Abstract
Introduction
The colon and rectum are common sites of food-related cancer in developed countries. Recent studies strongly suggest that red meat intake is associated with colon cancer, whereas for rectal cancer such an association still needs to be proved. The aim of the study was to assess the role of total amount and frequency of red meat intake in colorectal carcinogenesis based on published data using meta-analysis methods.
Material and methods
The literature published until 2009 was selected from: MEDLINE, PubMed, Scopus, Embase, CancerLit, Google Scholar and Cochrane Library databases. The used search terms were: colorectal cancer, colon cancer, rectal cancer, meat intake, red meat intake, red meat consumption, meat consumption, colorectal cancer risk, colon cancer risk, rectal cancer risk and lifestyle. Articles investigating red meat intake of more often than once a day or 50 g per day were reviewed and selected for further analysis.
Results
Twenty-two studies fulfilled the established criteria. A meta-analysis confirmed the carcinogenic effect of the consumption of over 50 g of red meat per day for the colon (relative risk 1.21, 1.07–1.37) but not for the rectum (relative risk 1.30, 0.90–1.89). Red meat intake more frequently than once a day can induce both colonic (relative risk 1.37, 1.09–1.71) and rectal cancer (relative risk 1.43, 1.24–1.64).
Conclusions
Red meat intake is associated with elevated risk of developing colorectal cancer. The frequency of red meat consumption rather than total amount of consumed meat is associated with a higher risk of colorectal carcinogenesis.
Keywords: diet, meat consumption, colorectal cancer, cancer risk
Introduction
Colorectal cancer is globally the most commonly diagnosed malignancy of the digestive tract. The highest incidence is observed in the United States of America, Australia, Japan and Western Europe. According to the World Health Organization, there are 945,000 newly diagnosed cases each year, of which 492,000 are fatal. The incidence for colorectal cancer is similar for men and women [1].
The cancer formation may be triggered by genetic as well as environmental factors, such as high alcohol intake and cigarette smoking [2]. However, the epidemiological studies conducted worldwide have demonstrated that the so-called “Western diet” rich in red meat may be the key stimulus for colonic and/or rectal carcinogenesis [3].
Cohort studies suggest a role of physical activity in prevention of colon rather than rectal cancers [1]. The tumour location and mucinous type may be dependent on some molecular characteristics of the tumour [4, 5]. The influence of environmental factors on histological type and cancer location through the colon and rectum have not been confirmed to date.
Although numerous epidemiological studies have aimed to confirm the positive relationship between the consumption of red meat and formation of colorectal cancer, their results were not strongly convincing [6–10]. The evaluation of red meat intake in colorectal cancer development was very difficult, due to many environmental factors modulating colorectal carcinogenesis, which has been reviewed recently [1].
A meta-analysis of seven cohort studies published up to 2004 estimated the summary effect of 1.43 (95% confidence interval [CI] 1.05–1.94) for red meat consumption per times per week [1]. Moreover, red meat consumption of over 100 g or 120 g per day elevates the risk of colorectal cancer development by 29% (95% CI 1.04–1.60) and 28% (95% CI 1.18–1.39), respectively [1]. Based on the data published in cohort studies the dose-dependent effect of red meat consumption is not clear. A change of dietary habits including more frequent consumption of red meat, breakfast sausages and pork chops/ham steaks elevates the risk of colorectal adenoma formation in a clinic-based case-control study [6, 7, 11–18]. We have not found in the literature a lower limit of amount of red meat intake which is not connected with elevation of colorectal cancer risk. Thus we test a relatively low daily intake (50 g) in comparison with frequency of red meat consumption against colorectal cancer risk.
In order to finally confirm or reject the hypothesis, we have meta-analysed the findings of the case-control and cohort studies carried out between 1994 and 2009. The outcome will be the ultimate confirmation of the assumption about the adverse effects of red meat intake of over 50 g or more frequently than once daily.
