Abstract
Introduction
High blood pressure (BP) leads to target organ damage. It is suggested that regression of early organ lesions is possible on condition of BP normalization. The study objective was to assess whether permanent reduction of BP to the recommended values modifies renal vascular response to acute angiotensin II inhibition in the Doppler captopril test (DCT) in patients with essential hypertension (EH).
Material and methods
Twenty-nine persons (58 kidneys) were found eligible for the study: 18 patients with EH and 11 healthy volunteers constituting the control group. Glomerular filtration rate estimation (eGFR), 24-h ambulatory BP monitoring (ABPM) and DCT with evaluation of renal resistive index change (ΔRI) were performed before and after a 6-month period of intensive antihypertensive therapy (IAT). Additional ABPM was performed at the end of IAT.
Results
The mean IAT period was 8.5 ±2.4 months. The mean 24-h values of systolic and diastolic BP in the EH group were significantly lower in the IAT period than at the beginning and at the end of the study. Significantly lower systolic and diastolic BP (p < 0.05) and improvement of renal function (eGFR 121 ±38 vs. 139 ±40 ml/min, p < 0.001) were found after IAT as compared to initial values. Before IAT, ΔRI was significantly lower in the EH group as compared to the controls, but no such differences were found after IAT.
Conclusions
In EH patients, intensive BP lowering to the recommended values was associated with improvement of renal function and normalisation of renal vascular response to acute angiotensin II inhibition.
Keywords: renal resistive index, Doppler captopril test
Introduction
Arterial hypertension is an epidemic disease. Essential hypertension (EH) constitutes 90–95% of cases of this disease, and its development is associated with the interaction of genetic, environmental and demographic factors. Progression of arterial hypertension leads to remodelling of the arterial wall and a further increase of already elevated peripheral resistance, especially in small vessels [1]. Structural changes of the vascular wall result in its decreased compliance, increased stiffness and acceleration of the pulse wave velocity and a further increase in vascular resistance. Intrarenal increase of arterial resistance and impaired renal autoregulation in EH is probably caused by local elevation of angiotensin II (Ang II), the greater concentration of which contributes to development of hypertension and renal injury [2]. Renal vascular resistance and arterial compliance are together expressed in the Doppler examination as the renal resistive index (RI) [3]. Change of RI due to acute inhibition of Ang II with captopril depends mainly on the level of local Ang II concentration and thus could reflect the efficacy of renal autoregulation [4]. In clinical conditions it is assessed in the Doppler captopril test (DCT) involving calculation of the difference between values of the renal resistive index before and after oral captopril administration [5–8]. In the studies conducted so far, a significant effect of blood pressure and duration of EH on elevated resistive index in intrarenal arteries, reflecting the advancement of organ lesions in the course of EH, was evidenced [9–13]. In view of these complications, the current guidelines for the management of hypertension consistently recommend lowering the mean daily blood pressure to<125–130/80 mmHg, and only values below 120/80 mmHg are considered optimal [14]. The study objective was to assess whether consistent reduction of blood pressure (BP) to the recommended values modifies renal vascular response to acute Ang II inhibition in the DCT in patients with EH.
Material and methods
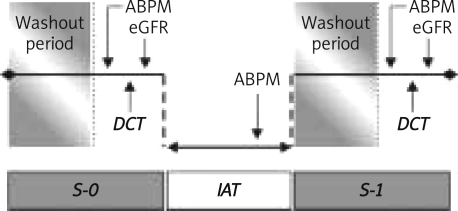
Twenty-nine persons (58 kidneys) were enrolled in the study and divided into two groups: 1) EH group – 18 patients with essential hypertension, 2) control group – 11 healthy volunteers. Patients were recruited from consecutive subjects who had been admitted to the Nephrology Department over a one-year period. Baseline characteristics of studied groups are shown in Table I. Exclusion criteria included renal insufficiency with serum creatinine levels>3 mg/dl; glomerular, tubulointerstitial and obstructive renal diseases; heart and liver failure; diabetes; renal artery stenosis; intolerance of or allergy to angiotensin-converting enzyme inhibitors; and ongoing treatment with non-steroidal anti-inflammatory drugs. The study was conducted in two stages (Figure 1), before (S-0) and after (S-1) a 6-month period of controlled and intensive antihypertensive therapy (IAT).
