Abstract
Objectives:
To evaluate the acute effects of colony-stimulating factor (G-CSF) on ventricular arrhythmias after coronary occlusion in rats.
Materials and Methods:
Male Wistar rats (10 weeks) received G-CSF (100 μg.kg-1) or vehicle. Thirty minutes later, animals were infarcted by coronary occlusion under artificial respiration. Electrocardiogram was monitored for 30 min to evaluate ventricular arrhythmias.
Results:
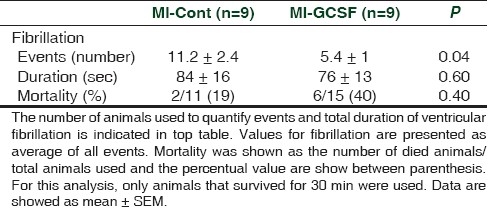
G-CSF treatment reduced the number of premature ventricular beats and the number and duration of ventricular tachycardia. The incidence of ventricular fibrillation was significantly reduced by G-CSF (MI-Cont: 11.2 ± 2.4 vs. MI-GCSF: 5.4 ± 1 events; P < 0.05). However, total duration of ventricular fibrillation was not altered (MI-Cont: 84 ± 16 vs. MI-GCSF: 76 ± 13 sec).
Conclusions:
Acute administration of G-CSF before coronary ligature in rats reduces the incidence of ventricular premature beats and ventricular tachycardia, suggesting a possible direct electrophysiological effect of this cytokine independently of its genomic effects. However, the data suggest that G-CSF treatment may affect the spontaneous recovery from ventricular fibrillation. Acute G-CSF administration acts directly on cardiac electrophysiology, different from chronic treatment.
Keywords: Arrhythmias, G-colony-stimulating factor, myocardial infarction
INTRODUCTION
Granulocyte colony-stimulating factor (G-CSF) is an endogenous cytokine that mobilizes several kinds of hematopoietic cells, such as the bone marrow-derived progenitor cells. Along with this cell-mobilizing property, G-CSF also improves cardiac function,[1] reduces fibrosis,[2] prevents apoptosis[3] and reduces acute to chronic mortality in rats.[4,5] In the electrophysiological field, Kulhmann et al,[6] showed that healed infarcted hearts treated with G-CSF plus stem cell factor depicted reduced number of inducible ventricular arrhythmias. In accordance, chronic treatment with G-CSF previously to coronary occlusion reduced the incidence of lethal ventricular arrhythmias during the early period of myocardial infarction in rats due to increased left ventricle connexin-43 expression.[5,7] Recently, Liu et al,[8] described that the electrophysiological effects of G-CSF are dissociated from its hemodynamic effects and could not be explained by improvements in the cardiac function. However, Lee et al,[9] identified a proarrhythmic effect associated to the chronic use of the G-CSF. These findings suggest that the arrhythmogenic effects of G-CSF may be due to a genomic-mediated effect of this cytokine in cardiac electrophysiological properties. However, the acute arrhythmogenic properties of G-CSF during ischemia are still missing. Thus, the aim of the present study was to evaluate the acute effects of G-CSF on the ischemia-induced early arrhythmias, without the background of a genomic-associated effect.
MATERIALS AND METHODS
Animals
Male Wistar rats (10 weeks) were acquired from the animal facility at Federal University of Espirito Santo. During the period of experimentation, animals were maintained under controlled temperature and humidity, in a maximum of four animals per cage, having free access to food and water. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996) and approved by the institutional ethical committee (CEUA 12/2010).
