Abstract
Objectives:
To find the efficacy of serial extracts of Anisomeles malabarica in inhibiting proliferation of and inducing apoptosis in human cervical cancer cells, SiHa and ME 180, that are HPV 16-positive.
Materials and Methods:
The whole plant was extracted in n-hexane, chloroform, ethyl acetate, n-butanol, methanol, and water. The cells were treated with the extracts at increasing concentrations to find the IC50, adopting MTT ([3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) assay. Acridine orange (AO) and ethidium bromide (EB) and Hoechst 33258 staining were adopted to assess the mode of cell death, Annexin V-Cy3 staining to evaluate one of the early apoptotic features, JC-1 staining to assess the mitochondrial membrane depolarization, comet assay for DNA fragmentation, and cell cycle analysis for the distribution of cells after treatment.
Results:
n-Hexane and chloroform extracts were cytotoxic to the cervical cancer cells in dose- and duration-dependent manner. The cells that responded to the treatments revealed typical apoptotic features. Early features of apoptosis, phosphatidyl serine translocation and loss of mitochondrial trans-membrane potential, were observed in the treated cells, and comet assay revealed DNA damage. In the FACS analysis, the cells accumulated in the sub-G0/G1 phase of the cell cycle, except in n-hexane- and chloroform extract–treated SiHa cells at 24 h, which showed arrest in S- and G2/M phases.
Conclusions:
n-Hexane and chloroform extracts of A. malabarica inhibit proliferation of and induce death in HPV16-positive cervical cancer cells, mostly by apoptosis and to some extent by necrosis.
Keywords: Anti-cancer, apoptosis, cell cycle arrest, cervical cancer
INTRODUCTION
Cervical cancer is the second largest cause of cancer deaths in women worldwide. Human papilloma viruses (HPVs) are the causative agents of over 99% of cervical cancers. The development of cervical cancers involves a coordinated targeting of multiple pathways by HPV oncoproteins, with each pathway having a distinct role in the malignant progression.[1] Based on the ability to induce malignant transformation, the HPVs are distinguished into low-risk and high-risk viruses. The high-risk HPVs are considered the primary causative agents of morbidity in cervical cancer, and HPV-16 and HPV-18 belong to this group. Integration of the HPV-16 and HPV-18 oncoproteins E6 and E7 is the critical step in the development of cancer and immortalization of the infected cells.[2] Most HPV-positive cervical cancer cells possess wild-type p53 and Rb genes, but their normal functions are disrupted by the expression of HPV's E6 and E7, respectively.[3] The HPV-E6 associates with ubiquitin-protein ligase E6-AP and, subsequently, interacts with p53, resulting in its degradation in the proteasome.[4] The E6 also increases the telomerase activity above its critical point, thus contributing to immortality of the cells.[5] The HPV-E7 protein interacts with retinoblastoma protein (Rb) and causes release of the transcription factor E 2 F bound to Rb, which in turn causes induction of expression of genes involved in cell proliferation. Therefore, restoration of p53 and Rb functions by blocking E6/p53 and E7/Rb pathways might be a potential therapeutic approach for these cancers.[6] Since the current chemotherapy drugs by themselves are rather inefficient against HPV-positive cervical cancers, new therapeutic approaches and newer drugs are needed.[7] It is believed that alternative interventions such as preparations from herbs can potentially replace or supplement the current regimens.[8]
A wide array of plants has been reported to possess substantial anticarcinogenic and antimutagenic activities. Plants have played an important role as sources of effective anticancer agents, and it is significant that over 60% of currently used anticancer agents are derived in one way or the other from natural sources, including plants, marine organisms, and microorganism.[9] Vincristine, etoposide, taxanes, and camptothecins are all examples of plant-derived anticancer compounds. In the present study, we treated the HPV-positive SiHa and ME180 cells with the extracts of Anisomeles malabarica and examined whether the extracts would be cytotoxic to these cancer cells and induce apoptotic cell death.