Table I.
Baseline characteristics of studied groups
| Parameter | Controls (n = 11) | EH group (n = 18) | Value of p |
|---|---|---|---|
| Age [years] | 34.4±12.7 | 37.3±11.3 | NS |
| Gender | 6 M+5 F | 11 M+7 F | NS |
| BMI [kg/m2] | 23.8±3.8 | 26.8±4.5 | NS |
| Smoking [years] | 0 | 0 (0–25) | NS |
| EH duration [years] | 0 | 2 (0.25–15.0) | <0.001 |
| LDLs [mg/dl] | 97.8±20.9 | 119.4±29.7 | <0.05 |
| Creatinines [mg/dl] | 0.85±0.16 | 0.99±0.39 | NS |
| CRPs [mg/dl] | 0.13±0.14 | 0.09±0.08 | NS |
| UAER [g/24 h] | 13.3±8.7 | 8.8 (4.0–265.0) | NS |
UAER – urinary albumin excretion rate, s – serum, NS – non-significant
Figure 1.
Study protocol diagram
Target BP level was 125/80 mmHg according to recommendations for ABPM measurements [14]. Antihypertensive treatment (β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors and thiazide diuretics) was modified according to BP control. Depending on the value of baseline 24-h ambulatory blood pressure monitoring (ABPM), new antihypertensive medications were added or changed; increasing the doses was less frequent. If home blood pressure was higher than 135/85 mmHg after the first month of treatment, only the doses of already prescribed medications were increased. The tests of each stage were performed after a 5-day washout period without antihypertensive drugs, except β-blockers if they were used. These tests included estimation of glomerular filtration rate (eGFR) by the Cockcroft-Gault formula, ABPM and the Doppler captopril test. 24-h Ambulatory blood pressure monitoring was also performed during controlled antihypertensive treatment, 7–10 days before S-1 tests.
Ultrasound examinations were performed using the LOGIQ 400 ultrasonograph (GE), with a convex transducer, with operating frequency of 3.5–5 MHz. The renal resistive index (RI) in segmental and interlobar arteries of the kidneys was calculated on the basis of Pourcelot's equation as the ratio of the difference between the maximum systolic velocity (Vs) and the end-diastolic velocity (Vd) to the maximum systolic velocity: RI=(Vs – Vd)/Vs [15]. Resitive index for each kidney was a mean of 3–5 measurements performed in the upper middle and lower regions of the renal sinus. The record of the Doppler spectrum was obtained after an angle correction of ≤ 60° to the vessel axis.
The Doppler captopril test involved RI assessment performed before (Phase 0) and 60 min after oral administration of 50 mg of captopril (Phase 1), with arterial pressure measurement on the brachial artery performed each time (Figure 2) [6–8, 16].
Figure 2.
Doppler captopril test diagram
Renal response to Ang II inhibition was calculated as the change of the renal resistive index (ΔRI) in the Doppler captopril test based on the formula: ΔRI=RI1 – RI0 [8]. The local bioethics committee approved the protocol of the study.
Statistical analysis
The variables examined were analysed with Student's t-test, Wilcoxon or Mann-Whitney test, as determined by meeting the condition of normal distribution and a relation between the analysed variables. Differences between RI values in the DCT were assessed by Student's t-test for related variables. The relationships between change of eGFR during IAT and variables (age, BMI, EH duration, LDL, urinary albumin excretion rate [UAER], baseline eGFR, magnitude of BP reduction and numbers of antihypertensive drugs) were examined by linear regression analyses.