Myocardial infarction production and arrhythmia analysis
Animals were treated with a single subcutaneous injection of G-CSF (MI-GCSF; n = 15) (100 μg.kg-1; Filgrastim, Biosintética) or saline (MI-Cont; n = 11), and 30 min later, the animals were infarcted under sodium pentobarbital (60 μg.kg-1; i.p.) anesthesia. Surgery was performed after tracheotomy and artificial ventilation with room air (64 strokes/min, 1.2–1.5 ml/100 g). The thorax was opened and myocardial infarction (MI) was produced by permanent occlusion of the left coronary artery as previously described.[5] A false operation (SO) was done in control animals (n = 6). Animals were maintained under artificial ventilation for 30 min after surgery. Electrocardiogram (ECG) was continuously recorded for 30 min following MI and SO surgery through metallic electrodes inserted in the subcutaneous space. ECG was acquired with an analogic-to-digital system (PowerLab, ADInstruments, Australia) and analyzed off-line. Arrhythmias were analyzed as previously described[10] and a modified and validated score to characterize severity of arrhythmias (including those animals that died during the register) was used.[5]
Three hours after surgery, animals were killed and the heart was rapidly excised, cut in four slices and incubated in a 1% triphenyltetrazolium chloride (TTC)-buffered solution (37°C, pH 7.4) during 5 minutes. Damage area was determined as percentage of TTC non-stained area in relation to the entire left ventricle area in the four slices by digital planimetry (ImageJ v1.41, NIH, USA).
Statistical analysis
Comparison of two independent means were done with the unpaired Student t-test. The Fisher exact test was used to compare proportions. Data are shown as mean ± standard error of mean (SEM) and P <0.05 was considered statistical significant.
RESULTS
Except for mortality, all variables were analyzed only in animals that survived 30 minutes after surgery (nine for each infarcted group, and six in the SO group). During ECG monitoring, heart rate was stable and similar in all groups (Before: SO = 375 ± 16, MI-Cont = 384 ± 13, MI-GCSF = 372 ± 17 beats/min; at 30 min after surgery: SO = 385 ± 19, MI-Cont = 398 ± 18, MI-GCSF = 401 ± 20 beats/min; P > 0.05) without significant differences throughout the experiment. No episodes of ventricular arrhythmias were observed in the SO group, and therefore, this group was excluded from Figures and Table. The incidence of ventricular premature beats (VPB) and ventricular tachycardia (VT) episodes during ECG monitoring are shown in Figures 1 and 2, respectively. Either phase-1 VPB's and VT's episodes were significantly reduced in the G-CSF group [Figures 1 and 2].
Figure 1.
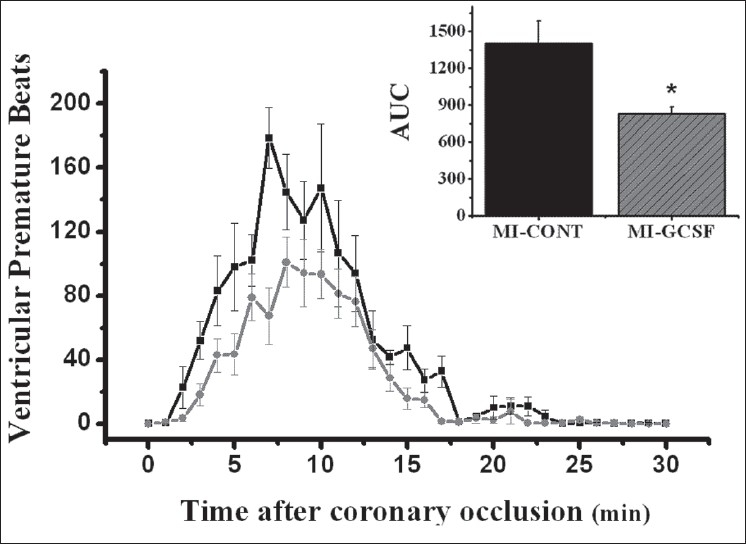
Temporal distribution of ventricular premature beats after coronary occlusion (nine animals for each group). The comparison was performed using the area under curve (AUC) (upper panel). * P < 0.05
Figure 2.