A. malabarica (L.) R. Br. is an herb belonging to the family Lamiaceae. This plant, commonly called Malabar catmint, is recommended in ancient medicine for use in catarrh, intermittent fever, bowel disorder, and cancer.[10] Traditional healers in some pockets in India use it in cancer therapy. The plant is also believed to possess antispasmodic, diaphoretic, antipyretic, and antiperiodic properties. The bitter infusion obtained from the leaves of the plant has been in common use in South India for stomach and bowel disorders, catarrhal afflictions, and intermittent fevers. The essential oil obtained from the leaf of this plant is externally applied for treatment of rheumatism.[11] The aqueous ethanolic (50%) extract of the plant has been shown to possess significant anticancer activity.[12] Another highly preliminary study revealed an anticancer effect of the ethanolic extract of this plant in 7,12-dimethylbenz[α]anthracene (DMBA)–induced carcinogenesis in male Swiss albino mice.[13] A. malabarica has been reported to contain secondary metabolites such as anisomelic acid, ovatodiolide, geranic acid, citral,[14–17] betulinic acid, and beta-sitosterol.[18] Ovatodiolide[19] and anisomelic acid[14,15] have been shown to exert cytotoxic effect in a few cancer cells. However, to date, A. malabarica has not been tested against any HPV-positive cervical cancer. Therefore, developing upon the ethnomedical and scientific information so far available, the present study was undertaken to evaluate the cytotoxic property of A. malabarica in HPV16-positive cervical cancer cell lines.
MATERIALS AND METHODS
Plant material
A. malabarica was collected from the outskirts of Tiruchirappalli, India [latitude: N 10° 16’–11°22’ and longitude: E 78° 15’–79° 16’] and identified by the Director of Rapinat Herbarium, St. Joseph's College, Tiruchirappalli, India, an authoritative botanical referral center. A voucher specimen (PRP-001) was deposited in the herbarium. The whole plant was washed, shade-dried, and powdered in a mixer.
Serial extraction
The powdered plant material (100 g) was serially extracted with solvents of increasing polarity, viz., n-hexane, chloroform, ethyl acetate, n-butanol, methanol, and water. All reagents were procured from Merck Chemicals, Mumbai, India. The extracts were concentrated to a paste in vacuum at 40°C using a rotary evaporator. The paste obtained was transferred to vials and kept at 4°C until use.
Cell cultures
Human cervical cancer cells SiHa and ME 180 were obtained from the National Center for Cell Science (NCCS), Pune, and maintained in DMEM medium (Biochrom AG, Berlin, Germany), supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), and with 100 μg/ml streptomycin and 100 U/ml penicillin (Himedia, Mumbai, India) as antibiotics. The cells were stored at 37°C in a humidified atmosphere of 5% CO2 in a CO2 incubator (Heraeus, Hanau, Germany).
Determination of the cytotoxic property of the extracts adopting MTT assay
To evaluate the cytotoxic property of A. malabarica extracts, MTT colorimetric assay was performed.[20] The extracts were dissolved in DMSO (dimethyl sulfoxide) (Sigma Chemical Co., St. Louis, MO, USA). The cells were seeded in 96-well plates at a density of 5 × 104 cells/well and treated with the n-hexane, chloroform, ethyl acetate, n-butanol, methanol, and aqueous extracts at concentrations ranging 0–200 μg/ml, at 37°C, for 24 h and 48 h. At the end of the exposure period, the cells were subjected to assessment of viability using the MTT assay. Data were collected for four replicates each and used to calculate the respective means and the standard deviations. The percentage inhibition was calculated from this data, using the formula:

The IC50 concentration was determined as the dose that would be required to kill 50% of the cells.
Fluorescent staining for morphological assessment
Acridine orange (AO) and ethidium bromide (EB) staining
The cervical cancer cells were seeded in 6-well plates and allowed to reach 70% confluence. The cells were then treated with the IC50 concentration of n-hexane and chloroform extracts (which produced the best results as revealed in the MTT assay) and incubated for 24 h and 48 h. The cells were trypsinized and pelleted and then suspended in PBS (Phosphate Buffered Saline). A drop of cell suspension was placed on a glass slide and stained with AO and EB (Sigma Chemical Co., St. Louis, MO, USA), and a cover slip was laid over it to reduce light diffraction. At random 300 cells were observed in a fluorescent microscope (Carl Zeiss, Jena, Germany) fitted with a 377–355 nm filter and examined at ×400 magnification. The percentage of cells reflecting pathological changes was calculated. Data were collected for four replicates and used to calculate the respective means and the standard deviations.