Results
Eighteen patients with EH examined in both stages were found eligible for the study. The median time from EH diagnosis was 2 years (range: 3 months to 15 years). 24-h Ambulatory blood pressure monitoring during intensive antihypertensive therapy was performed in 15 patients. The mean period of IAT was 8.5±2.4 months. The mean number of antihypertensive medications taken before S-1 was significantly higher than before S-0 (1.8±1.0 vs. 1.0±1.0, p=0.002). Modification of antihypertensive drugs during IAT is presented in Table II.
Table II.
Modification of antihypertensive treatment during IAT
| Number of drugs per patient | Before IAT (n=18) | IAT (n=18) | ||
|---|---|---|---|---|
| n | Treatment | n | Treatment | |
| 0 | 7 | – | 0 | – |
| 1 | 6 | (1×BB) | 11 | (5×BB) |
| (1×CCB) | (2×CCB) | |||
| (4×ACE-I) | (4×ACE-I) | |||
| 2 | 3 | (2×ACE-I+D) | 4 | (2×BB+D) |
| (1×CCB+D) | (1×ACE-I+D) | |||
| (1×CCB+D) | ||||
| 3 | 2 | (1×CCB+ACE-I+D) | 1 | (1×CCB+ACE-I+D) |
| (1×BB+CCB+D) | ||||
| 4 | 0 | 2 | (2×BB+CCB+ACE-I+D) | |
BB – β-blocker, CCB – calcium channel blocker, ACE-I – inhibitor of angiotensin-converting enzyme, D – thiazide diuretic
Due to ethical reasons, the control group was tested only in S-0. The DCT results for the control group and the EH group in both stages are presented in Table III.
Table III.
Comparison of DCT scores
| Group | RI | ΔRI | Value of p (phase 0 : 1) | |
|---|---|---|---|---|
| Phase 0 | Phase 1 | |||
| Control (n = 11) | 0.606±0.049 | 0.616±0.054 | 0.0129±0.0114 | <0.004 |
| EH (n = 18) S-0 | 0.601±0.033 | 0.593±0.047 | m.−0.0045 (*) | |
| (−0.075 : 0.048) | NS | |||
| EH (n = 18) S-1 | 0.591±0.042 | 0.592±0.034 | m. 0.0030 | |
| (−0.0455 : 0.0675) | NS | |||
S-0 – stage 0, S-1 – stage 1
*significance level of p <0.05 vs. the control group, NS – non-significant
In both groups and both stages, captopril administration resulted in significant lowering of systolic and diastolic blood pressure (p<0.05) but a significant increase in RI was found only in the control group. In the EH group, the change of the RI was not significant in either of the two stages. Parameters of renal function and ABPM values in both stages and also ABPM in the treatment intensification period are presented in Table IV.
Table IV.
Comparison of ABPM and eGFR in stages and IAT period
| Variable | Control group (n = 11) | EH group (n=18) | Value of p EH group | ||
|---|---|---|---|---|---|
| S-0 | IAT | S-1 | |||
| SBP [mmHg] | 117.5±8.3 | 135.0**±12.3 | 125.5*±8.5 | 131.3*±9.8 | S-0 : S-1 – NS |
| S-0 : IAT –<0.05 | |||||
| P-1 : IAT –<0.05 | |||||
| DBP [mmHg] | 69.9±5.3 | 85.0**±11.5 | 77.4*±7.0 | 81.7**±8.5 | S-0 : S-1 – NS |
| NS S-0 : IAT –<0.05 | |||||
| S-1 : IAT –<0.05 | |||||
| eGFR [ml/min] | 116.4±21.4 | 121.0±38.0 | – | 138.7±39.7 | S-0 : S-1 –<0.001 |
S-0 – stage 0, S-1 – stage 1, IAT – period of intensive antihypertensive treatment, *significance level vs. the control group:
*for p < 0.05
**for p < 0.001, NS – non-significant
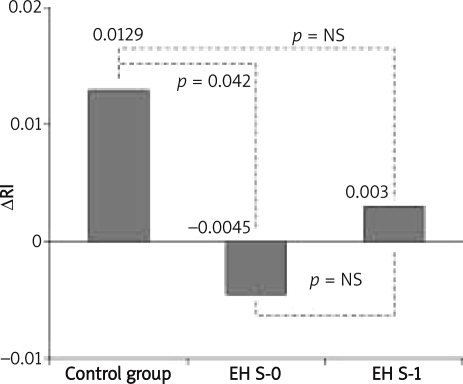
In S-0, in the period of intensive antihypertensive therapy and in S-1, the values of 24-h BP were significantly higher in the EH group as compared to the control group. At the same time, the mean 24-h values of systolic and diastolic pressure in the EH group were significantly lower in the IAT period than at the beginning and at the end of the study. Tests of renal function showed a slight but significant improvement of organ function after IAT. In multiple regression analyses only duration of hypertension (β=1.464, p=0.002), magnitude of BP reduction during IAT (β=1.327, p=0.004) and baseline eGFR (β=–0.668, p=0.02) significantly affected the improvement of eGFR (R2=0.600, p<0.015). Considering ΔRI variability in study groups and stages, a significantly lower value of ΔRI was found in the EH group in S-0 as compared to the controls. In S-1, the difference in ΔRI values was not significant (Figure 3).