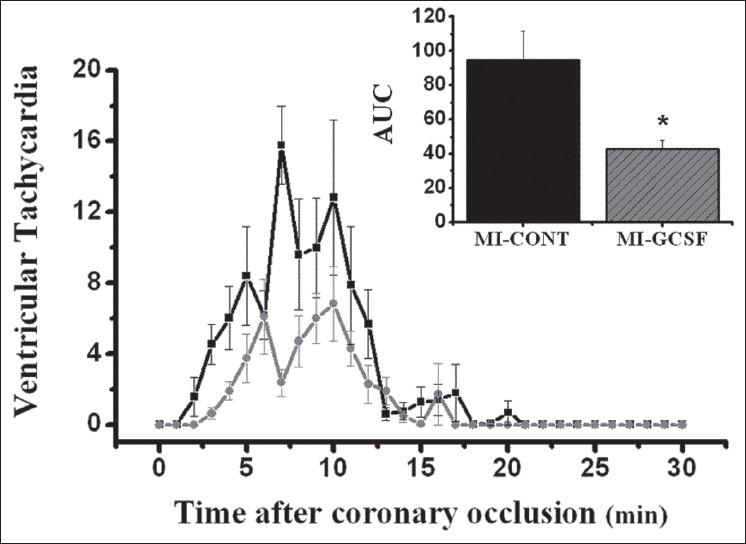
Events of ventricular tachycardia in the first 30 minutes following coronary occlusion (nine animals per group). Area under curve (AUC) was used to compare temporal distribution of the events (upper panel). * P < 0.05
Despite the beneficial effect on reduction of VPB's and VT's [Figures 1 and 2], only animals that survived to the whole period of the register were considered for analysis. When a score was used, the analysis of the whole sample (including those which died during register) did not show significant effects of G-CSF treatment (MI-Cont = 5 ± 0.19 vs MI-GCSF = 5.3 ± 0.16; P = 0.10).
All infarcted animals depicted at least one fibrillatory event during the first 30 min. G-CSF treatment did not increase mortality due to ventricular fibrillation (6 out of 15 rats in the MI-GCSF group vs 2 out of 11 rats in the MI-Cont group; P = 0.40) [Table 1].
Table 1.
Ventricular fibrillation and mortality rate
TTC non-stained area was unaffected by G-CSF treatment (41 ± 3 % in MI-GCSF vs. 42 ± 4 % in MI-Cont; P > 0.05) suggesting that the acute use of G-CSF, unlike its chronic use,[3] did not affect the myocardial damage produced by coronary ligature.
DISCUSSION
It is well described that G-CSF has an important involvement in the postinfarction remodeling, improving cardiac structure and function, and increasing the acute and chronic survival. In fact, despite initial studies suggest the regenerative hypothesis to explain these results, a great amount of recent studies suggest a direct effect of G-CSF on cardiac cells.[11]
Phase-1 ventricular arrhythmias following coronary occlusion are due to increased extracellular potassium concentration, and/or electrical uncoupling in the ischemic area.[12] The acute loss of K+ in ischemic tissue is mediated, in part, by activation of ATP-sensitive potassium channels (KATP),[13] predisposing to reentrant arrhythmias. Inhibition of KATP channel opening prevents action potential shortening, and may be a useful strategy to suppress lethal arrhythmias and sudden death early after coronary occlusion. It was reported that KATP channels are downstream in PI3K pathway, depicting defibrillatory properties in a rat model of ischemia followed by reperfusion.[14] Moreover, inhibition of KATP channels by glibenclamide did not reduce ventricular fibrillation incidence, but enhanced the probability of spontaneous recovery from fibrillation.[15] It was described that G-CSF directly activates PI3K in cardiomyocytes.[16] In our study, the number of ventricular fibrillatory episodes was significantly reduced in rats given acute G-CSF injection. Moreover, total duration of all fibrillatory episodes was similar in both infarcted groups, suggesting that recovery from fibrillatory status seems to be difficult under G-CSF treatment. Despite a reduced number of evidences on electrophysiological effects of G-CSF, a possible interaction with KATP channels could justify these results.