Material and methods
Search strategy
We conducted an electronic search of MEDLINE, Pub Med, Cochrane Library, Embase, CancerLit, Scopus and Google Scholar databases up to the end of September 2009. The search was done using the following keywords: (red meat OR minced meat OR ham OR bacon OR sausages OR lifestyle OR diet) AND (colorectal cancer OR colon cancer OR rectal cancer OR colorectal neoplasm OR colon neoplasm OR rectal neoplasm). A function extracting related articles was used during the search process. The full versions of the English-language analysed articles and abstracts of all found papers were available during the selection process.
Study selection
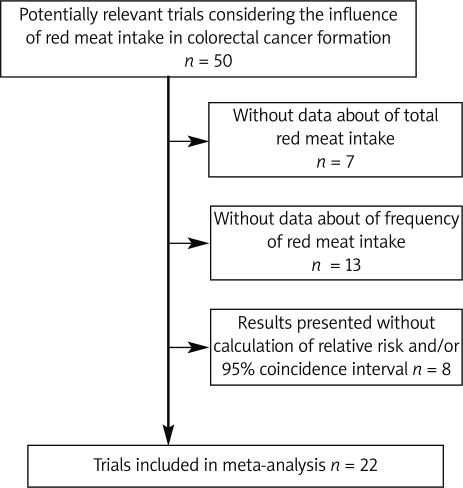
In the primary search we found 290 records. During the first selection we evaluated 50 of them as potentially relevant articles considering the influence of red meat intake in colorectal cancer formation. Studies with results supported by calculated variables RR (relative risk) and/or 95% confidence interval were selected for further analysis. During the second selection 7 articles were excluded due to lack of data referring to total red meat intake. Another 13 were also rejected due to insufficient data concerning frequency of red meat intake. The results of 8 studies were presented without calculated relative risk and/or 95% confidence interval ratio (Figure 1). The remaining 22 studies were included in the meta-analysis. All studies selected for the meta-analysis process investigate the association between risk of colon and/or rectal cancer development and red meat intake of over 50 g/day or more frequently than once per day. The selected studies were cohort or case-control with questionnaires used as a tool. The age of analysed populations ranged from 30 to 80. Studies that failed to meet our criteria were not taken into consideration.
Figure 1.
Study selection process of articles considering red meat intake and risk of colorectal cancer development
Quality of studies
The studies were qualified using the Protocol Enhancement Project: with questions to assist the critical appraisal of an interventional study without randomisation or a non-experimental study. Out of the 22 selected studies assessing the influence of red meat consumption of over 50 g/day or more frequently than once daily on the development of colon and/or rectal cancer, 12 and 10 were case-control and cohort, respectively.
Data extraction
The data were abstracted from analysed studies using forms standardized by both authors. Abstracted data included study design, setting, and population; nutritional habits, especially red meat consumption; and outcome with the incidence of colorectal cancer.
Statistical analysis
The analysis was performed using the meta-analysis software Stat Direct v. 2.6.2. StatSoft LTD, UK based on the random effects model. The results are presented graphically including relative risk (RR) and 95% confidence intervals (95% CI). The heterogeneity between trials was tested using χ2 tests. Value of p≤0.05 indicates significant heterogeneity. Studies that failed to meet the established criteria were not taken into consideration.