Hoechst 33258 staining
The cell suspension, as above, was also stained with Hoechst 33258 (Sigma Chemical Co., St. Louis, MO, USA) and incubated at 37°C for 15 min. At random, 300 cells were observed in the fluorescent microscope at ×400 magnification and the percentage of cells reflecting pathological changes was calculated. Data were collected for four replicates and used to calculate the respective means and the standard deviations.
Measurement of mitochondrial transmembrane potential (ΔΨm)
Mitochondrial transmembrane potential, ΔΨm, is an important parameter of mitochondrial function that is used as an indicator of cell health. JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolcarbocyanine iodide) (Sigma Chemical Co., St. Louis, MO, USA) is a lipophilic, cationic dye that can selectively enter the mitochondria and reversibly change color from green to red as the membrane potential increases. The cells were grown in glass cover slips placed in 6-well plates and treated with the IC50 concentrations of n-hexane and chloroform extracts. The cells were stained with JC-1 dye after 12-h and 24-h exposure. The mitochondrial depolarization patterns of the cells were observed in the fluorescent microscope at ×400 magnification and the percentage of cells reflecting pathological changes was calculated. Data were collected for four replicates and used to calculate the means and the standard deviations.
Apoptosis assay adopting Annexin V-Cy3 staining
Phosphatidylserine translocation from the inner to the outer leaflet of the plasma membrane is one of the early features of apoptosis.[21] Cell surface phosphatidylserine was detected by phosphatidylserine-binding protein annexin V conjugated with Cy3 using the commercially available annexin V - Cy3 apoptosis detection kit (APOAC apoptosis detection kit, Sigma). Cells were cultured on cover slips and treated with the IC50 concentrations of n-hexane and chloroform extracts and incubated for 12 h and 24 h. After the incubation period, the cells were processed according to the instructions in the kit and observed in the fluorescent microscope. Data were collected in three individual experiments, each in tetraplicate, and used to calculate the respective means and the standard deviations.
Comet assay
DNA fragmentation is a hallmark of apoptosis. To detect this, we used a single-cell gel electrophoresis technique that detects DNA migration in individual cells. DNA damage was quantified using the comet assay.[22] Photographs were obtained using the fluorescent microscope. From each treatment 150 cells were digitalized and analyzed using CASP (Comet Assay Software Project) software. The images were used to estimate the DNA content of individual nuclei and to evaluate the degree of DNA damage as a fraction of the total DNA in the tail. The cells were assigned to five classes: 0 (<7% of the DNA in the tail undamaged), 1 (7%–15%), 2 (15%–22%), 3 (22%–30%), and 4 (>30%, maximally damaged).
Sub-G0/G1 apoptosis assay and cell cycle analysis
The cells were seeded in 25 cm2 tissue culture flasks at a density of 1 × 106 cells/flask. After 24 h, the cells were treated with IC50 concentrations of n-hexane and chloroform extracts and incubated for 24 h and 48 h. After incubation, the cells were trypsinized, harvested, and fixed in 1 ml 80% cold ethanol and incubated at 4°C for 15 min. After incubation, cells were centrifuged at 1500 rpm for 5 min and the cell pellets were resuspended in 500 μl propidium iodide (10μg/ml) containing 300 μg/ml RNase (Sigma Chemical Co., St. Louis, MO, USA). The cells were then incubated on ice for 30 min and filtered in 53 μm nylon mesh. Cell cycle distribution was analyzed using FACScan (fluorescent activated cell sorter) (Becton-Dickinson, Sanjose, CA, USA) with 15-mW, 488-nm argon ion laser. PI (Propidium Iodide) signals were collected using a 585/42-band pass filter. The data were acquired and analyzed using CellQuest™ software.
Statistical analysis
Numerical data are expressed as mean ± standard deviation (SD). Statistical differences were evaluated by a one-way analysis of variance (ANOVA) using Statistical Package of Social Sciences (SPSS™) software package for Windows™ (Version 11.5; SPSS Inc., Chicago, IL, USA). Post-hoc test was performed for comparisons, using the least significant differences (LSD) test. Differences were considered statistically significant when P≤0.05.
