Abstract
Aim:
The aim of this study was to evaluate a very short-term in vitro and in situ effect of 2% chlorhexidine-digluconate-based (CHX) cavity cleanser on the disinfection of dentin demineralized by cariogenic bacteria.
Materials and Methods:
Human dentin slabs were randomly allocated and used in 2 distinct phases, in vitro and in situ, for obtaining demineralized dentin. In vitro, the slabs (n=15) were immersed for 5 days in BHI broth inoculated with Streptococcus mutans CTT 3440. In situ, a double-blind design was conducted in one phase of 14 days, during which 20 volunteers wore palatal devices containing two human dental dentin slabs. On 5th day in vitro and 14th day in situ, the slabs were allocated to the two groups: Control group (5 μl of 0.9% NaCl solution) and CHX group (5 μl of 2% chlorhexidine digluconate solution, Cavity Cleanser™ BISCO, Schaumburg, IL, EUA), for 5 minutes. The microbiological analyses were performed immediately before and after the treatments.
Results:
The log reductions means found for CHX treatment on tested micro organisms were higher when compared to Control group either in vitro or in situ conditions.
Conclusions:
Our results showed that CHX was effective in reducing the cultivable microbiota in contaminated dentin. Furthermore, although the use of chlorhexidine-digluconate-based cavity disinfectant did not completely eliminate the viable microorganisms, it served as a suitable agent to disinfect tooth preparations.
Keywords: Atomic force microscopy, chlorhexidine, dentin, micro organisms
INTRODUCTION
The principle of maximum preservation of tooth structure is based on the early diagnosis of caries lesions with focus on the remineralizing action of dentinal zones affected by the carious process.[1,2] There are several perceptions about the quantity and quality of the carious tissue to be removed.[3] Dentin caries occurs in different layers of the tooth, in which the outer layer is highly infected with bacteria, resulting in an impossibility of remineralization. Nevertheless, the inner layer is less frequently contaminated with bacteria and although bacteria may dissolve the mineralized tissue, the cross-banded ultra structure of the collagen matrix is preserved. Accordingly, the dentin of this carious layer can be preserved and remineralized if these bacteria are removed or killed.[4,5]
Antimicrobial substances may be a potential choice for dentin disinfection, thus decreasing the possibility of infected tissue existence after tooth preparation. Thus, the use of antimicrobial solutions for cleaning the cavity after its preparation has been recommended. Chlorhexidine is a promising antimicrobial agent due to its ability to significantly reduce the levels of oral microorganisms.[6] Different formulations of chlorhexidine have been used for reducing the number of cariogenic bacteria in the saliva.[7,8] However, the very short-term antimicrobial effect of 2% chlorhexidine digluconate has not been evaluated. This is because in all the previous in vivo studies, chlorhexidine-based agents were analyzed after weeks or months[9] and in combination with subsequent restorations.[10]
The objective was to evaluate a very short-term in vitro and in situ effect of 2% chlorhexidine-digluconate-based cavity cleanser on the disinfection of dentin contaminated with cariogenic bacteria. The study hypothesis is that 2% chlorhexidine-digluconate is effective in reducing total viable microorganisms, total streptococci, mutans streptococci and lactobacilli in the tested conditions.
MATERIALS AND METHODS
Ethical aspects and study population
This study was performed in conformity with the norms of the Research and Ethics Committee of Federal University of Ceará (Process # 185/08). For the experiment, 85 extracted human third molars were stored in 0.01% (v/v) thymol solution at 4°C prior to use.[11] For the in situ phase, twenty adult volunteers who were able to comply with the experimental protocol were invited to participate, and an informed consent was obtained from all the participants.