Results
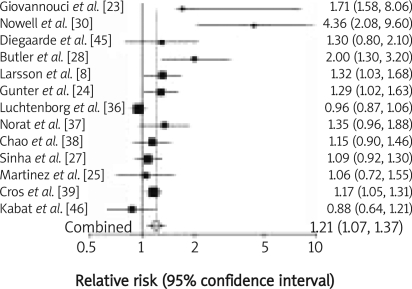
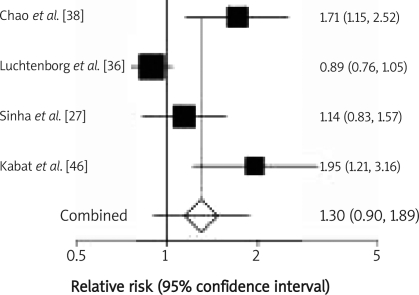
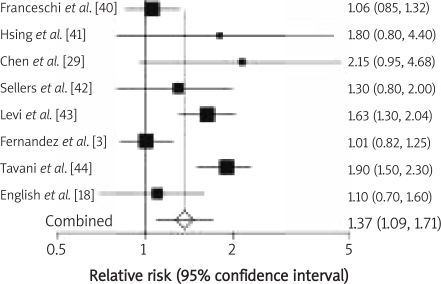
Twenty-two studies investigating the red meat consumption of over 50 g/day or more frequently than once daily were analysed. Twelve were performed in the United States of America, 8 in European countries including the UK, Spain, Switzerland, the Netherlands, Sweden, France and Italy, 1 was conducted in Australia, and 1 in Canada. Eleven of them investigated the association between red meat intake and the risk of colonic cancer, whereas only 1 study analysed the influence of red meat consumption on possible development of rectal cancer. The results of 10 studies referred to the effect of red meat intake on the development of both types of cancer. Red meat intake of over 50 g/day was analysed in 13 studies. The meta-analysis of selected studies demonstrated that red meat intake of over 50 g/day triggers colonic carcinogenesis (RR 1.21, 95% CI 1.07–1.37; Figure 2); the evidence for rectal cancer is not conclusive (RR 1.30, 95% CI 0.90–1.89; Figure 3). Consumption of red meat more frequently than once per day was analysed in 9 studies. The meta-analysis of these studies showed that red meat intake more frequently than once per day induces colonic cancer (RR 1.37, 95% CI 1.09–1.71; Figure 4) as well as rectal cancer (RR 1.43, 95% CI 1.24–1.64; Figure 5).
Figure 2.
Red meat intake over 50 g/day and relative risk of colon cancer development. Summary metaanalysis plot with random effect
Figure 3.
Red meat intake over 50 g/day and relative risk of rectal cancer development. Summary metaanalysis plot with random effect
Figure 4.
Red meat intake more frequent than once a day and relative risk of colon cancer development. Summary meta-analysis plot with random effect
Figure 5.
Red meat intake more frequent than once a day and relative risk of rectal cancer development. Summary meta-analysis plot with random effect
Discussion
Inappropriate dietary habits may lead to numerous serious diseases. It is generally accepted that a high-calorie “Western” diet is the cause of such conditions as obesity, diabetes, hypertension and cancer. The higher incidence of colorectal cancer in developed countries prompted researchers to conduct epidemiological studies aiming to confirm or reject the hypothesis of a relationship between dietary habits and the development of cancer [19].
Colorectal cancer formation is dependent on both genetic and environmental factors stimulating the remodelling of bowel mucosa [20]. The strong relationships between instability of some genes and adenomatous polyposis or hereditary non-polyposis colorectal cancers are already well known. To date, in the majority of diagnosed cancers located within the colon and rectum, hereditary mutations have not been observed. These findings are probably associated with the instabilities of low-penetrance genes, e.g. APC∣1307K, TGFbR1, BLM, HRAS1 [20]. It has been widely accepted that in the case of genes with low penetrance potentially involved in carcinogenesis, the role of environmental factors is the most probable.
A review of the literature on this subject suggests a positive association between the consumption of red meat and colon and/or rectal cancer. High intake of red meat causes an increasing concentration of biliary acids in the bowel content. The biliary acids can upset the balance between the maturing and apoptotic cells [10, 21]. The products of their metabolism, including deoxycholic acid, have the property of destroying cytoplasmic membrane in epithelial cells of the large intestine. That process indirectly results in increased cell proliferation and their higher sensitivity to mutagenic factors; consequently, it may be the cause of malignant transformations [21–24]. The process of neoplasm formation with the participation of the biliary acids may also be associated with their ability to activate cyclooxygenase 2, which may, in turn, lead to excessive prostaglandin production. This results in resistance to apoptosis and stimulates malignancy and invasiveness [22].
During high-temperature processing of red meat many substances such as heterocyclic amines (HCA) and polycyclic aromatic hydrocarbons (PAH) are formed [25–27]. Their carcinogenic effect still needs further investigations. The carcinogenic effect of HCA has been confirmed in several studies [25–30]. Metabolism of heterocyclic amines with the participation of N-acetyltransferase (NAT)2 and (NAT)1 damages cell DNA, which may lead to colonic cancer formation [2]. Polycyclic aromatic hydrocarbons, formed during high temperature processing of meat, are considered as possible carcinogenic factors. No studies have confirmed these observations so far [25, 27].