Figure 3.
Variability of ΔRI in the studied groups
Discussion
Normal values of the resistive index in intrarenal arteries in adults are contained within the limits of 0.56–0.70 [9, 17]. Elevated RI values in the elderly are attributed to lower arterial compliance and an increase in pulse pressure, characteristic for old age [18, 19]. Although in our study the groups differed significantly in 24-h BP values, the baseline measurement of the RI in patients with EH did not significantly differ from healthy persons. An increase in the value of RI after captopril administration was significant only in the group of healthy persons.
Examining 15 healthy persons Taniwaki et al. showed that RI values increased significantly after an oral dose of captopril (25 mg) in comparison to the baseline [8]. Veglio et al. obtained similar findings [20]. In a group of 45 EH patients and 15 healthy persons, they found a significant increase in RI after captopril only in healthy persons and patients with mild arterial hypertension. These authors also reported that in patients with more advanced hypertension, the RI change after captopril was not significant. These reports correspond to the results of our study, where no significance of RI change in the DCT was found in the essential hypertension group, in stages S-0 and S-1. Nevertheless, ΔRI obtained in S-0 was significantly lower in the EH group as compared to the controls. Undoubtedly, the fact that the mean value of RI after captopril administration decreased, which may be attributed to vascular alterations and haemodynamic impairment due to elevation of intrarenal Ang II concentration, contributed to this result. A similar phenomenon, but much more marked, is observed in haemodynamically significant renal artery stenosis when RI lowering after captopril administration exceeds 0.05 [6, 7]. In their studies, Veglio et al. found that the initial values of the renal resistive index in stenosed renal arteries did not differ significantly from RI in arteries without stenosis, and after captopril administration the RI value significantly lowered in kidneys with a stenosed artery, while in kidneys with a normal artery an increase of the RI was found [16]. In our study, in the EH group, we found a post-captopril reduction of the resistive index in DCT in S-0 and a slight increase of this index in S-1. The stages were separated by an over 8-month period of intensive antihypertensive therapy, where the recommended target values of 24-h BP were reached.
To assess the probable effect of intensive BP reduction (and avoid the effect of the drugs) on vascular properties, both evaluations of DCT were performed after suitable, although relatively short, washout periods of 5 days covering double the half-life time of the used drugs. Most probably, as a result of intensive antihypertensive therapy, the renal vascular response to Ang II inhibition, which did not differ significantly in S-1 from the result found in healthy persons, in contrast to S-0, improved. This cause-and-effect relationship seems to be supported by a significant improvement of renal function in the EH group in the S-1 stage as compared to S-0.
