Abstract
Objectives:
To study the effect of acute and repeated dose administration of lyophilized aqueous extract of the dried fruits of Tribulus terrestris (LAET) on sexual function in sexually sluggish male albino rats.
Materials and Methods:
Aphrodisiac activity of the test drug was evaluated in terms of exhibited sexual behavior. In order to assess the effect of chronic T. terrestris exposure on the hypothalamus--pituitary--gonadal axis, testosterone level estimation and sperm count were carried out. Twenty-eight-day oral toxicity studies were carried out to evaluate the long-term effects of the LAET administration on different body systems.
Results:
A dose-dependent improvement in sexual behavior was observed with the LAET treatment as characterized by an increase in mount frequency, intromission frequency, and penile erection index, as well as a decrease in mount latency, intromission latency, and ejaculatory latency. The enhancement of sexual behavior was more prominent on chronic administration of LAET. Chronic administration of LAET produced a significant increase in serum testosterone levels with no significant effect on the sperm count. No overt body system dysfunctions were observed in 28-day oral toxicity study.
Conclusions:
Findings of the present study validate the traditional use of T. terrestris as a sexual enhancer in the management of sexual dysfunction in males.
Keywords: Aphrodisiac, penile erection index, sexual behavior, testosterone, Tribulus terrestris
INTRODUCTION
Male sexual dysfunction, which includes erectile dysfunction (ED) and premature ejaculation, is the most common problem that contributes to infertility, distress, relationship problems, deterioration of self-image, and quality of life.[1,2] Erectile dysfunction has been identified as the persistent inability to attain and maintain penile erection sufficient for satisfactory sexual performance.[3] The predisposing factors for ED include chronic heart disease, high cholesterol, diabetes mellitus, smoking, alcohol, drug abuse, stress, food habits, and increasing age. Epidemiological studies have demonstrated a high prevalence of ED in developed countries, and therefore it is considered to be an important health problem.[1] Currently, modalities including psychotherapy, surgery, mechanical devices, drugs, and penile implants are used for the management of ED.[1] Drug therapy today mainly focuses on phosphodiesterase type 5 inhibitors, which increase the levels of cyclic Guanosine Mono-Phosphate (cGMP) in the cavernosal vasculature, leading to facilitation and prolongation of penile erection.[4]
In traditional medicine, asphaltum, Tribulus terrestris, Asparagus racemosus, and Sida cordifolia have been reported to possess aphrodisiac activity and are some of the oldest modalities for enhancing sexual function in humans.[5] T. terrestris L. (family: Zygophyllaceae) is a flowering plant found in the temperate and tropical regions of Southern Asia, Africa, and Australia. In Sanskrit, it is known as Gokshura and finds references in many Ayurvedic formulations for the treatment of sexual dysfunction in males.[6,7]
Experimental studies using T. terrestris extracts have demonstrated an increase in sexual function in rats which has been attributed to an increase in testosterone, dihydrotestosterone, and dehydroepiandrosterone.[8,9] One clinical study has also demonstrated that T. terrestris extract increases body's natural testosterone levels and thereby improves male sexual performance and helps build muscle.[10] However, as sexual dysfunction has been shown to be more prevalent in the aging population,[1] the evaluation of investigational drugs in aged and sexually sluggish males for their aphrodisiac and sexual tonic activity is a better predictor for their efficacy in the clinical setting. The present study was thus carried out to evaluate the aphrodisiac activity of single and repeated dose administration of the lyophilized aqueous extract of the dried fruits of T. terrestris (LAET) in aged and sexually sluggish male albino rats. Additionally, in order to evaluate the effect of the LAET treatment on testosterone levels and its feedback inhibition of gonadotropin release,[11] we estimated the testosterone levels and sperm count after repeated dose LAET administration.
MATERIALS AND METHODS
Animals
Female (14–16 week old) and male (55 - 60 week old) albino rats of the Wister strain from our institutional breeding stock were used in this study. All animal experimentation was carried out in compliance with the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines and was approved by the Institutional Animal Ethics Committee. The animals were housed separately in clean polypropylene cages at 24 ± 2°C and had free access to standard pellet feed and drinking water. Animals were acclimatized to a reversed 12 h/12 h light and dark cycle (lights on from 20:00 to 08:00) for 10 days before the start of experiments. Behavioral studies were carried out during the reversed dark phase (between 11:00 and 16:00 using a dim red light for illumination).
Drugs
Standardized LAET (whole plant extract containing ≥20% w/w total saponins) was provided by Sanat Products Ltd., New Delhi, India. Estradiol benzoate was procured from Organon India Ltd., Kolkata, India, and progesterone was procured from Cadilla Healthcare Ltd., Daman, India.
