Abstract
Aim:
To evaluate the bonding ability of composite to unset glass-ionomer cement (GIC) using different self-etching bonding systems.
Materials and Methods:
One hundred samples of composite bonded to unset GIC were prepared and were divided into four groups. In Group A, composite was bonded to unset GIC employing a strong (pH 1) self-etch primer was used. In Group B, intermediary strong (pH 1.4) self-etch primer was employed. In Group C and D, mild (pH 2) and (pH 2.2) self-etch primer was employed. Shear bond strength analysis was performed at a cross-head speed of 0.5 mm/min.
Results:
Statistical analysis performed with one way analysis of variance and Tukey's test showed that the bond strength of composite to unset GIC was significantly higher for the mild self-etch primer group. In addition, energy dispersive x-ray (EDX) analysis was used to determine the composition of various structural phases identified by FE-SEM along the GIC-bonding agent interfaces.
Conclusion:
Hence this present study concludes that clinically the use of mild self-etching bonding agent over unset GIC has improved bond strength compared to the use of strong and intermediate self-etching bonding agent.
Keywords: Bilayered/laminate restoration, composite resin, pH, self-etch bonding agent, unset glass-ionomer cement
INTRODUCTION
The use of bilayered restorations is one of the recommended composite restorative techniques used in dentistry, and they are known by various names such as double laminate technique or sandwich restorations.[1] The concept of the lamination technique is to use two different restorative materials to form one restoration. The rationale behind the technique is to make the most of the physical and esthetic properties of each material as it combines the dentin-adhesion and fluoride release of glass ionomer as well as the aesthetics and polishability of resin.[2]
The first laminated restorations used conventional auto-cure GIC that develops mechanical interlock between it and composite resin. McLean et al.[3] described bonding composite resin to GIC by etching the set GIC with phosphoric acid prior to applying resin bond. The clinical technique described by Mount[4] suggests etching the initially set GIC for 15 s prior to placing a layer of resin bond to develop a mechanical bond between the two materials. However, failure occurred due to sensitivity to moisture and the progressive loss of the GIC.[5]
The bond strength between conventional GICs and composites is limited by the low cohesive strength of glass-ionomers due to the lack of chemical bonding. This could be attributed to the difference in the setting reactions between dental composites and conventional GICs.[6] Bond strengths improve if the GIC is etched after 24 h of maturation.[7,8] However, this procedure requires an additional clinical visit to complete a restoration.
Knight et al.[9] have proved that the co-cured Resin modified Glass ionomer (RMGIC) bonding system (i.e., sequential layering of GIC, RMGIC and composite resin prior to photo-polymerization, and before the initial set of GIC) eliminates several placement steps and produces a significantly strong chemical bond between GIC and composite resin than the etch and rinse technique.
Gopikrishna et al.[10] provided two alternative techniques for the laminate restorations instead of employing the traditional total etch system. A clinician can employ a glass-ionomer adhesive system after the initial set of GIC or a self-etch primer over the unset GIC. Clinically the latter technique would be more useful as it not only do away with the etch and rinse procedure, but also saves valuable clinical time as it can be employed immediately after the placement of GIC in the cavity.
A self-etch approach involves either a two- or one-step application procedure. Depending on etching aggressiveness, they can be subdivided in to strong, intermediary, and mild self-etch adhesives.[11] “Strong” self-etch adhesives usually have a pH of 1 or below. This high acidity results in rather deep demineralization effects. At enamel, the resulting acid etch pattern resembles a phosphoric-acid treatment following an etch and rinse approach. At dentin, collagen is exposed and nearly all hydroxyapatite is dissolved.[12] Such low-pH self-etch adhesives have often been documented with rather low bond strength values, especially at dentin. Mild self-etching adhesive systems generally have a pH of approximately 2, and induce only a shallow partial demineralization no deeper than 1 μm in dentin. Micromechanical interlocking is obtained through hybridization of the microporous hydroxyapatite-coated collagen network.[13,14]
However, only few studies have reported the efficacy of bonding systems on GIC surface before the application of the resin composite in laminate restorations.[15] Hence, the aim of our study was to measure the shear bond strength of four different pH self-etching bonding agents to unset GIC and composite. The null hypothesis tested was that the pH of self-etching bonding agents has no effect on shear bond strength.