The analysis demonstrated the strongest association between red meat intake of more than once daily and the risk of both rectal (RR 1.43, 95% CI 1.24–1.64) and colonic cancer (RR 1.37, 95% CI 1.09–1.71) occurrence. Red meat consumption of ≥ 50 g/day may also lead to colon cancer (RR 1.21, 95% CI 1.07–1.37), while dependence between red meat consumption of over 50 g daily and rectal cancer is not conclusive (RR 1.30, 95% CI 0.90–1.89). According to the presented results of our meta-analysis we speculate that the frequency of red meat consumption is a crucial risk factor for carcinogenesis within the colon and rectum. The relative risk calculated for the total amount of meat consumed daily is lower than calculated for regular consumption. The difference between these two groups of patients – with consumption of over 50 g/day and more frequently than once daily – is difficult to estimate. Frequent consumption of meat may be lower than 50 g; high intake of more than 50 g can also be consumed as one meal. We hypothesize that permanent exposure to some carcinogenic agents contained in red meat as well as stimulation by bile efflux may be responsible for remodelling of colorectal mucosa and tumour development.
High red meat intake can implicate high energy intake and chronic overeating. The protein/ fat/energy can be balanced by physical activity. Physical activity reduces cancer formation in the colon and rectum although the evidence is stronger for the colon than for the rectum [1]. Low physical activity can increase the risk of colorectal cancer development by approximately 20% [31]. Moreover, cigarette smoking and high alcohol intake are connected with elevated risk of colorectal cancer formation.
The established association between red meat consumption and colonic cancer poses the question of what type of diet could prevent the disease. Dietary fibre is undoubtedly a protective factor [32]. A diet rich in fruits and vegetables has a beneficial effect on the human body and may indeed protect against colonic and/or rectal cancer [32]. There are insufficient data about the role of a vegetarian diet in prevention of colonic malignancy formation [33]. It is still not obvious whether the benefits of a vegetarian diet are due to the high amount of vegetable intake or due to the elimination of meat consumption. It is a known phenomenon that a red meat enriched diet is connected with lower consumption of poultry, vegetables and fish. Thus the beneficial effect of vegetables or fish is partially related to the low amount or less frequent red meat consumption. In our opinion red meat consumption should be evaluated as an indicator of dietary habits when the direct and independent role of particular elements of the diet is very difficult to prove. These data should be elucidated in randomized trials including precise calculations of the total energy intake.
Published clinical studies suggest that calcium may protect the mucous membrane of the large intestine from excessive concentration of biliary acids and, consequently, prevent neoplasm formation [34]. Similarly, magnesium, whose activity is dependent on the concentration of calcium ions in the body, seems to play a significant role in the protection against colonic and/or rectal cancer [35]. Unfortunately, the results of these studies are not explicit. Further clinical and epidemiological studies are necessary to confirm the hypothesis concerning the importance of calcium and magnesium in the prevention of colonic and/or rectal cancer.
In conclusion, we confirm in our meta-analysis that high-frequency red meat intake is an important factor increasing the risk of colorectal cancer development. There are insufficient data that would allow us to confirm that a high level of red meat consumption of more than 50 g/day increases the risk of rectal cancer development. In contrast to this observation, high daily red meat consumption elevates the incidence of colon cancer formation. We suggest that frequent regular red meat consumption rather than a high amount elevates the risk of colorectal cancer development. Changes in dietary habits with less frequent consumption of red meat may be a useful observation for planning a cancer preventive diet in the future.
