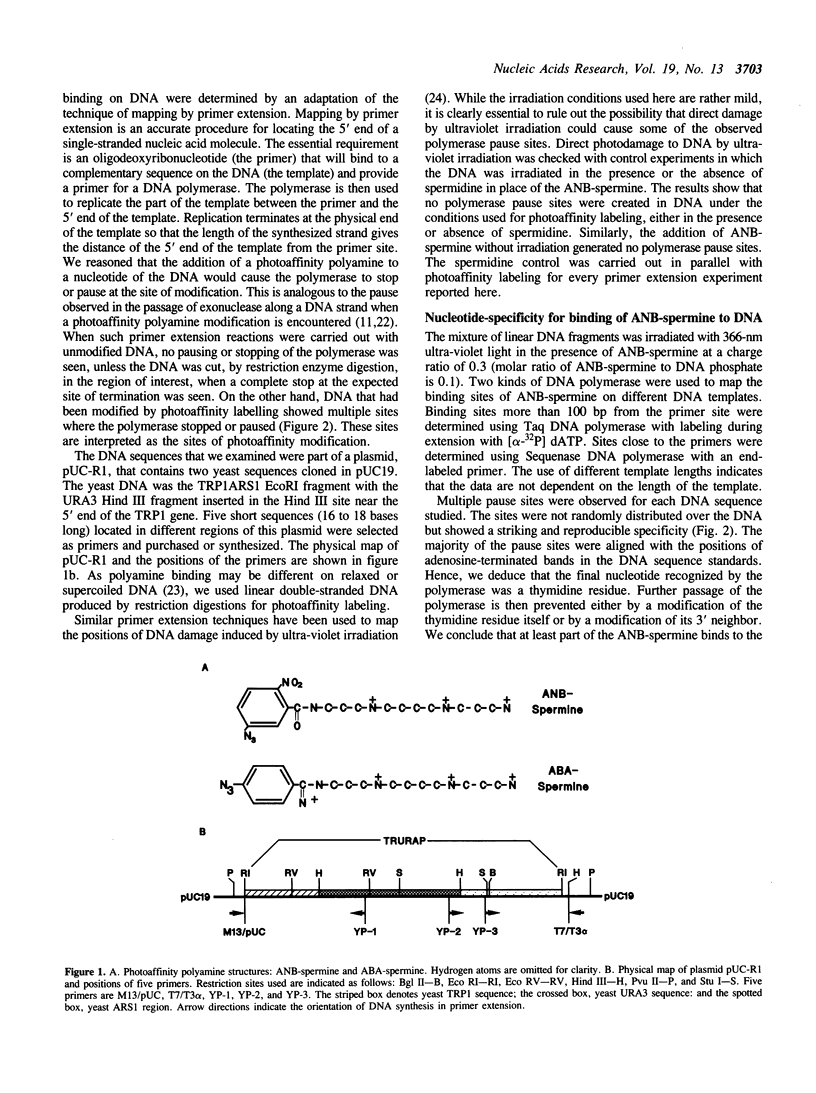
Abstract
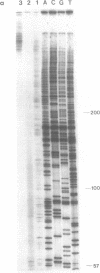
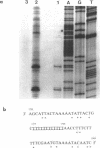
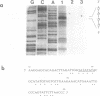
ANB-spermine is a photoaffinity analog of the naturally-occurring polyamine, acetylspermine. ANB-spermine was used to determine its binding sites on naked double stranded DNA, at the nucleotide level, using a modification of the primer extension technique. A total of 1,275 nucleotides was examined in 5 sequences of DNA from Saccharomyces cerevisiae. Binding sites were non-random. The primary determinant of binding was the presence of a thymidine residue. Secondary determinants appeared to depend on the secondary structure of the DNA, with runs of thymidines providing unusually poor binding sites while TA and, especially, TATA providing the strongest binding sites. The 'TATA element' upstream of the URA3 gene from S. cerevisiae was the strongest binding site. The data indicate that ANB-spermine binding to DNA is a probe for DNA secondary structure and suggest a role for polyamines in regulating the structure of chromatin in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu H. S., Schwietert H. C., Feuerstein B. G., Marton L. J. Effects of variation in the structure of spermine on the association with DNA and the induction of DNA conformational changes. Biochem J. 1990 Jul 15;269(2):329–334. doi: 10.1042/bj2690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu H. S., Shafer R. H., Marton L. J. A stopped-flow H-D exchange kinetic study of spermine-polynucleotide interactions. Nucleic Acids Res. 1987 Jul 24;15(14):5873–5886. doi: 10.1093/nar/15.14.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Clark E., Swank R. A., Morgan J. E., Basu H., Matthews H. R. Two new photoaffinity polyamines appear to alter the helical twist of DNA in nucleosome core particles. Biochemistry. 1991 Apr 23;30(16):4009–4020. doi: 10.1021/bi00230a028. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Drew H. R., McCall M. J., Calladine C. R. Recent studies of DNA in the crystal. Annu Rev Cell Biol. 1988;4:1–20. doi: 10.1146/annurev.cb.04.110188.000245. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. Structural junctions in DNA: the influence of flanking sequence on nuclease digestion specificities. Nucleic Acids Res. 1985 Jun 25;13(12):4445–4467. doi: 10.1093/nar/13.12.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Wing R. M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler W., Corr R. Polyamine effects on DNA-directed RNA polymerases in the ciliate Tetrahymena thermophila. In vivo- and in vitro-experiments suggesting highly specific regulative interactions. Biol Chem Hoppe Seyler. 1989 May;370(5):451–466. doi: 10.1515/bchm3.1989.370.1.451. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Basu H. S., Marton L. J. Theoretical and experimental characterization of polyamine/DNA interactions. Adv Exp Med Biol. 1988;250:517–523. doi: 10.1007/978-1-4684-5637-0_46. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990 Mar 11;18(5):1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Spermine-DNA interactions: a theoretical study. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5948–5952. doi: 10.1073/pnas.83.16.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J. M., Nissen K. A., Smerdon M. J. UV-induced formation of pyrimidine dimers in nucleosome core DNA is strongly modulated with a period of 10.3 bases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6644–6648. doi: 10.1073/pnas.84.19.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. A., Mandecki W. Gene synthesis by serial cloning of oligonucleotides. DNA. 1988 Oct;7(8):571–577. doi: 10.1089/dna.1.1988.7.571. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. S., Jue C. Direct sequencing of lambda-gt11 recombinant clones. Biotechniques. 