RESULTS
Yield of A. malabarica extracts
The yield obtained from the serial extraction was as follows: n-hexane extract (1.3 g), chloroform extract (2.4 g), ethyl acetate extract (2.9 g), n-butanol extract (1.6 g), methanol extract (2.2 g), and water extract (1.9 g).
Effect of A. malabarica extracts on viability of cells
MTT assay was conducted as an indirect measure of the viability of cells treated with the various extracts. The cytotoxic property was determined according to the dose values of the extracts required to bring down the viability of cells to 50% (IC50). The n-hexane and chloroform extracts caused 50% inhibition of SiHa and ME180 cells at concentrations below 100 μg/ml [Figure 1; Table 1]. The IC50 of the other three extracts were found to be >200 μg/ml. Therefore, further experiments were carried out with n-hexane and chloroform extracts only.
Figure 1.
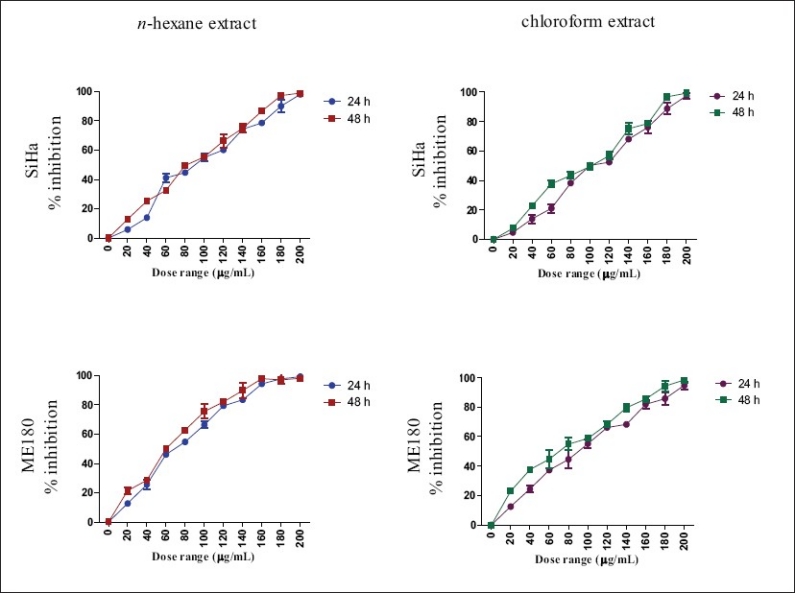
Effect of n-hexane and chloroform extracts on viability of SiHa and ME-180 cells. Cytotoxicity was determined by MTT assay and calculated as percentage of inhibition of cell proliferation
Table 1.
IC50 values for A. malabarica extracts on cervical cancer cell lines

Mode of cell death induced by the treatments
AO and EB and Hoechst staining were adopted to find out if the cells responded to the treatment with n-hexane and chloroform extracts with apoptosis and/or necrosis. Staining of cells with AO and EB indicated typical apoptotic morphology, such as condensed nuclei and formation of apoptotic bodies [Figure 2a]. Hoechst 33258 staining revealed that the nuclear chromatin was affected. In untreated cells the nuclei fluoresced faint blue and the color was homogenous. In the treated cells that succumbed to the treatment, the blue emission was much brighter than in the unaffected cells. Condensed chromatin could be visualized in many treated cells and some of them formed apoptotic bodies [Figure 2b].
Figure 2a.