Our results showed a reduction in VPB's and VT in G-CSF treated rats. These reductions were similar to those found with chronic treatment.[11] However, despite the related electrophysiological effects mediated by chronic G-CSF treatment are due to connexin-43 expression as reported,[6,7,11] we do not believe that the acute effects mediated by G-CSF reported here should be due to gene or protein expression, since the treatment was initiated just 30 min before surgery. This suggests that the effects reported in this work are related to a direct action mediated by G-CSF in the electrophysiological properties.
Liu et al,[8] have recently reported that both early and delayed administration of G-CSF after myocardial infarction improved electrophysiological properties 3 months after surgery. These results are of fundamental importance, once cardiac remodeling was not blunted by G-CSF, showing that electrophysiological effects mediated by this cytokine are independently associated with improvements in cardiac function and/or structure.
Another effect related to G-CSF was a proarrhythmic effect under electrical stimulation in heart failure rats.[9] The authors observed an increase in sympathetic reinervation that contributed to enhanced norepinephrine levels and increased arrhythmogenic response. The related effect on sympathetic activation by G-CSF appears to be a chronic adaptative process, associated with long-term treatment and not an acute effect of this cytokine, as shown here. Moreover, in our study, heart rate remained unchanged. Thus, a direct effect on sympathetic drive and/or facilitation of norepinephrine release seems not to be an explanation for the effects observed on ventricular fibrillation.
In a small clinical study, it was reported that one infarcted patient who received G-CSF treatment developed ventricular fibrillation.[17] Conversely, no changes were detected on ECG parameters in neutropenic[18] or cardiopathic patients[19] treated with G-CSF. It is worth to note that even though G-CSF appears to be safe and associated with left ventricular structure and function improvements after infarction, electrophysiological properties described in this and others studies deserve to be carefully observed.
It is noteworthy that the effects related in our study occur precocious to coronary occlusion. These results are important once the effects of G-CSF on ventricular function in infarcted patients verified by clinical studies are more evidenced as soon as G-CSF administration.[20]
It is important to note that infarct area measured by TTC staining was a limitation in this study, since 3 hours is a short period to determine with precision the infarcted area. Besides, the blue dye perfusion technique to normalize the area at risk was not done.
In summary, even though reduction in the incidence of ventricular arrhythmias should be an important goal during the acute treatment period, our results suggest that acute G-CSF-treated rats presented reduced defibrillatory properties in the acute phase of MI. In conclusion, our results showed that acute G-CSF administration depicted an antiarrhythmic effect, similar to those observed with chronic treatment. Thus, our data suggest that this cytokine can interfere in the defibrillatory properties.
ACKNOWLEDGMENTS
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Espírito Santo (FAPES/PRONEX).
Footnotes
Source of Support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Espírito Santo (FAPES/PRONEX)
Conflict of Interest: None declared.