MATERIALS AND METHODS
Shear bond strength
One hundred samples were prepared and were divided into four groups of 25 samples each. A split Teflon mold (6 mm in diameter, 6 mm in height) was used to prepare glass ionomer cylinders (Fuji IX) of the dimension (6 mm in diameter, 3 mm in height). Composite material (Solare) was added to the surface of GIC to a height of 3 mm in increments following the application of the appropriate bonding agent to that group [Table 1].
Table 1.
Self-etching bonding agents used in the study
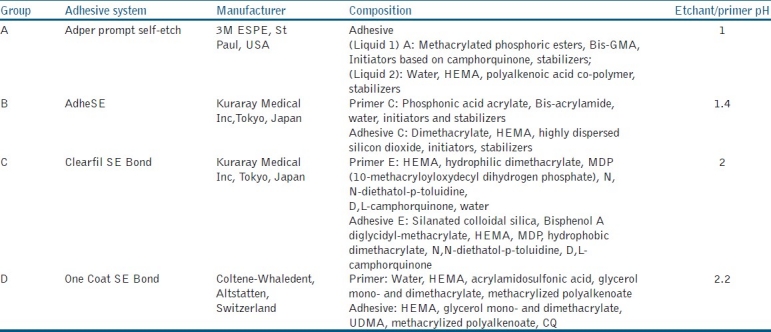
Group A
Capsulated GIC was mixed for 10 s using a capsule mixer and placed into the vertical mould. A freshly mixed adper prompt self-etch liquid A and liquid B were applied over the unset GIC with a microbrush, using continuous rubbing with light pressure for 15 s.[9] The adhesive was air dried with oil-free compressed air from an air syringe until it became a thin film and was light-cured for 10 s. Composite material was then added in increments to a height of 3 mm and each increment was light cured for 40 s.
Group B
Capsulated GIC was mixed for 10 s using capsule mixer and placed into the vertical mould. AdheSE Primer was applied over the unset GIC with a microbrush, using continuous rubbing with light pressure for 15 s. The excess primer was dispersed and air dried with oil-free compressed air from an air syringe until the mobile liquid film disappeared. The bonding resin AdheSE Bond was applied, gently air blown and light cured for 10 s. Composite material was then added in increments to a height of 3 mm and light cured for 40 s.
Group C
Capsulated GIC was mixed for 10 s using a capsule mixer and placed into the vertical mould. Clearfil SE Primer was applied over the unset GIC with a microbrush, using continuous rubbing with light pressure for 20 s. The excess primer was dispersed and air dried with oil-free compressed air from an air syringe until it became a thin film. The bonding resin Clearfil SE Bond was applied, gently air dried and light cured for 20 s. Composite material was then added in increments to a height of 3 mm and each increment was light cured for 40 s.
Group D
Capsulated GIC was mixed for 10 s using capsule mixer and placed into the vertical mould. One coat SE bond applied in two consecutive coats over the unset GIC with a microbrush, using continuous rubbing with light pressure for 20 s. It is gently air dried with oil-free compressed air from an air syringe and light cured for 10 s. Composite material was then added in increments to a height of 3 mm and each increment was light cured for 40 s.
The samples were removed from the mould and were mounted in square metal jig (1 × 1 inch) filled with auto cure acrylic resin. The samples were then placed in 100% humidity at room temperature for 48 h. The shear bond strength was determined using the Universal testing machine (Instron 8500, Instron Corporation, Canton, USA) at a cross-head speed of 1 mm/min, utilizing a device constructed to direct the shearing force on the glass-ionomer composite interface.