1990 Feb;8(2):156–160. [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Lipanov A. A., Chuprina V. P. The structure of poly(dA):poly(dT) in a condensed state and in solution. Nucleic Acids Res. 1987 Jul 24;15(14):5833–5844. doi: 10.1093/nar/15.14.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Marczynski G. T., Jaehning J. A. A transcription map of a yeast centromere plasmid: unexpected transcripts and altered gene expression. Nucleic Acids Res. 1985 Dec 9;13(23):8487–8506. doi: 10.1093/nar/13.23.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet R., Houssier C. Different binding modes of spermine to A-T and G-C base pairs modulate the bending and stiffening of the DNA double helix. Biochem Pharmacol. 1988 May 1;37(9):1857–1858. doi: 10.1016/0006-2952(88)90481-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Blankenship J. W., Matthews H. R. Association constants for the interaction of double-stranded and single-stranded DNA with spermine, spermidine, putrescine, diaminopropane, N1- and N8-acetylspermidine, and magnesium: determination from analysis of the broadening of thermal denaturation curves. Arch Biochem Biophys. 1986 Apr;246(1):225–232. doi: 10.1016/0003-9861(86)90467-4. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Blankenship J. W., Matthews H. R. Polyamines and acetylpolyamines increase the stability and alter the conformation of nucleosome core particles. Biochemistry. 1987 Jun 16;26(12):3643–3649. doi: 10.1021/bi00386a058. [DOI] [PubMed] [Google Scholar]
- Negri R., Costanzo G., Venditti S., Di Mauro E. Linkage reduction allows reconstitution of nucleosomes on DNA microdomains. J Mol Biol. 1989 Jun 5;207(3):615–619. doi: 10.1016/0022-2836(89)90469-5. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Plum G. E., Bloomfield V. A. Effects of spermidine and hexaamminecobalt(III) on thymine imino proton exchange. Biochemistry. 1990 Jun 26;29(25):5934–5940. doi: 10.1021/bi00477a008. [DOI] [PubMed] [Google Scholar]
- Saluz H. P., Jost J. P. Genomic footprinting with Taq polymerase. Nature. 1989 Mar 16;338(6212):277–277. doi: 10.1038/338277a0. [DOI] [PubMed] [Google Scholar]
- Satchwell S. C., Drew H. R., Travers A. A. Sequence periodicities in chicken nucleosome core DNA. J Mol Biol. 1986 Oct 20;191(4):659–675. doi: 10.1016/0022-2836(86)90452-3. [DOI] [PubMed] [Google Scholar]
- Stewart K. D. The effect of structural changes in a polyamine backbone on its DNA-binding properties. Biochem Biophys Res Commun. 1988 May 16;152(3):1441–1446. doi: 10.1016/s0006-291x(88)80447-9. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F. Protein-DNA interactions and nuclease-sensitive regions determine nucleosome positions on yeast plasmid chromatin. J Mol Biol. 1986 Jul 20;190(2):177–190. doi: 10.1016/0022-2836(86)90291-3. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- Viswamitra M. A., Kennard O., Jones P. G., Sheldrick G. M., Salisbury S., Favello L., Shakked Z. DNA double helical fragment at atomic resolution. Nature. 1978 Jun 22;273(5664):687–688. doi: 10.1038/273687a0. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Frederick C. A., Ughetto G., Rich A. Ternary interactions of spermine with DNA: 4'-epiadriamycin and other DNA: anthracycline complexes. Nucleic Acids Res. 1990 Sep 25;18(18):5533–5541. doi: 10.1093/nar/18.18.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A., Scott J. F. Construction, replication, and chromatin structure of TRP1 RI circle, a multiple-copy synthetic plasmid derived from Saccharomyces cerevisiae chromosomal DNA. Mol Cell Biol. 1982 Mar;2(3):221–232. doi: 10.1128/mcb.2.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska K., Pullman B. Spermine-nucleic acid interactions: a theoretical study. Biopolymers. 1986 Mar;25(3):375–392. doi: 10.1002/bip.360250302. [DOI] [PubMed] [Google Scholar]