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Nutrition. 2008;24:393–398. [Google Scholar]
- 2.Chan AT, Tranah GJ, Giovannucci EL, Willett WC, Hunter DJ, Fuchs CS. Prospective study of N-acetylotransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. Int J Cancer. 2005;115:648–52. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez E, Negri E, La Vecchia C, Franceschi S. Diet diversity and colorectal cancer. Prev Med. 2000;31:11–4. doi: 10.1006/pmed.2000.0667. [DOI] [PubMed] [Google Scholar]
- 4.Paluszkiewicz P, Berbeć H, Pawłowska-Wakowicz B, Cybulski M, Paszkowska A. p53 protein accumulation in colorectal cancer tissue has prognostic value only in left-sided tumours. Cancer Detect Prev. 2004;28:252–9. doi: 10.1016/j.cdp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Hanski C, Tiecke F, Hummel M, et al. Low frequency of p53 gene mutations and protein expression in mucinous colorectal carcinomas. Cancer Lett. 1996;103:163–70. doi: 10.1016/0304-3835(96)04208-5. [DOI] [PubMed] [Google Scholar]
- 6.Chiu BC, Gasptur SM. Changes in diet during adult life and risk of colorectal adenomas. Nutr Cancer. 2004;49:49–58. doi: 10.1207/s15327914nc4901_7. [DOI] [PubMed] [Google Scholar]
- 7.Robertson DJ, Sandler RS, Haile R, et al. Fat, fiber, meat and the risk of colorectal adenomas. Am J Gastroenterol. 2005;100:2789–95. doi: 10.1111/j.1572-0241.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. Int J Cancer. 2005;113:829–34. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 9.Le Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): lipids and foods of animal origin. Cancer Causes Control. 1997;8:637–48. doi: 10.1023/a:1018406716115. [DOI] [PubMed] [Google Scholar]
- 10.Kampman E, Slattery ML, Bigler J, et al. Meat consumption, genetic susceptibility, and colon cancer risk: a United States multicenter case-control study. Cancer Epidemiol Biomarkers Prev. 1999;8:15–24. [PubMed] [Google Scholar]
- 11.Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst. 1992;84:91–8. doi: 10.1093/jnci/84.2.91. [DOI] [PubMed] [Google Scholar]
- 12.Mathew A, Peters U, Chatterjee N, Kulldorff M, Sinha R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer. 2004;108:287–92. doi: 10.1002/ijc.10984. [DOI] [PubMed] [Google Scholar]
- 13.Tiemersma EW, Kampman E, Bas Bueno de Mesquita H, et al. Meat consumption, cigarette smoking, and genetic susceptibility in the etiology of colorectal cancer: results from a Dutch prospective study. Cancer Causes Control. 2002;13:383–93. doi: 10.1023/a:1015236701054. [DOI] [PubMed] [Google Scholar]
- 14.Satia AJ, Keku T, Galanko JA, et al. Diet, lifestyle, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14:429–36. doi: 10.1158/1055-9965.EPI-04-0486. [DOI] [PubMed] [Google Scholar]
- 15.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148:761–74. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Giovannucci E, Byrne C, et al. Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2006;15:1120–5. doi: 10.1158/1055-9965.EPI-05-0782. [DOI] [PubMed] [Google Scholar]
- 17.Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. II. Meat, poultry, seafood, dairy foods and eggs. Int J Cancer. 1993;53:720–7. doi: 10.1002/ijc.2910530503. [DOI] [PubMed] [Google Scholar]
- 18.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, Giles GG. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1509–14. [PubMed] [Google Scholar]
- 19.Irigaray P, Newby JA, Clapp R, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61:640–58. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 20.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–80. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 21.Cheng K, Raufman JP. Bile acid-induced proliferation of human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–47. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Rosignoli P, Fabiani R, De Bartolomeo A, Fuccelli R, Pelli MA, Morazzi G. Genotoxic effect of bile acid on human normal and tumour colon cells and protection by dietary antioxidants and butyrate. Eur J Nutr. 2008;47:301–9. doi: 10.1007/s00394-008-0725-8. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–7. [PubMed] [Google Scholar]
- 24.Gunter MJ, Probst-Hensch NM, Cortessis VK, Kulldorff M, Haile RW, Sinha R. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis. 2005;26:637–42. doi: 10.1093/carcin/bgh350. [DOI] [PubMed] [Google Scholar]