Sexual behavior study
Females were ovariectomized under ether anesthesia, and after full recovery, they were brought into the estrous state by the sequential subcutaneous administration of 10 μg/kg body weight of estradiol benzoate and 500 μg/kg body weight of progesterone at 48 h and 4 h prior to copulatory studies, respectively.[8] They were checked for sexual receptivity with sexually active adult males (different from the ones used in the study), and only those showing copulatory behavior, that is, solicitation and lordosis in response to mounting, were used in the study.[12] A preliminary screening was carried out to identify the sexually sluggish males. Briefly, the male rats were placed singly with sexually receptive females for 30 min at seven different occasions, with a gap of 5 days between each exposure. Male animals that failed to achieve ejaculation during any of the last three exposures were considered to be sexually sluggish[13] and used in the present study.
Acute study
A total of 18 sexually sluggish male Wistar rats were selected and housed separately. They were randomly divided into three groups of six animals each. Group I received 2 ml/kg vehicle (1% gum acacia) and served as control, group II received 50 mg/kg of LAET, and group III received 100 mg/kg LAET per orally. Fifty minutes after the administration of drug/vehicle, the animals were placed in a glass cage (40 × 50 × 40 cm). After an adaptation period of 10 min, a sexually receptive female was presented to the male by dropping into the cage. The following sexual behavioral parameters, as described by Agmo,[14] were recorded for 30 min by a trained observer who was unaware of the treatment given to each group:
Mount latency: Time duration (in seconds) from the introduction of the female into the cage till the first mount.
Intromission latency: Time duration (in seconds) from the introduction of the female into the cage till the first intromission (vaginal penetration).
Ejaculation latency: Time duration (in seconds) from the first intromission till ejaculation.
Mount frequency: Total number of mounts preceding ejaculation.
Intromission frequency: Total number of intromission preceding ejaculation.
Penile erection index: It is determined by multiplying the percentage of rats exhibiting at least one episode of penile erection during 30 min of the observation period with the mean number of penile erections.[15,16]
Penile erection index = % rats exhibiting erection × mean number of erections
After testing each animal, the cage was thoroughly cleaned with ethanol in order to mask the odor left by the animal.
Chronic study
The same set of animals was used for the chronic study. The drug/vehicle treatment was continued for 13 more days. Fifty minutes after the drug/vehicle administration on day 14, sexual behavior of animals was measured as described above under ‘Acute study’. After behavioral study, the animals were anesthetized with diethyl ether and terminal blood collection was done by exsanguination. Serum was separated and stored at –20°C, which was used for the testosterone level estimation by using an ELISA kit (Cayman Chemical Company, Ann Arbor, Michigan, USA). The left and right epididymis of each rat was dissected out and placed into a petri dish with 5 ml of 1% sodium citrate solution. It was teased thoroughly with a needle and forceps until a milky suspension was obtained. This suspension was filtered through an 80-μm mesh and the final volume was made up to 10 ml with an aqueous diluting medium containing sodium bicarbonate (5% w/v), formalin (1% v/v), and a saturated aqueous solution of gentian violet (0.5% v/v).[17] This suspension was then used for sperm count with a Neubauer chamber (Roche, Germany) and a microscope.
Twenty-eight-day oral toxicity study
In order to assess the effect of chronic administration of LAET on various body systems, a 28-day oral toxicity study was carried out according to the Organisation for Economic Co-operation and Development guidelines for testing of chemicals.[18] Sixteen male Wister rats (each weighing 180-200 g) were divided into two groups (n = 8). Group I received the vehicle (1% gum acacia) and served as the normal control. Group II received the test drug at a dose of 500 mg/kg (five times the highest tested dose). The drug/vehicle administration was continued for 28 days. Animals were observed on all days for behavioral abnormalities till 3 hours after drug/vehicle administration.
Data analysis
All values were expressed as mean ± standard error. A comparison between groups was done by using either unpaired Student's t test (for toxicity study) or one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison. A P value of < 0.05 was considered significant.
RESULTS
Acute study
The LAET administration produced a dose-dependent increase in sexual behavior of treated animals [Table 1]. At both the doses tested (50 and 100 mg/kg), LAET significantly decreased mount latency, intromission latency, and ejaculatory latency as compared with the control (vehicle-treated) rats. There was also a statistically significant increase in mounting frequency, intromission frequency, and penile erection index, which are the indicators of enhanced sexual behavior.
Table 1.