Finite element scanning electron microscopy/Energy dispersive X-ray analysis
Three samples (Set GIC, Unset GIC with strong and mild pH) were prepared. The samples were examined at 30 kv without being coated,[16] with the temperature of the Peltier stage fixed at 4°C and the vapor pressure of the specimen chamber varying between 5.4 and 5.9 Torr to generate a relative humidity of 96–99.9%. In each sample, FESEM/EDX analysis was performed in three randomly selected spots. FESEM was used to determine the effects of different pH environments on morphological characteristics of cured cement disks. The criteria for evaluation included the presence of cracks and micropores at the surface of the material. Quantitative elemental changes of the material surface were evaluated using EDX.
RESULTS
The mean shear bond strength and standard deviation were computed and analyzed by one-way analysis of variance (ANOVA) and Tukey's test at significance level 0.05. The mean shear bond strength and standard deviation are shown in Table 2.
Table 2.
Mean shear bond strength of study groups (n=25)
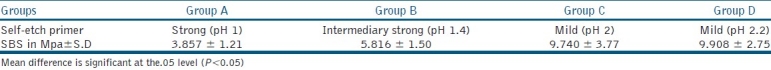
Group C and D, (mild self-etch primer pH 2 and 2.2) showed significantly higher bond strength than group A and B (P<0.05). Group D showed no significant difference in bond strength from Group C. Group B (intermediary strong self etch primer pH 1.4) showed significant bond strength from group A. Group A (strong self etch primer pH 1) showed the least bond strength values.
FESEM analysis showed morphological differences between the three samples. More homogenous material structure was observed in set GIC sample [Figure 1]. Destruction of the material surface was evident in GIC with strong self-etch sample compared to other samples. FESEM analysis revealed the presence of a large number of voids and cracks in GIC with strong self-etch sample [Figure 2]. Although microporosities were present, yet destructions like large cracks were not seen in GIC with mild self-etch sample [Figure 3]. Elemental analysis showed significant reduction in the weight percentage of elements in GIC with the strong self-etch sample, whereas elemental composition is same in both GIC sample and GIC with mild self-etch sample.
Figure 1.
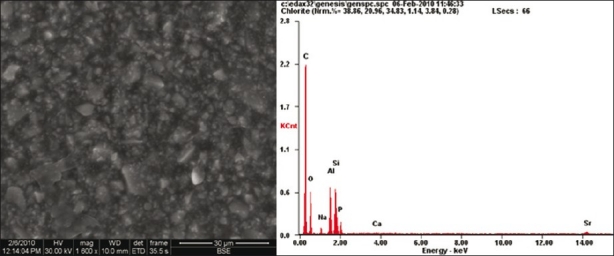
FESEM / EDX showing set glass ionomer cement and its elemental composition
Figure 2.
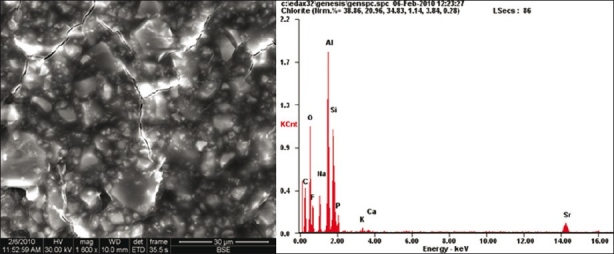
FESEM/EDX showing unset glass ionomer cement with strong self-etch bonding agent (pH 1)
Figure 3.
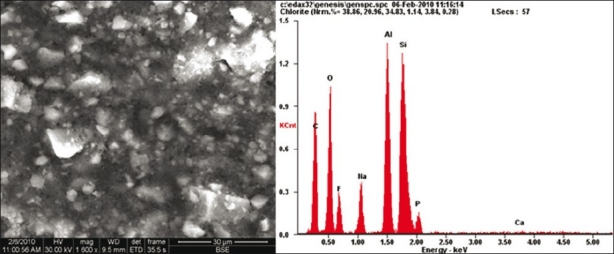
C. FESEM / EDX showing unset glass ionomer cement with mild self-etch bonding agent (pH 2.2)
DISCUSSION
Bond strength of composite resin to glass-ionomer cement can be better when a self-etching primer is employed over unset GIC when compared to the use of total-etch adhesive.[10] The unset form of glass ionomer cement provides a more conducive medium for effective chemical reaction.