Procedures employed
Specimen preparation
In order to remove the occlusal enamel of the teeth, a polishing device (Arotec, São Paulo, SP, Brazil) was used with silicon carbide waterproof 100 grit paper. 45 slabs of coronal dentin (5 × 5 × 2 mm3) were obtained using a water-cooled diamond saw and a cutting machine (IsoMet™ Low Speed Saw, Buehler, Lake Bluff, IL, USA). The specimens were then polished and immersed in distilled water, followed by sterilization by autoclaving to 121°C for 15 minutes.[12] The slabs were fixed in the lids of glass container vessels with plastic rods, and only the occlusal dentin face was used for the experiment. For the in situ study, the other 40 dentin slabs were selected and inserted into the palatal appliances. An intra oral removable acrylic palatal appliance was fabricated, in which 2 cavities (6 × 6 × 3 mm3) were prepared on the left and right sides; and one slab was attached with wax in each cavity [Figure 1].
Figure 1.
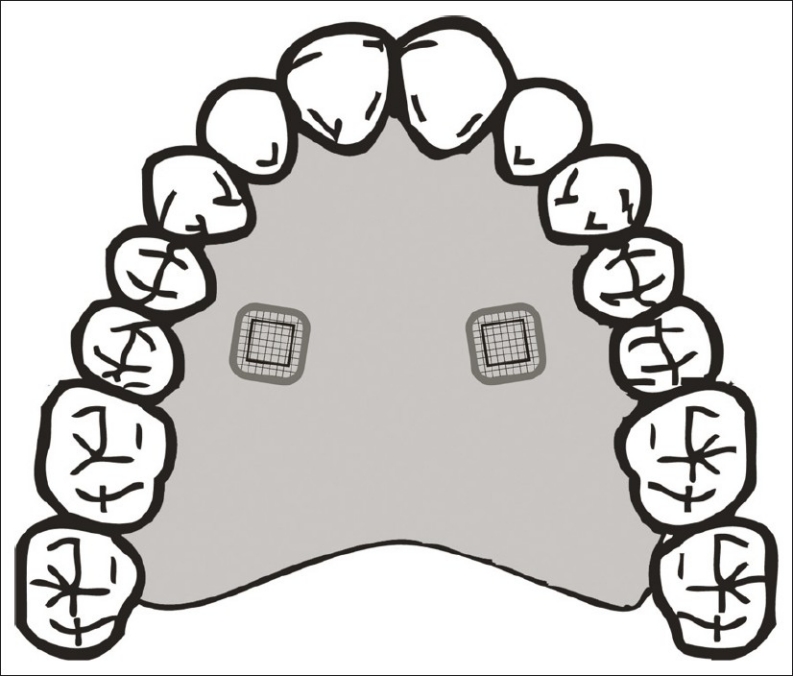
Schematic representation of the intra oral palatal device used in the study
Experimental design
In vitro experiment
Forty-five human dentin slabs were randomly allocated to 3 groups, with 15 experimental units per group, according to a computer generated randomization list.[13] The experimental design was performed in triplicate (n = 5). In addition to this, the study involved 2 set conditions in which carious dentin was exposed to 0.9% sodium chloride (NaCl) solution (Control) or 2% chlorhexidine digluconate (CHX) for 5 minutes, and a sterile group (untreated) was performed.
In vitro lesion production
Streptococcus mutans CTT 3440 was evaluated in this study. To prepare the inoculum, Streptococcus mutans was first grown in an overnight culture of brain heart infusion (BHI) (Difco Lab. Detroit, MI, USA) in a 10% carbon dioxide (CO2) atmosphere (Thermo Fisher Scientific Inc, Waltham, MA, USA). An Ultraviolet – visible (UV–Vis) spectrophotometer was used on the bacterial growth; a specific optical density was determined, and used for all samples to adjust the inoculum to the same cell number. The dentin slabs were immersed in sterile BHI containing 5% sucrose (w/v). All BHI-containing recipients, except the not inoculated group (untreated), were inoculated with 80 μl of 1-2 × 108 colony forming units (CFU)/ml overnight cultures of Streptococcus mutans. The dentin specimens were transferred to a fresh medium every day for 5 days. In addition, each BHI-containing recipient was streaked onto a new fresh BHI agar media plated and incubated at 37°C in an atmosphere of 10% CO2 for 24 hours to check for purity.