Effect of single-dose LAET administration on copulatory behavior of sexually sluggish rats

Chronic study
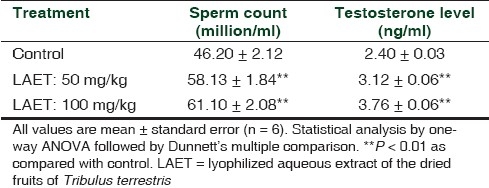
Effects of chronic LAET administration on sexual behavior were similar to the results obtained in acute study. Chronic administration also produced a dose-dependent increase in sexual behavior, which was significant as compared with the control animals [Table 2]. As in the acute study, chronic LAET administration also produced a significant decrease in mount latency, intromission latency, and ejaculatory latency while producing a significant increase in mounting frequency, intromission frequency, and penile erection index. However, chronic administration of LAET produced a greater increase in sexual behavior as compared with single dose administration. Serum testosterone level and sperm count in LAET-treated rats were also found to significantly increased, as compared with the control animals [Table 3].
Table 2.
Effect of multiple-dose LAET administration on copulatory behavior of sexually sluggish rats

Table 3.
Effect of multiple-dose LAET administration on sperm count and testosterone level in sexually sluggish rats
Toxicity profile of LAET
Twenty-eight-day oral administration of LAET did not produce any mortality or behavioral changes in the tested animals during the entire observation period. Even though there was no difference in the food and water intake between the groups, a significant increase in body weight was observed in the LAET-treated group as compared with normal control (data not shown). There were no changes in blood chemistry, bleeding time, coagulation time, hepatic enzymes, or histology of kidney, liver, testes, and penis. However, LAET-treated animals showed a slight increase in the percentage testicular weight as compared with the normal control, but this increase was not statistically significant.
DISCUSSION
The present study was carried out to evaluate the traditional claim of aphrodisiac and sexual stimulant activities of T. terrestris in an experimental model of sexual dysfunction. Even though the stimulant activity of T. terrestris on sexual behavior has been evaluated in castrated rats,[8] this induced condition does not completely mimic age-related and disease-related sexual dysfunctions, because in these conditions there is a decrease in sexual behavior even though there is no complete absence of testosterone.
Therefore, in the present study we have evaluated the sexual stimulant activity of the test drug in aged and sexually sluggish Wistar rats. LAET administration increased the sexual behavior of sexually sluggish animals, as observed by a decrease in mount, intromission, and ejaculation latencies and an increase in intromission and mount frequencies. This enhancement of sexual behavior in the tested animals was more prominent after chronic administration of the test drug. T. terrestris administration has been reported to increase the blood levels of testosterone, dehydroepiandrosterone, dihydrotestosterone, and dehydroepiandrosterone. This hormonal modulation has been attributed to the presence of the steroidal saponin protodioscin in the plant extract.[8,10] Protodioscin has been shown to improve libido, sexual activity, and intracavernous pressure in experimental animals. It is believed that the hormonal effects of protodioscin are mediated through its metabolic conversion into dehydroepiandrosterone.[19] Besides central effects, testosterone has been shown to act peripherally by facilitating the nitrergic neurotransmission, accentuating nitric oxide synthase activity and nitric oxide release in the cavernosa, all of which contribute toward penile erection.[20,21]
A concern that arises with the sustained elevation in testosterone levels is the feedback inhibition of gonadotropin release.[11] However, chronic administration (14 days) of LAET in our study did not produce any decrease in sperm count in the treated animals. On the contrary, there was a significant and dose-dependent increase in sperm count in the LAET-treated animals. These findings are similar to the findings reported earlier.[22] Although at this moment it is not clear as to how the testosterone levels and sperm count remain elevated even in the presence of high circulating testosterone levels, it could partly be attributed to the initial diminished hormonal status of the experimental animals, as all the animals were aged and sexually sluggish. Another plausible reason for the absence of this feedback inhibition could be the leutinizing-hormone-releasing property of LAET.[23] Oral toxicity studies also demonstrated the safety of LAET on chronic administration. Only the body weight and testicular weight of LAET-treated animals showed an increase as compared with those of normal control. Both these changes can be attributed to the androgenic activity of the plant extract.
The present study demonstrates the effectiveness of LAET in reversing aging-induced sexual dysfunction in experimental rats. On the basis of our results, we conclude that LAET has the potential to be used as a safe therapeutic alternative to current modalities for the management of sexual dysfunction in males.
ACKNOWLEDGMENT
The authors thank M/s Sanat Products Ltd., New Delhi, India, for supporting the research work in the form of a grant.