- 25.Martínez ME, Jacobs ET, Ashbeck EL, et al. Meat intake, preparation methods, mutagens and colorectal adenoma recurrence. Carcinogenesis. 2007;28:2019–27. doi: 10.1093/carcin/bgm179. [DOI] [PubMed] [Google Scholar]
- 26.Murtaugh MA, Ma KN, Sweeney C, Caan BJ, Slattery ML. Meat consumption patterns and preparation, genetic variants of metabolic enzymes, and their association with rectal cancer in men and women. J Nutr. 2004;134:776–84. doi: 10.1093/jn/134.4.776. [DOI] [PubMed] [Google Scholar]
- 27.Sinha R, Peters U, Cross AJ, et al. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res. 2005;65:8034–41. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- 28.Butler LM, Sinha R, Millikon RC, et al. Heterocyclic amines, meat intake, and association with colon cancer in population based study. Am J Epidemiol. 2003;157:434–45. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Stampfer MJ, Hough HL, et al. A prospective study of N-acetylotransferase genotype, red meat intake, and risk of colorectal cancer. Cancer Res. 1998;58:3307–11. [PubMed] [Google Scholar]
- 30.Nowell S, Coles B, Sinha R, et al. Analysis of total meat intake and exposure to individual heterocyclic amines in case-control study of colorectal cancer: contribution of metabolic variation to risk. Mutat Res. 2002;506–507:175–85. doi: 10.1016/s0027-5107(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 31.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr ChL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 32.Asano TK, McLeod RS. Dietary fibre for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2002;2:CD003430. doi: 10.1002/14651858.CD003430. [DOI] [PubMed] [Google Scholar]
- 33.Sanjoaquin MA, Appleby PN, Thorogood M, Mann JI, Key TJ. Nutrition, lifestyle and colorectal cancer incidence: a prospective investigation of 10998 vegetarians and non-vegetarians in the United Kingdom. Br J Cancer. 2004;90:118–21. doi: 10.1038/sj.bjc.6601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;1:CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Q, Shrubsole MJ, Ness RM, et al. The relation of magnesium and calcium intake and genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr. 2007;86:743–51. doi: 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lüchtenborg M, Weijenberg MP, de Goeij AF, et al. Meat and fish consumption, APC gene mutations and hMLH1 expression in colon and rectal cancer: a prospective cohort study (The Netherlands) Cancer Causes Control. 2005;16:1041–54. doi: 10.1007/s10552-005-0239-0. [DOI] [PubMed] [Google Scholar]
- 37.Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906–16. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–82. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 39.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat in relation to cancer risk. PloS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franceschi S, Favero A, La Vecchia C, et al. Food groups and risk of colorectal cancer in Italy. Int J Cancer. 1997;72:56–61. doi: 10.1002/(sici)1097-0215(19970703)72:1<56::aid-ijc8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Hsing AW, McLaughlin JK, Chow WH, et al. Risk factors for colorectal cancer in a prospective study among U.S. white men. Int J Cancer. 1998;77:549–53. doi: 10.1002/(sici)1097-0215(19980812)77:4<549::aid-ijc13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Sellers TA, Bazyk AE, Bostick RM, et al. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa United States) Cancer Causes Control. 1998;9:357–67. doi: 10.1023/a:1008886715597. [DOI] [PubMed] [Google Scholar]
- 43.Levi F, Pasche C, LaVecchia C, Lucchini F, Franceschi S. Food groups and colorectal cancer risk. Br J Cancer. 1999;79:1283–7. doi: 10.1038/sj.bjc.6690206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavani A, Vecchia CL, Gallus S, et al. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425–8. doi: 10.1002/(sici)1097-0215(20000501)86:3<425::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Diergaarde B, Braam H, van Muijen GNP, Ligtenberg MJ, Kok FJ, Kampman E. Dietary factors and microsatellite instability in sporadic colon carcinomas. Cancer Epidemiol Biomarkers Prev. 2003;12:1130–6. [PubMed] [Google Scholar]
- 46.Kabat GC, Miller AB, Jain M, Rohan TE. A cohort study of dietary iron and heme intake and risk of colorectal cancer in women. Br J Cancer. 2007;97:118–22. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]