In situ experiment
A double-blind in situ design was conducted in one phase of 14 days, during which 20 volunteers wore palatal devices containing two human dental dentin slabs. At the end of the clinical phase, the slabs were randomly allocated into one of the following treatments: 0.9% NaCl solution (Control) and 2% chlorhexidinedigluconate (CHX).
Intraoral phase
For each volunteer, an acrylic palatal device containing two human dentin slabs was fabricated. In order to allow biofilm accumulation and to protect it from mechanical disturbance, a plastic mesh was positioned on the acrylic resin, leaving a 1-mm space from the slab surface.[14] The cariogenic challenge was provided by dripping a 40% sucrose solution[15] onto all dentin slabs 10 times a day at a pre-determined schedule.[16,17] The dentifrice treatment was performed 3 times a day, after meal times[18,19] and all volunteers consumed fluoridated water (0.70 mg F.l-1).
Treatments and microbiological analysis
On 5th day in vitro and 14th day in situ, the slabs were allocated to the two groups: Control group (5 μl of 0.9% NaCl solution) and CHX group (5 μl of 2% chlorhexidine digluconate solution, Cavity Cleanser™ BISCO, Schaumburg, IL, EUA), for 5 minutes. The microbiological analyses were performed immediately before and after the treatments. One baseline dentin sample was harvested from one intact half of each slab, using a #5 carbide bur in a low speed drill (Labor dental, São Paulo, SP, Brazil).[19,20] The second sample was obtained in the same way after performing the respective treatment. The dentin samples were analytically weighed using pre-weighed microcentrifuge tubes containing the bur, to which 0.9% NaCl solution was added (1 ml.mg-1 dentin). The suspension was serially diluted in 0.9% NaCl solution. Afterwards, samples were plated in triplicate in BHI (in vitro study); mitissali varius agar; and, mitissali varius agar plus 0.2 units of bacitracin ml-1; Rogosa agar and blood agar was used for in situ study. The plates were incubated for 48 hours at 37°C in a partial atmosphere of 10% CO2. Representative colonies were counted, and the results were expressed in CFU.mg-1 of dentin.
Atomic force microscopy
In order to verify changes in the Streptococcus mutans morphology of treated and non-treated bacterial cells, an Atomic force microscopy (AFM) analysis was utilized from the three samples of each group. Moreover, this analysis aimed to verify the presence of Streptococcus mutans inside the dentinal tubules and over dentin after biofilm removal, besides microbiological analyses. AFM images of the dentin substrate were obtained from the slabs submitted to the in vitro model. The slabs were randomly selected and scanned by AFM in different areas. All images were collected in the contact mode using sharpened silicon nitride cantilevers with experimentally determined spring constants of 0.15.Nm-1 and a tip radius of < 20 nm and at a scan rate of 0.5 Hz, using a Nanoscope III A Bioscope [(Veeco Digital Instruments, Santa Barbara, California, United States of America (USA)].
Statistical analysis
The log reduction results were calculated by subtraction of the initial values from the final values of CFU.mg/- 1 of dentin after being transformed by Log10. In order to compare differences between the treatments in vitro, a t-test for independent samples was applied. In the in situ data, a one-way analysis of variance (ANOVA) followed by a Tukey-Kramer test was used. The significance level was set at 5% (P<0.05). The software BioStat 2007 Professional (Analyst Soft Robust business solutions company, Vancouver, Canada) was used.
RESULTS
The log reductions means found for CHX treatment on tested microorganisms were higher when compared to Control group either in vitro or in situ conditions, as showed in Figure 2. In addition, no differences were found in the susceptibility of the several microorganisms collected from dentinal caries produced in situ, since very similar log reductions were verified ranging from 3.32 to 3.95 (P=0.720). Similarly, no difference was found between susceptibility for Streptococcus mutans to chlorhexidine in vitro and in situ, which presented log reductions of 3.71 and 3.95 respectively.
Figure 2.
