxillofacial pathology (#389069)
Conflict of Interest: None declared.
REFERENCES
- 1.Oliveira-Neto HH, Leite AF, Costa NL, Alencar RC, Lara VS, Silva TA, et al. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral Oncol. 2007;43:484–90. doi: 10.1016/j.oraloncology.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Costa NL, Oton-Leite AF, Cheim-Junior AP, Alencar Rde C, Bitar GO, Silva TA, et al. Density and migration of mast cells in lip squamous cell carcinoma and actinic cheilitis. Histol Histopathol. 2009;24:457–65. doi: 10.14670/HH-24.457. [DOI] [PubMed] [Google Scholar]
- 3.Michailidou EZ, Markopoulos AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;28:126–32. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macluskey M, Chandrachud LM, Pazouki S, Green M, Chisholm DM, Ogden GR, et al. Apoptosis, proliferation, and angiogenesis in oral tissues: Possible relevance to tumour progression. J Pathol. 2000;191:368–75. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH652>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Sharma B, Sriram G, Saraswathi TR, Sivapathasundharam B. Immunohistochemical evaluation of mast cells and angiogenesis in oral squamous cell carcinoma. Indian J Dent Res. 2010;21:260–65. doi: 10.4103/0970-9290.66655. [DOI] [PubMed] [Google Scholar]
- 6.Elpek GO, Gelen T, Aksoy NH, Erdogan A, Dertsiz L, Demircan A, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–4. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantarak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 8.Jaafari Ashkavandi Z, Moshref M, Mashhadi-Abbas F, Sargolzaie S, Taghavi N. Evaluation of CD31 expression and mast cell count in dysplastic lesions and squamous cell carcinoma of the oral cavity. J Iran Red Crescent Med. 2010;12:272–6. [Google Scholar]
- 9.Eshghyar N, Motahhari P, Rahrotaban S. Evaluation of microvascular density by CD34 in squamous cell carcinoma of the tongue and its relationship with cervical lymph node metastasis. J Dental Med. 2009;21:233. [Google Scholar]
- 10.Moriyama M, Kumagai S, Kawashiri S, Kojima K, Kakihara K, Yamamoto E. Immunohistochemical study of tumor angiogenesis in oral squamous cell carcinoma. Oral Oncol. 1997;33:369–74. doi: 10.1016/s1368-8375(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 11.Pazouki S, Chisholm DM, Adi MM, Carmichael G, Farquharson M, Ogden GR, et al. The association between tumour progression and vascularity in the oral mucosa. J Pathol. 1997;183:39–43. doi: 10.1002/(SICI)1096-9896(199709)183:1<39::AID-PATH1088>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Hasina R, Lingen MW. Angiogenesis in oral cancer. J Dent Educ. 2001;65:1282–90. [PubMed] [Google Scholar]
- 13.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 14.Brocker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41:562–7. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- 15.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–13. [PubMed] [Google Scholar]
- 16.Salvesen HB, Akslen LA. Significance of tumor-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumor angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84:538–43. doi: 10.1002/(sici)1097-0215(19991022)84:5<538::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993;342:148–9. doi: 10.1016/0140-6736(93)91348-p. [DOI] [PubMed] [Google Scholar]
- 18.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 19.Puszati L, Clover LM, Cooper K, Starkey PM, Lewis CE, McGee JO. Expression of tumor necrosis factor alpha and its receptors in carcinoma of the breast. Br J Cancer. 1994;70:289–92. doi: 10.1038/bjc.1994.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liss C, Fekete MJ, Hasina R, Lam CD, Lingen MW. Pracrine angiogenic loop between head-and-neck squamous cell carcinomas and macrophages. Int J Cancer. 2001;93:781–5. doi: 10.1002/ijc.1407. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Abbas AK, Fausto N. Robbins and cotran pathologic basis of disease. 7th ed. Philadelphia: Saunders Co; 2005. p. 309. [Google Scholar]