The concept of self-etch primers was first introduced with Scotchbond 2 (3M) in the early 1990s. However, this system was advocated only to be applied on dentin alone, and therefore required clinically selective-enamel etching in a separate step. The current self-etch adhesives provide monomer formulations for simultaneous conditioning and priming of both enamel and dentin. Most common self-etch adhesives involve two application steps with the self etch primer followed by an adhesive resin, resulting in two-step self-etch adhesives. Recently, one-step self-etch or so-called all-in-one adhesives combining the conditioning, priming and the application of an adhesive resin into a single application have been marketed.
Besides, on the basis of the number of application steps, self-etch adhesives should also be subdivided into mild, intermediary strong, and strong self-etch adhesives, depending on their pH and thus etching potential.[17] “Intermediary strong” self-etch adhesives exhibited significantly superior shear bond strength values compared to both “strong” and “mild” self-etch systems, which implies that pH of the adhesive has a significant effect on the bond strength.[15]
The bonding mechanisms of restorative materials to tooth tissues are often explained in the literature.[7] Yet, few studies have addressed aspects regarding the restorative materials used in sandwich techniques.[18] Hence, the purpose of this study is to evaluate the influence of pH in bonding between GIC and composite using the different self-etch bonding systems.
From the results of the study, Group C and D mild self-etching systems showed the highest bond strength values. Elemental analysis of GIC with mild self-etch showed similar ion concentration to that of set GIC without any bonding agent and FESEM showed few microporosities without any destructions like large cracks as seen on GIC with a strong self-etch sample. Based on organic chemistry, when there is only a mild acid attack, there will be minimal flushing of ions, and hence salt crumps formation will be minimal.[19] As a consequence, unflushed cations such as Na+, Ca2+, Al3+ ions will be available in sufficient large amounts to effectively interact, especially in a conducive reaction medium (i.e., unset GIC) toward establishing effective and strong ionic interactions with available bonding agents.[20,21]
The results of the study showed that group A with a strong self-etch bonding system, the bond strength values were least. With stronger acids there will be more neutralisation of the cations and formation of salt crumps (crusts) which is a weaker structure and fragile form, thereby resulting in a weaker bonding.[19,22] The neutralization of ions was evident with the elemental analysis showing significant reduction in the weight percentage of ions in GIC with a strong self-etch sample. FESEM analysis provided direct visibility of the changes at the material surface like the presence of a large number of voids and cracks that could have resulted in increased bond failure.
CONCLUSION
In conclusion, this study proves that clinically the use of mild self-etching bonding agent over unset GIC has improved bond strength compared to the use of strong and intermediate self-etching bonding agent.
Clinical relevance
The bond strength of resin composite to glass-ionomer cement can be improved when a mild self-etching-bonding agent is employed over unset GIC compared to the use of strong self-etching bonding agent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Giachetti L, Bertini F, Bambi C, Scaminaci Russo D. A rationale use of dental materials in posterior direct resin restorations in order to control polymerisation shrinkage stress. Minerva Stomatol. 2007;56:129–38. [PubMed] [Google Scholar]
- 2.Farah CS, Orton VG, Collard SM. Shear bond strength of chemical and light-cured glass ionomer cements bonded to resin composites. Australian Dent J. 1998;43:81–6. doi: 10.1111/j.1834-7819.1998.tb06095.x. [DOI] [PubMed] [Google Scholar]