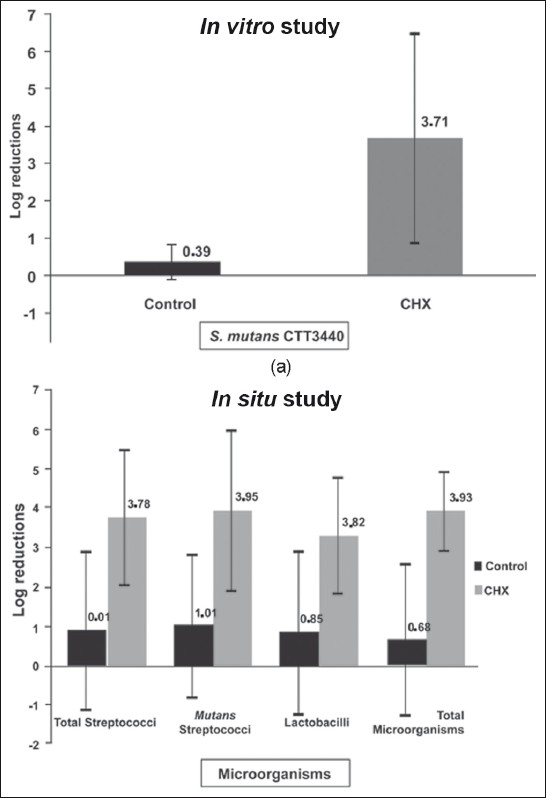
Log reductions and standard deviation obtained according to the treatments for in vitro and in situ phases. Chlorhexidine showed significantly higher bacterial reduction
With regard to atomic force microscopy analysis, data showed no morphological differences for Streptococcus mutans chains between the tested groups. However, the presence of the bacteria was evidenced over carious dentin as well as inside the dentin tubules [Figure 3].
Figure 3.
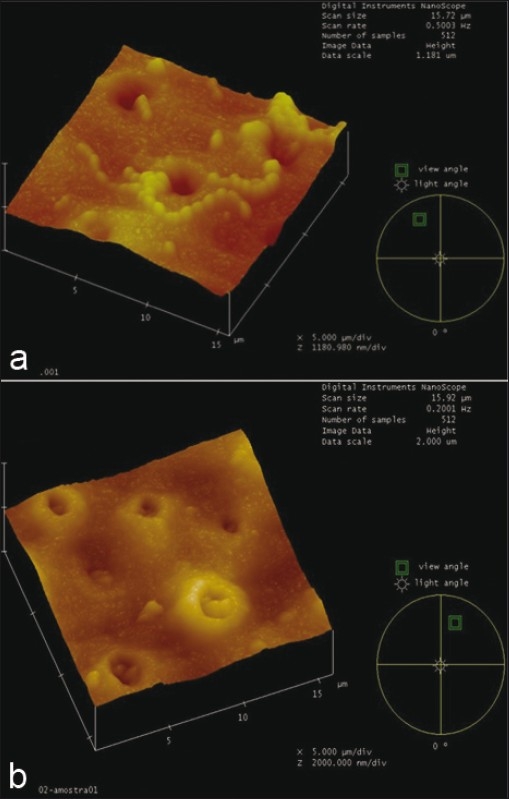
Dentine slab infected by Streptococcus mutans in vitro (a), highlighting streptococci mutans inside dentine tubules (b)
DISCUSSION
The antibacterial active substance CHX is widely considered to be the most effective agent against dental plaque and gingivitis.[21] Thus, all “new” antibacterial products have been compared with the “gold standard” CHX, for their effect on oral microorganisms.[22] However, in most former methods used, the examined bacteria were not available on/inside dentin substrate. The primary end point was infection of the dentin, quantitatively assessed by microbiological counts and qualitatively showed by AFM images, where Streptococcus mutans could be observed inside dentinal tubules in vitro. However, data showed no morphological differences for Streptococcus mutans chains among the tested groups and differences in its quantity were not expected, since bacterial cells were present even after death. Similar to this study, in previous in vitro studies, CHX vehicles were shown to be effective. They inhibited the development of the biofilm with respect to its maturation as well as the metabolism of the exposed bacteria was inhibited.[7,23]
Log reduction means obtained by CHX treatment for all tested microorganisms were similar suggesting that this antimicrobial agent is as efficient against Streptococcus mutans as it is against lactobacilli and total viable microorganisms. These results for lactobacilli are in contrast to those found by Ersin and colleagues,[9] who could not demonstrate a significant superior effect of chlorhexidine on lactobacillus. It should be emphasized that our study microbiological analysis was performed 5 min after CHX application in contrast to the 6 months period used by the latter authors. In addition, our study utilized a two fold more concentrated CHX solution, and Botelho[24] supplied evidence that lactobacilli are not insensitive to chlorhexidine and the inhibition follows a dose-response manner. Accordingly, one should assume that higher levels of the chlorhexidine digluconate directly applied onto tooth surfaces significantly reduce the counts of lactobacilli, as seen in this investigation. Furthermore, the in vivo methodology performed by Ersin and colleagues,[9] against the in situ methodology used in our study should be considered, since some species of Lactobacillus are more sensitive to chlorhexidine, whereas some of them are less sensitive.