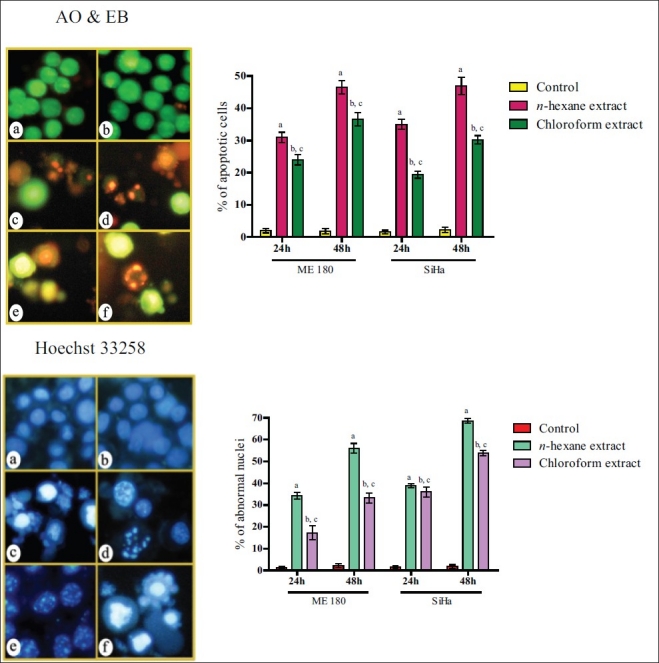
Photomicrographs showing the untreated (a, b), n-hexane extract (c, d) and chloroform extract (e, f) (IC50 concentration) – treated SiHa cells. For statistical analysis, error bars, SD; P<0.05. a, respectively, indicates statistically significance difference between control and n-hexane extract – treated cells; b, between control and chloroform extract-treated cells and c, between n-hexane- and chloroform extract-treated cells.
Figure 2b.
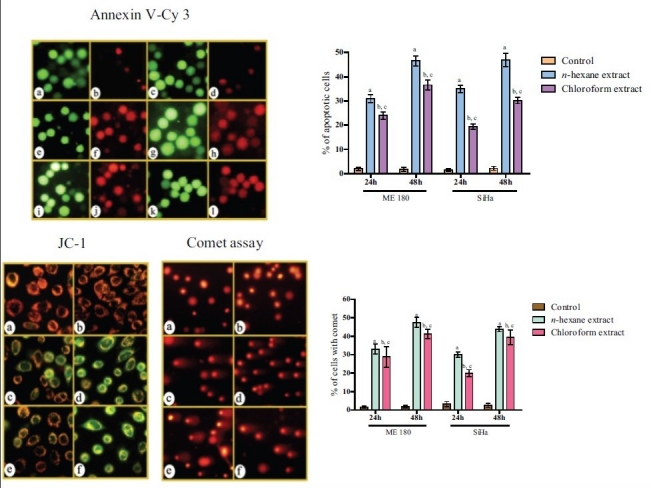
Photomicrographs showing Annexin V – Cy3 stained untreated (a-d), n-hexane extract (e-h) and chloroform extract (i-l) treated SiHa cells. Cells stained with JC-1 and analyzed by comet assay - untreated (a, b), n-hexane extract (c, d) and chloroform extract (e, f) (IC50 concentration) –treated SiHa cells. For statistical analysis, error bars, SD; P<0.05. a, respectively, indicates statistically significance difference between control and n-hexane extract –treated cells; b, between control and chloroform extract-treated cells and c, between n-hexane- and chloroform extract-treated cells. For JC-1 staining, quantification was not done.
Change in mitochondrial transmembrane potential (ΔΨm)
Early apoptosis is always accompanied by disruption of mitochondrial ΔΨm, resulting in a rapid collapse in the electrochemical gradient.[23] In this study, we explored the effect of the extracts on ΔΨm by using a mitochondrion-specific dye, JC-1. The control cells emitted red fluorescence due to high ΔΨm. The cells treated with n-hexane and chloroform extracts showed progressive loss of red J-aggregate fluorescence and cytoplasmic diffusion of green monomer fluorescence at 24 h due to mitochondrial transmembrane depolarization. There was complete loss of red fluorescence and retention of only green fluorescence at 48 h due to total loss of mitochondrial ΔΨm [Figure 2b].
Phosphatidylserine translocation
During apoptosis, phosphatidylserine (PS) is translocated from the cytoplasmic face of the plasma membrane to the cell surface. The live untreated cells were stained only with 6-CFDA, and emitted green fluorescence. The treatment of cells with the extracts increased the number of cells double-stained with annexin V-Cy3 and 6-CFDA, emitting red and green fluorescence, respectively, indicating early apoptosis. Some cells were stained only with annexin V-Cy3, emitting red fluorescence only, suggesting necrotic cell death. The responses were much higher in the treated cells than in the control [Figure 2b].