Footnotes
Source of Support: M/s Sanat Products Ltd., New Delhi, India
Conflict of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
REFERENCES
- 1.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on Male Sexual Dysfunction: Erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Wagner G, Saenz de TI. Clinical review. Update on male erectile dysfunction. Br Med J. 1998;316:678–82. doi: 10.1136/bmj.316.7132.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinhardt W, Kropman RF, Vermeji P. Comparative tolerability and efficacy of treatments of impotence. Drug Saf. 1999;20:133–46. doi: 10.2165/00002018-199920020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Khare CP. Berlin: Springer-Verlag; 2007. Indian Medicinal Plants. An illustrated dictionary. [Google Scholar]
- 6.Adaikan PG, Gauthaman K, Prasad RN. History of herbal medicines with and insight on Pharmacological properties of Tribulus terrestris. Ageing Male. 2001;4:163–9. [Google Scholar]
- 7.Ministry of Health and Family Welfare; Govt of India. The Ayurvedic Pharmacopoeia of India. 2001 [Google Scholar]
- 8.Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002;71:1385–96. doi: 10.1016/s0024-3205(02)01858-1. [DOI] [PubMed] [Google Scholar]
- 9.Gautaman K, Ganesan AP. The hormonal effects of Tribulus terrestris and its role in the management of erectile dysfunction: An evaluation using primates, rabbits and rat. Phytomedicine. 2008;15:44–54. doi: 10.1016/j.phymed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Brown GA, Vukovich MD, Reifenrath TA, Parsons KA, Sharp RL, King DS. Effects of anabolic precursors on serum testosterone concentration and adaptations to resistance training in young men. Int J Sport Nutr Exer Metabol. 2000;10:340–59. doi: 10.1123/ijsnem.10.3.340. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto AM. Effects of chronic testosterone administration in normal men: Safety and efficacy of high dosage testosterone and parallel dose-dependent suppression of luteinizing hormone, follicle-stimulating hormone, and sperm production. J Clin Endocrinol Metab. 1990;70:282–7. doi: 10.1210/jcem-70-1-282. [DOI] [PubMed] [Google Scholar]
- 12.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanoli P, Rivasi M, Zavatti M, Brusiani F, Vezzalini F, Baraldi M. Activity of single component of Ferula hermonis on male rat sexual behaviour. Int J Impot Res. 2005;17:513–8. doi: 10.1038/sj.ijir.3901346. [DOI] [PubMed] [Google Scholar]
- 14.Agmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203–9. doi: 10.1016/s1385-299x(96)00036-0. [DOI] [PubMed] [Google Scholar]
- 15.Benassi-Benelli A, Ferrari F, Quarantotti BP. Penile Erection induced by apomorphine and N-n-propyl-norapomorphine in rats. Arch Int Pharmacodyn Ther. 1979;242:241–7. [PubMed] [Google Scholar]
- 16.Ang HH, Sim MK. Effects of Eurycoma longifolia jack on penile erection index and homosexual mounting in rats. J Pharm Sci. 1997;3:117–9. [Google Scholar]
- 17.Ahmed KA, Venkataraman BV, Mitra SK. Assessment of a polyherbal ayurvedic medicine for sexual activity in rats. Indian Drugs. 1999;36:576–82. [Google Scholar]
- 18.OECD. OECD Guidelines for the testing of chemicals. - 407. 2008 [Google Scholar]
- 19.du Plessis SS, de Jongh PS, Franken DR. Effect of acute in vivo sildenafil citrate and in vitro 8-bromo-cGMP treatments on semen parameters and sperm function. Fertil Steril. 2004;81:1026–33. doi: 10.1016/j.fertnstert.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 20.Aversa A, Mazzilli F, Rossi T, Delfino M, Isidori AM, Fabbri A. Effects of sildenafil (Viagra) administration on seminal parameters and post ejaculatory refractory time in normal males. Hum Reprod. 2000;15:131–4. doi: 10.1093/humrep/15.1.131. [DOI] [PubMed] [Google Scholar]
- 21.Lefievre L, De Lamirande E, Gagnon C. The cyclic GMP-specific phosphodiesterase inhibitor, sildenafil, stimulates human sperm motility and capacitation but not acrosome reaction. J Androl. 2000;21:929–37. [PubMed] [Google Scholar]
- 22.Tomova M, Gjulemetova R, Zarkova S, Peeva S, Pangarova T, Simova M. Varna, Bulgaria: International Conference of Chemistry and Biotechnology of Biologically Active Natural Products; 1981. Steroidal saponins from Tribulus terrestris L. with a stimulating action on the sexual functions. [Google Scholar]
- 23.Burger M, Sikka SC, Bivalacqua TJ, Lamb DJ, Hellstrom WJ. The effect of sildenafil on human sperm motion and function from normal and infertile men. Int J Impot Res. 2000;12:229–34. doi: 10.1038/sj.ijir.3900551. [DOI] [PubMed] [Google Scholar]