With regards to the total viable microorganism count, the present results are in contrast to Wicht and colleagues,[10] who found that the total viable counts were not significantly affected after the application of chlorhexidine varnish as a cavity disinfectant onto the carious dentin. A possible explanation for this might be the different treatment procedures and materials. Furthermore, they suggested that using a larger sample, would reflect a more significant influence on the total viable microorganism counts by chlorhexidine. No other in situ studies have evaluated the antibacterial effects of CHX-based cavity disinfectant on cariogenic bacteria in dentin. According to our results, 2% digluconate chlorhexidine may be an appropriate disinfecting agent to be used in dentinal caries. Dentin tissue could be better preserved, thereby making patient treatment easier for the dentist and more comfortable for patients, and enabling lesions to be restored with minimal tissue removal, thus improving the long-term prognosis for the repaired tooth.[25] Our results showed that CHX was effective in reducing the cultivable microbiota. Furthermore, although the use of chlorhexidine-digluconate-based cavity disinfectant did not completely eliminate the viable microorganisms, it served as a suitable agent in reducing the residual microbiota. However, further clinical trials are necessary to determine its antibacterial and clinical effects in short and long term time frames.
Footnotes
Source of Support: This research received the fi nancial support of Grant # 477070/2008-6 from National Council for Scientifi c and Technological Development (CNPq). This paper was based on a thesis submitted by the fi rst author to the Faculty of Pharmacy, Dentistry and Nursing of Federal University of Ceará, in partial fulfi llment of the requirements for a MS degree in Dentistry.
Conflict of Interest: None declared.
REFERENCES
- 1.Mount GJ. A new paradigm for operative dentistry. Aust Dent J. 2007;52:264–70. doi: 10.1111/j.1834-7819.2007.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 2.Ten Cate J. Remineralization of caries lesions extending into dentin. J Dent Res. 2001;80:1407–11. doi: 10.1177/00220345010800050401. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee A, Kidd EA, Watson TF. In vitro validation of carious dentin removed using different excavation criteria. Am J Dent. 2003;16:228–30. [PubMed] [Google Scholar]
- 4.Ogushi K, Fusayama T. Electron microscope structure of the two layers of carious dentin. J Dent Res. 1975;54:1019–26. doi: 10.1177/00220345750540050301. [DOI] [PubMed] [Google Scholar]
- 5.Zavgorodniy AV, Rohanizadeh R, Swain MV. Ultrastructure of dentine carious lesions. Arch Oral Biol. 2008;53:124–32. doi: 10.1016/j.archoralbio.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 7.Petti S, Hausen H. Caries-preventive effect of chlorhexidine gel applications among high-risk children. Caries Res. 2006;40:514–21. doi: 10.1159/000095651. [DOI] [PubMed] [Google Scholar]
- 8.Van Strijp AJ, Gerardu VA, Buijs MJ, Van Loveren C, Ten Cate JM. Chlorhexidine efficacy in preventing lesion formation in enamel and dentine: An in situ study. Caries Res. 2008;42:460–5. doi: 10.1159/000170587. [DOI] [PubMed] [Google Scholar]
- 9.Ersin NK, Uzel A, Aykut A, Candan U. Inhibition of cultivable bacteria by chlorhexidine treatment of dentin lesions treated with the ART technique. Caries Res. 2006;40:172–7. doi: 10.1159/000091120. [DOI] [PubMed] [Google Scholar]