DNA damage caused by treatment with extracts
The comet assay or single-cell gel electrophoresis (SCGE) is a rapid, sensitive, and relatively simple method for detecting DNA damage at the level of individual cells.[22] The control cells showed little, if any, comets. But the treatments produced high percentage of damaged cells forming comets at 24 h and 48 h, and the effect was also duration dependent [Figure 2b].
DNA content and cell cycle analysis adopting flow cytometry
Apoptotic cells can be recognized by flow cytometry through their diminished stainability with the DNA-specific fluorochrome propidium iodide (PI), in which the hypodiploid population can be quantified by DNA content frequency histograms.[24] Treatment of SiHa cells with n-hexane and chloroform extracts for 24 h led to significant decrease of G0+G1 cells and increase of S-phase and G2+M cells, revealing cell cycle arrest at these two phases. On the other hand, n-hexane extract treatment led to decrease of S-phase cells and increase of sub-G0+G1 population at 48 h. However, chloroform extract treatment for 48 h led to massive killing of G0+G1-, S-, and G2/M-phase cells, as a result of which the sub-G0+G1 population was significantly increased. ME180 cells treated with n-hexane extract showed significant decrease of S-phase and G2/M-phase cells at 24 h and also a significant decrease of G0+G1-phase cells at both 24 h and 48 h. Consequently, there was increase in the sub-G0+G1 population after 24 h and 48 h treatment. Treatment with chloroform extract resulted in significant decrease of G0+G1 cells and dramatic increase of sub-G0+G1 population at 24 h and 48 h. Thus, both the extracts induced cell cycle arrest and cell death, mostly apoptosis [Figure 3].
Figure 3.
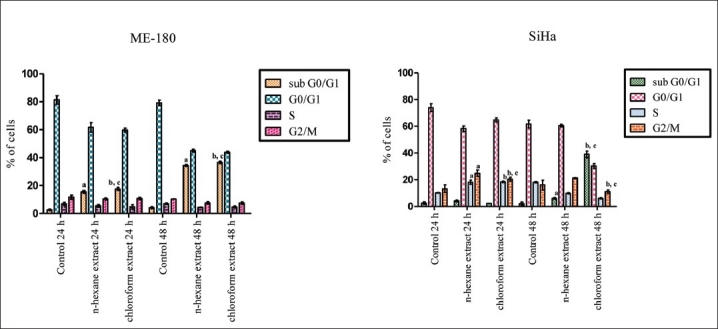
Assessment of n-hexane and chloroform extract–induced apoptotic cells by hypodiploid DNA content and S-phase and G2+M-phase arrest in ME-180 and SiHa cells using three independent experiments each. Error bars, SD; P<0.05. a, respectively indicates statistical significance difference between control and n-hexane extract treated cells; b, between control and chloroform extract treated cells and c, between n-hexane and chloform extract treated cells
DISCUSSION
Plant-based formulations have been used as remedies against diverse ailments because they offer an overwhelming variety of metabolites that demonstrate multiple medicinal and pharmaceutical properties, including synergism and additive effects.[25] In line with this, the results of this study demonstrate that n-hexane and chloroform extracts of A. malabarica are cytotoxic to HPV16-positive cervical cancer cells, as revealed in the various assays.
The tumor suppressor protein p53 plays a pivotal role in the DNA damage response and is defective in >50% of human tumors, which has generated substantial interest in developing p53-targeted cancer therapies. The HPV E6 protein promotes the degradation of p53 and, thus, inhibits the stabilization and activation of p53 that would normally occur in response to HPV E7 oncogene expression.[26] Restoration of p53 function in these cells by blocking this pathway should promote a selective therapeutic effect. The extracts produced killing of cervical cancer cells which express the E6 viral protein. Thus, the results suggest that the extracts inhibit the degradation of p53 protein or upregulate a downstream event. The extracts also induced cell cycle arrest, which shows that the inhibitors of cell cycle that are usually degraded by E7 viral protein are activated and the degradation is inhibited, leading to a functional pathway. Thus, the extracts offer potential for application in HPV-positive cervical cancers.