- 3.Mclean JW, Powis DR, Prosser HJ, Wilson AD. The use of glass ionomer cements in bonding composite resin to dentine. Br Dent J. 1985;158:410–4. doi: 10.1038/sj.bdj.4805621. [DOI] [PubMed] [Google Scholar]
- 4.Mount GJ. Clinical placement of modern glass-ionomer cements. Quintessence Int. 1993;24:99–107. [PubMed] [Google Scholar]
- 5.Wellbury RR, Murray JJ. A clinical trial of the glass ionomer cement-composite resin “sandwich technique” in class II cavities in permanent premolar and molar teeth. Quintessence Int. 1990;21:507–12. [PubMed] [Google Scholar]
- 6.Li J, Liu Y, Soremark R, Sundstrom F. Flexural strength of resin-modified glass ionomer cements and their bond strength to dental composites. Acta Odontol Scand. 1996;54:55–8. doi: 10.3109/00016359609003510. [DOI] [PubMed] [Google Scholar]
- 7.Kingsford Smith ED, Martin FE. Acid etching of glass ionomer cement base. Aust Dent J. 1990;35:236–40. doi: 10.1111/j.1834-7819.1990.tb05400.x. [DOI] [PubMed] [Google Scholar]
- 8.Taggart SE, Pearson GJ. The effect of etching on glasspolyalkenoate cements. J Oral Rehabil. 1991;18:31–42. doi: 10.1111/j.1365-2842.1991.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 9.Knight GM, McIntyre JM, Mulyani Bond strengths between composite resin and auto cure glass ionomer cement using the co-cure technique. Aust Dent J. 2006;51:175–9. doi: 10.1111/j.1834-7819.2006.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 10.Gopikrishna V, Abarajithan M, Krithikadatta J, Kandaswamy D. Shear bond strength evaluation of resin composite bonded to GIC using three different adhesives. Oper Dent. 2009;34:467–71. doi: 10.2341/08-009-L. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems.I: Depth of penetration beyond dentin smear layers. Dent Mater. 2001;17:296–308. doi: 10.1016/s0109-5641(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 12.Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives, part II: Etching effects on unground enamel. Dent Mater. 2001;17:430–44. doi: 10.1016/s0109-5641(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 13.Perdigao J, May KN, Jr, Wilder AD, Jr, Lopes M. The effect of depth of dentin demineralization on bond strengths and morphology of the hybrid layer. Oper Dent. 2000;25:186–94. [PubMed] [Google Scholar]
- 14.Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, et al. Microtensile bond strength of eleven modern adhesives to dentin. J Adhes Dent. 2001;3:237–45. [PubMed] [Google Scholar]
- 15.Bharadwaj N, Karthikeyhan KS, Sukumaran VG. Comparative shear bond strength analysis of strong, intermediary strong and mild ‘self- etch’ systems, and a hypothesis on their chemical behavior on dentin. J Conserv Dent. 2005;8:24–30. [Google Scholar]
- 16.Yiu CK, Tay FR, King NM, Pashley DH, Sidhu SK, Neo JC, et al. Interaction of Glass-ionomer Cements with Moist Dentin. J Dent Res. 2004;83:283–9. doi: 10.1177/154405910408300403. [DOI] [PubMed] [Google Scholar]
- 17.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Adhesion to Enamel and Dentin: Current status and furture challenges. Oper Dent. 2003;283:215–35. [PubMed] [Google Scholar]
- 18.Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83:454–8. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]
- 19.Morrison RT, Boyd RN. Organic chemistry. 5th ed. New Delhi: Prentice-Hall India Ltd; 1987. p. 25. [Google Scholar]
- 20.Arora V, Kundabala M, Parolia A, Thomas MS, Pai V. Comparison of the shear bond strength of RMGIC to a resin composite using different adhesive systems: An in vitro study. J Conserv Dent. 2010;13:80–3. doi: 10.4103/0972-0707.66716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutinho E., Landuyt K. Van, De Munck J., Poitevin A., Yoshida Y., Inoue S., Peumans M., Suzuki K., Lambrechts P., Van Meerbeek B. Development of a Self-etch Adhesive for Resin-modified Glass Ionomers. J Dent Res. 2006;85:349. doi: 10.1177/154405910608500413. [DOI] [PubMed] [Google Scholar]
- 22.Crisp S, Wilson AD. Reactions in Glass Ionomer Cements: III. The Precipitation Reaction. J Dent Res. 1974;53:1420–4. doi: 10.1177/00220345740530062101. [DOI] [PubMed] [Google Scholar]