- 10.Wicht MJ, Haak R, Schütt-Gerowitt H, Kneist S, Noack MJ. Suppression of caries-related microorganisms in dentine lesions after short-term chlorhexidine or antibiotic treatment. Caries Res. 2004;38:436–41. doi: 10.1159/000079624. [DOI] [PubMed] [Google Scholar]
- 11.Gilmour AS, Edmunds DH, Newcombe RG. Prevalence and depth of artificial caries-like lesions adjacent to cavities prepared in roots and restored with a glass ionomer or a dentin-bonded composite material. J Dent Res. 1997;76:1854–61. doi: 10.1177/00220345970760120801. [DOI] [PubMed] [Google Scholar]
- 12.Pantera EA, Schuster GS. Sterilization of extracted human teeth. J Dent Educ. 1990;54:283–5. [PubMed] [Google Scholar]
- 13.Bolliger CT, Zellweger JP, Danielsson T, Van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: Double blind, randomized clinical trial of efficacy and safety. BMJ. 2000;321:329–33. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cury JA, Rebello MA, Del Bel CA, Derbyshire MT, Tabchoury CP. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34:491–7. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- 15.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre-dos-Santos M, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur J Oral Sci. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Duggal MS, Toumba KJ, Amaechi BT, Kowash MB, Higham SM. Enamel demineralization in situ with various frequencies of carbohydrate consumption with and without fluoride toothpaste. J Dent Res. 2001;80:1721–4. doi: 10.1177/00220345010800080801. [DOI] [PubMed] [Google Scholar]
- 17.Ccahuana-Vásquez RA, Tabchoury CP, Tenuta LM, Del Bel CA, Vale GC, Cury JA. Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride. Caries Res. 2007;41:9–15. doi: 10.1159/000096100. [DOI] [PubMed] [Google Scholar]
- 18.Sousa RP, Zanin IC, Lima JP, Melo MA, Beltrão HC, Rodrigues LK. In situ effects of restorative materials on dental biofilm and enamel demineralization. J Dent. 2009;37:44–51. doi: 10.1016/j.jdent.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Kidd EA, Joyston-Bechal S, Beighton D. Marginal ditching and staining as a predictor of secondary caries around amalgam restorations: A clinical and microbiological study. J Dent Res. 1995;74:1206–11. doi: 10.1177/00220345950740051001. [DOI] [PubMed] [Google Scholar]
- 20.Melo MA, De-paula DM, Lima JP, Borges FM, Steiner-Oliveira C, Nobre-dos-Santos M, et al. In vitro photodynamic antimicrobial chemotherapy in dentine contaminated by cariogenic bacteria. Laser Phys. 2010;20:1–10. [Google Scholar]
- 21.Auschill TM, Hein N, Hellwig E, Follo M, Sculean A, Arweiler NB. Effect of two antimicrobial agents on early in situbiofilm formation. J ClinPeriodontol. 2005;32:147–52. doi: 10.1111/j.1600-051X.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S, Giertsen E, Guggenheim B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002;36:93–100. doi: 10.1159/000057866. [DOI] [PubMed] [Google Scholar]
- 24.Botelho MG. The minimum inhibitory concentration of oral antibacterial agents against cariogenic organisms. Microbios. 2000;103:31–41. [PubMed] [Google Scholar]
- 25.Wilson M. Lethal photosensitization of oral bacteria and its potential application in the photodynamic therapy of oral infection. PhotochemPhotobiol Sci. 2004;3:412–8. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]