The mode of cell death due to the cytotoxic property of the extracts is principally apoptosis as revealed in the AO and EB and Hoechst staining. The comet assay revealed that induction of apoptosis is preceded by DNA damage. Apoptosis is a genetically controlled cell-death process, which is characterized by chromatin condensation, DNA fragmentation to oligonucleosome-sized particles, membrane blebbing, cell shrinkage, and formation of apoptotic bodies.[27] The observation in this study revealed all these features in the cells treated with the extracts. One of the early features of apoptosis, phosphatidylserine translocation, was observed in both the n-hexane– and chloroform extract–treated cells. These results further substantiate the potential of the plant to induce apoptosis in cervical cancer cells.
The treatment of the cells with the extracts also led to the loss of mitochondrial outer membrane potential (MOMP). Mitochondria are well known targets for chemotherapeutic agents because they play a central role in the induction and regulation of apoptotic cell death.[28] Shortly after the discovery that MOMP is frequently impaired in cancer, mitochondria have become an attractive target to induce apoptosis and to overcome resistance to chemotherapy. More than 20 mitochondrion-targeted compounds have been reported to induce apoptosis selectively in malignant cell lines, and some of these are already being used in phase II/III clinical trials or validated in in vitro preclinical settings. The conventional anticancer agents such as doxorubicin, cisplatin, and paclitaxel cause loss of MOMP in an indirect manner by activating proapoptotic second messengers, for example p53, ceramide/GD3 pathways, and the Fas/FasL systems. However, there are a number of agents, including arsenite, lonidamine, and betulinic acid, that induce cell death by acting directly on mitochondrial membranes.[29]
Inhibition of cell proliferation and induction of apoptosis are highly correlated with the activation of a variety of intracellular signaling pathways that arrest the cell cycle in the G1, S, or G2 phases. Certain antioxidants, such as flavonoids, have been reported to have inhibitory effects on PI3-kinase, protein kinase C, protein tyrosine kinase, and some transcription factors, and such inhibition in turn arrests cell growth and induces cell death in several carcinoma cell lines.[30] Our data indicate that the extracts caused increase of cells in sub-diploid (sub-G0+G1) regions with decrease of cells in diploid regions (G1, S, and G2 phases). This again indicates cell death beyond sub-G0+G1 region. But treatment of SiHa cells with n-hexane and chloroform extracts caused arrest at S and G2/M phases of the cell cycle at 24 h but increase of sub-G0 cell population at 48 h, thus revealing that the extracts caused cell cycle arrest at an earlier hour and apoptosis at a later hour.
CONCLUSION
In conclusion, we have demonstrated that the n-hexane and chloroform extracts of A. malabarica inhibit proliferation of and induce death in HPV16-positive cervical cancer cells, mostly by apoptosis and to some extent by necrosis. Isolation, identification, and characterization of the various compounds present in the n-hexane and chloroform extracts of this plant and investigation of the anti-cervical cancer property of the major compounds are in progress and the findings will be the subject of future publications.
ACKNOWLEDGMENT
The authors thank the Central Image-Flow facility of National Center for Biological Sciences, Bangalore, India, for providing flow cytometry facility. The DST-FIST [grant No. SR/FST/LSI -112/2002] and UGC-SAP [grant No. F. 3 - 5/2007 (SAP- II)] support for improvement of infrastructure facility at Animal Science Department of Bharathidasan University is gratefully acknowledged. Preethy Paul acknowledges the UGC-JRF.
Footnotes
Source of Support: The DST-FIST [grant No. SR/FST/LSI -112/2002] and UGC-SAP [grant No. F. 3 - 5/2007 (SAP- II)]
Conflict of Interest: None declared.