Regression of lesions in intrarenal arteries formed in the course of arterial hypertension is questionable. Radermacher et al. investigated a group of 138 patients with hypertension in the course of haemodynamically significant renal artery stenosis and found that angioplasty of the stenosed renal artery in patients with RI>0.8 does not bring the expected clinical benefits such as improved renal function, better control of BP and longer survival [21]. In this clinical study, the RI value>0.8 was to reflect irreversibility of organ lesions in the course of hypertension and disqualified patients from invasive treatment. In response to these reports, in a group of 50 patients with RI>0.8 selected among 340 patients with unilateral or bilateral renal artery stenosis, Zeller et al. observed a significant improvement of renal function after angioplasty with stenting [22]. Haemodynamically significant renal artery stenosis is an extreme example of renal vascular pathology. Due to the chronic nature and haemodynamic significance of the process, some lesions in the renal vascular-interstitial region are irreversible. In earlier stages of hypertensive disease, the probability of regression of functional and structural alterations of intrarenal vessels should be much higher, which was suggested by Derchi et al. [23]. Strict BP control is necessary for renoprotection. In our study, we demonstrated that significant BP lowering to the recommended values is associated with a significant improvement of intrarenal arterial functional properties and renal function. We found significant improvement of eGFR (Cockcroft-Gault formula) corresponding with decrease of serum creatinine (0.99 vs. 0.92 mg/dl, p=0.03) in patients with EH after IAT. This finding is probably related to the better control of BP. It is suggested that some groups of antihypertensive medications such as ACE-I and ARB can prevent decline of renal function, irrespective of BP reduction. In our study, none group of antihypertensive drugs correlated with rise of eGFR. However, in multivariate linear regression duration of hypertension, magnitude of BP reduction during IAT and baseline eGFR significantly affected the magnitude of eGFR rise after intensive antihypertensive treatment. This finding suggests that improvement of renal function during antihypertensive therapy is possible when hypertension-related kidney damage is not so substantial, and is functional and vascular rather than glomerular and interstitial (longer duration of hypertension, higher BP reduction during IAT, but a low baseline eGFR). These data clearly suggest a beneficial effect of strict BP reduction. On the other hand, the short period of our study might have had a crucial impact on such optimistic results. If the time of observation was longer, there could be only stabilization or even lowering of eGFR observed due to time-dependent decline of renal function.
Undoubtedly, the value of the results of our study is limited by the young age of investigated persons, the small size of the study groups, relatively short washout period and lack of DCT evaluation at the end of IAT. These facts arise partially from the necessity to standardise the DCT and withdrawal of antihypertensive medicines, as well as from the burdensome nature of the conducted tests and frequent follow-up visits. On the other hand, an advantage of such a study protocol is the results of the DCT, which are devoid of an effect of other drugs blocking the renin-angiotensin-aldosterone system and BP lowering agents. Owing to the performance of Doppler tests by the same person, we eliminated the bias arising from variable measurement methods.
The results of our study confirm the benefits arising from intensive antihypertensive therapy and BP reduction to the recommended target values in patients with EH.
If our results are confirmed in a larger randomised study, we will have a strong argument for more intensive antihypertensive treatment of patients with EH.
In conclusion, in the group of patients with recently diagnosed EH, significant lowering of blood pressure to the recommended values was associated with a significant improvement of renal function and normalisation of renal vascular response to acute angiotensin II inhibition.