REFERENCES
- 1.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, et al. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:27–36. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Takemura G, Okada H, Miyata S, Esaki M, Maruyama R, et al. Treatment with granulocyte colony-stimulating factor ameliorates chronic heart failure. Lab Invest. 2006;86:32–44. doi: 10.1038/labinvest.3700367. [DOI] [PubMed] [Google Scholar]
- 3.Baldo MP, Davel AP, Damas-Souza DM, Nicoletti-Carvalho JE, Bordin S, Carvalho HF, et al. The antiapoptotic effect of granulocyte colony-stimulating factor reduces infarct size and prevents heart failure development in rats. Cell Physiol Biochem. 2011;28:33–40. doi: 10.1159/000331711. [DOI] [PubMed] [Google Scholar]
- 4.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldo MP, Davel AP, Nicoletti-Carvalho JE, Bordin S, Rossoni LV, Mill JG. Granulocyte colony-stimulating factor reduces mortality by suppressing ventricular arrhythmias in acute phase of myocardial infarction in rats. J Cardiovasc Pharmacol. 2008;52:375–80. doi: 10.1097/FJC.0b013e31818a2bb0. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, et al. G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med. 2006;203:87–97. doi: 10.1084/jem.20051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwabara M, Kakinuma Y, Katare RG, Ando M, Yamasaki F, Doi Y, et al. Granulocyte colony-stimulating factor activates Wnt signal to sustain gap junction function through recruitment of β-catenin and cadherin. FEBS Lett. 2007;581:4821–30. doi: 10.1016/j.febslet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Liu HM, Luo T, Zhou X, Cai L, Huang TG, Jiang TM, et al. Disassociation between left ventricular mechanical and electrical properties in ischemic rat heart after G-CSF treatment. Cardiovasc Drugs Ther. 2011;25:203–14. doi: 10.1007/s10557-011-6294-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee TM, Chen CC, Chang NC. Granulocyte colony-stimulating factor increases sympathetic reinnervation and the arrhythmogenic response to programmed electrical stimulation after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2009;297:512–22. doi: 10.1152/ajpheart.00077.2009. [DOI] [PubMed] [Google Scholar]
- 10.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–55. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 11.Baldo MP, Rodrigues SL, Mill JG. Granulocyte colony-stimulating factor for ischemic heart failure:should we use it? Heart Fail Rev. 2010;15:613–23. doi: 10.1007/s10741-010-9188-0. [DOI] [PubMed] [Google Scholar]
- 12.Billman GE. Role of ATP sensitive potassium channel in extracellular potassium accumulation and cardiac arrhythmias during myocardial ischaemia. Cardiovasc Res. 2004;28:762–9. doi: 10.1093/cvr/28.6.762. [DOI] [PubMed] [Google Scholar]
- 13.Kantor PF, Coetzee WA, Carmeliet EE, Dennis SC, Opie LH. Reduction in ischemic K+ loss and arrhythmias in rat hearts: Effect of glibenclamide, a sulfonylurea. Circ Res. 2006;66:478–85. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- 14.Vajda S, Baczkó I, Leprán I. Selective cardiac plasma-membrane KATP channel inhibition is defibrillatory and improves survival during acute myocardial ischemia and reperfusion. Eur J Pharmacol. 2007;577:115–23. doi: 10.1016/j.ejphar.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Baczkó I, Leprán I, Papp JG. KATP channel modulators increase survival rate during coronary occlusion-reperfusion in anaesthetized rats. Eur J Pharmacol. 1997;324:77–83. doi: 10.1016/s0014-2999(97)00064-2. [DOI] [PubMed] [Google Scholar]
- 16.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, et al. Granulocyte colony-stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol. 2006;26:e108–13. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 17.Hüttmann A, Dührsen U, Stypmann J, Noppeney R, Nückel H, Neumann T, et al. Granulocyte colony-stimulating factor-induced blood stem cell mobilization in patients with chronic heart failure.Feasibility, safety and effects on exercise tolerance and cardiac function. Basic Res Cardiol. 2006;101:78–86. doi: 10.1007/s00395-005-0556-1. [DOI] [PubMed] [Google Scholar]
- 18.Guneysel O, Onur OE, Denizbazi A. Effects of recombinant human granulocyte colony-stimulating factor (Filgrastim) on ECG parameters in neutropenic patients: A single-center, prospective study. Clin Drug Investig. 2009;29:551–5. doi: 10.2165/00044011-200929080-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wolfram O, Jentsch-UllRich K, Wagner A, Hammwöhner M, Steinke R, Franke A, et al. G-CSF-induced mobilization of CD34+ progenitor cells and proarrhythmic effects in patients with severe coronary artery disease. PACE. 2007;27:166–9. doi: 10.1111/j.1540-8159.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 20.Takano H, Ueda K, Hasegawa H, Komuro I. G-CSF therapy for acute myocardial infarction. Trends Pharmacol Sci. 2007;28:512–7. doi: 10.1016/j.tips.2007.09.002. [DOI] [PubMed] [Google Scholar]