REFERENCES
- 1.Moody CA, Lamins LA. Human papillomavirus oncoproteins: Pathways to transformation. Nat Rev Cancer. 2010;10:550–60. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 protein. Oncogene. 2002;20:7888–98. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 4.Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–56. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 5.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 6.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–28. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 7.Chou RH, Huang H. Restoration of p53 tumor suppressor pathway in human cervical carcinoma cells by sodium arsenite. Biochem Biophys Res Commun. 2002;293:298–306. doi: 10.1016/S0006-291X(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM, Snader K. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 9.Kinghorn AD, Chin Y, Swanson SM. Discovery of natural product anticancer agents from biodiverse organisms. Curr Opin Drug Discov Devel. 2009;12:189–96. [PMC free article] [PubMed] [Google Scholar]
- 10.Bakshi GD, Sensarma P, Pal DC. Calcutta, India: Naya Prokash; 1999. A Lexicon of Medicinal Plants in India; p. 152. [Google Scholar]
- 11.Kirtikar KR, Basu BD. Allahabad: Basu; 1935. Indian Medicinal Plants; p. 2011. [Google Scholar]
- 12.Dhar ML, Dhawan BN, Prasad CR, Rastogi RP, Singh KK, Tandon JS. Screening of Indian plants for biological activity. Indian J Exp Biol. 1974;12:512–23. [PubMed] [Google Scholar]
- 13.Jeyachandran R, Mahesh A, Cindrella L. DEN-induced cancer and its alleviation by Anisomeles malabarica (L.) R.Br. ethanolic leaf extract in male albino mice. Int J Cancer Res. 2007;3:174–9. [Google Scholar]
- 14.Arisawa M, Nimura M, Ikeda A, Hayashi T, Morita N, Momose Y, et al. Biologically active macrocyclic diterpenoids from Chinese drug” Fáng Féng Cáo ”. I. Isolation and structure 1. Planta Med. 1986a;52:38–41. doi: 10.1055/s-2007-969063. [DOI] [PubMed] [Google Scholar]
- 15.Arisawa M, Nimura M, Fujita A, Hayashi T, Morita N, Koshimura S. Biologically active macrocyclic diterpenoids from Chinese drug “Fáng Féng Cáo”. II. Derivatives of ovatodiolids and their cytotoxity 2. Planta Med. 1986b;52:297–9. doi: 10.1055/s-2007-969157. [DOI] [PubMed] [Google Scholar]
- 16.Devi G, Kapil RS, Popli P. Chemical Investigation of Anisomeles malabarica: Part I – Structures of Macrocyclic Diterpenes, Anisomelolide, Malabaric Acid, 2-Acetoxymalabaric Acid, Anisomelyl Acetate and Anisomelol. Indian J Chem. 1997;16:441–5. [Google Scholar]
- 17.Purushothaman KK, Rao RB, Kalyani K. Ovatodioilide and anisomelic acid, two diterpenoid lactones from Anisomeles malabarica R. Br. Indian J Chem. 1975;13:1357–8. [Google Scholar]
- 18.Anjaneyulu B, Babu RV, Ganguly A, Govindachari K, Joshi TR, Kamat S, et al. Chemical investigation of some Indian plants. Indian J Chem. 1965;3:237–8. [Google Scholar]
- 19.Hou Y, Wu M, Hwang Y, Chang F, Wu Y, Wu C. The natural diterpenoid ovatodiolide induces cell cycle arrest and apoptosis in human oral squamous cell carcinoma Ca9-22 cells. Life Sci. 2009;85:26–32. doi: 10.1016/j.lfs.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 22.Mohankumar MN, Janani S, Prabhu BK, Kumar PR, Jeevanram RK. DNA damage and integrity of UV-induced DNA repair in lymphocytes of smokers analyzed by the comet assay. Mutation Res. 2002;520:179–87. doi: 10.1016/s1383-5718(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 23.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: Different modes of dying. Biochem (Moscow) 2005;70:231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 24.Bao W, Pan H, Lu M, Ni Y, Zhang R, Gong X. The apoptotic effect of sarsasapogenin from Anemarrhena asphodeloides on HepG2 human hepatoma cells. Cell Biol Int. 2007;31:887–92. doi: 10.1016/j.cellbi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Singh B, Bhat TK, Singh B. Potential therapeutic applications of some antinutritional plant secondary metabolites. J Agric Food Chem. 2003;51:5579–97. doi: 10.1021/jf021150r. [DOI] [PubMed] [Google Scholar]
- 26.Werness BA, Levine AJ, Howley PM. Assosciation of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 27.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 28.Debatin KM, Poncet D, Kroemer G. Chemotherapy: Targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 29.Constantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2002;92:1042–53. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 30.Fruehauf JP, Meyskens FL. Reactive Oxygen Species: A Breath of Life or Death? Clin Cancer Res. 2007;13:789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]