References
- 1.Gryglewska B. Influence of arterial hypertension on structure and function of artery vessels. In: Grodzicki T, Kocemba J, editors. Arterial hypertension in elderly. Gdansk, Via Medica; 2000. pp. 49–55. [Google Scholar]
- 2.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411–7. doi: 10.1148/radiology.211.2.r99ma48411. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ND, Allan D, Kifor I, et al. Responses to converting enzyme and renin inhibition. Role of angiotensin II in humans. Hypertension. 1994;23:44–51. doi: 10.1161/01.hyp.23.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Lubas A, Żelichowski G, Obroniecka I, Wankowicz Z. Influence of controlled hypotensive therapy on renal autoregulation efficiency in the Doppler Captopril Test in patients with chronic glomerulonephritis. Pol Merkuriusz Lek. 2008;142:289–93. [PubMed] [Google Scholar]
- 6.Oliva VL, Soulez G, Lesage D, et al. Detection of renal artery stenosis with Doppler sonography before and after administration of captopril: value of early systolic rise. AJR Am J Roentgenol. 1998;170:169–75. doi: 10.2214/ajr.170.1.9423626. [DOI] [PubMed] [Google Scholar]
- 7.René PC, Oliva VL, Bui BT, et al. Renal artery stenosis: evaluation of Doppler US after inhibition of angiotensin-converting enzyme with captopril. Radiology. 1995;196:675–79. doi: 10.1148/radiology.196.3.7644628. [DOI] [PubMed] [Google Scholar]
- 8.Taniwaki H, Ishimura E, Kawagishi T, et al. Intrarenal hemodynamic changes after captopril test in patients with type 2 diabetes: a duplex Doppler sonography study. Diabetes Care. 2003;26:132–7. doi: 10.2337/diacare.26.1.132. [DOI] [PubMed] [Google Scholar]
- 9.Pontremoli R, Viazzi F, Martinoli C, et al. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant. 1999;14:360–5. doi: 10.1093/ndt/14.2.360. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Itoh T, Hougaku H, et al. Clinical usefulness of duplex ultrasonography for the assessment of renal arteriosclerosis in essential hypertensive patients. Hypertens Res. 2001;24:13–7. doi: 10.1291/hypres.24.13. [DOI] [PubMed] [Google Scholar]
- 11.Alterini B, Mori F, Terzani E, et al. Renal resistive index and left ventricular hypertrophy in essential hypertension: a close link. Ann Ital Med Int. 1996;11:107–13. [PubMed] [Google Scholar]
- 12.Ogata Ch, Horio T, Kamide K, Takiuchi S, Kawano Y. Association between left ventricular diastolic dysfunction and renal hemodynamic change in patients with treated essential hypertension. Hypertens Res. 2003;26:971–98. doi: 10.1291/hypres.26.971. [DOI] [PubMed] [Google Scholar]
- 13.Lubas A, Wankowicz Z. Usefulness of ultrasonographic renal resistive index in monitoring efficacy of hypotensive therapy in haemodialysed patients – case report. Pol Merkuriusz Lek. 2002;13:403–5. [PubMed] [Google Scholar]
- 14.Mancia G, Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 15.Pourcelot L. Applications cliniques de l'examen Doppler transcutané. In: Peronneau P, editor. Velocimetrie ultrasonore Doppler. Paris: Seminare Institut National de la Santé et de la Recherche Médicale; 1975. pp. 213–40. [Google Scholar]
- 16.Veglio F, Frascisco M, Melchio R, et al. Assessment of renal resistive index after captopril test by Doppler in essential and renovascular Hypertension. Kidney Int. 1995;48:1611–6. doi: 10.1038/ki.1995.455. [DOI] [PubMed] [Google Scholar]
- 17.Krzanowski M, Plichta A. 2000. Atlas of vessels ultrasonography. Medycyna Praktyczna, Kraków. [Google Scholar]
- 18.Terry JD, Rysavy JA, Frick MP. Intrarenal Doppler: characteristics of aging kidneys. J Ultrasound Med. 1992;11:647–51. doi: 10.7863/jum.1992.11.12.647. [DOI] [PubMed] [Google Scholar]
- 19.Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? Am J Roentgenol. 2003;180:885–92. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 20.Veglio F, Provera E, Pinna E, et al. Renal resistive index after captopril test by echo-Doppler in essential hypertension. Am J Hypertens. 1992;5:431–6. doi: 10.1093/ajh/5.7.431. [DOI] [PubMed] [Google Scholar]
- 21.Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–7. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 22.Zeller T, Frank U, Müller C, et al. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: long-term results from a prospective registry of 456 lesions. J Endovasc Ther. 2004;11:95–106. doi: 10.1583/03-1062.1. [DOI] [PubMed] [Google Scholar]
- 23.Derchi LE, Leoncini G, Parodi D, et al. Mild renal dysfunction and renal vascular resistance in primary hypertension. Am J Hypertens. 2005;18:966–71. doi: 10.1016/j.amjhyper.2005.01.018. [DOI] [PubMed] [Google Scholar]