Abstract
After a diagnosis of HIV infection is made, the patient needs to be monitored using both clinical assessment and laboratory markers. HIV/AIDS monitoring is essential in guiding when to recommend initiation of therapy. Clinical monitoring will include staging of the HIV/AIDS disease using either the presence or absence of HIV-related signs and symptoms using the WHO staging system. Various laboratory methods can be used to monitor the disease progression and to guide whether the patient will need antiretroviral therapy or not. Laboratory monitoring for patients who are not on drugs is done to provide information about the stage of illness; to enable the clinician to make decisions on treatment and to give information on prognosis of the patient. Patients on drugs are monitored to assess their response to treatment with antiretroviral drugs and to detect any possible toxicity and improvement associated with the antiretroviral drugs.
Keywords: ART, β-2 microglobin, CD4+/ CD8+ cell count, HIV, neoptrin, viral load
Introduction
The AIDS epidemic continues to spread in the South-East Asia (SEA) Region. The South-East Asia is the second most affected WHO region in the world, after sub-Saharan Africa. To date, close to 40 million people throughout the world have been infected with human immunodeficiency virus (HIV). Of these, almost 6.4 million are in the SEA Region. Over 99 per cent of cases have been reported from four countries - Thailand, India, Indonesia and Myanmar1,2. The estimated adult HIV prevalence was 0.32 per cent in 2008 and 0.31 per cent in 2009. The states with high HIV prevalence rates include Manipur (1.40%), Andhra Pradesh (0.90%), Mizoram (0.81%), Nagaland (0.78%), Karnataka (0.63%) and Maharashtra (0.55%)3. As per the estimates, 40,000 cases in the country are eligible for ART. As part of the “3 by 5” initiative, India has started the ART roll-out in April 2004. Currently, 1,500 cases of AIDS are on ART, with drugs being available for a total of 8,000 patients. By January 2010 about 3,00,743 cases would be under treatment in Andhra Pradesh, Karnataka, Maharashtra, Tamil Nadu, Gujarat and Uttar Pradesh. This is being provided through 8 designated centres spread across higher prevalence areas. It is planned to increase the number to 254.
The general consensus among those fighting AIDS worldwide is that HIV testing should be carried out voluntarily, with the consent of the individual concerned. This view has been supported by the Indian government and NACO, who have helped to establish hundreds of integrated counselling and testing centres (ICTCs) in India. By the end of 2009 there were 5135 ICTCs in India, compared to just 62 in 1997. By 2009 these centres tested had tested 13.4 million people for HIV, an increase from 4 million in 20065.
The Global Fund for AIDS, Tuberculosis and Malaria has also identified ART as one of the priority areas. With efforts being initiated to provide ART, close monitoring of patients on these drugs is also essential to ascertain response. For various issues pertaining to prevention and control of HIV/AIDS, laboratory support is the most essential with reliable laboratory support at clinical and public health areas. The WHO has developed guidelines to ensure optimal utilization of laboratory support in providing quality care and reliable diagnostic support to various interventions against HIV/AIDS. Monitoring includes clinical, immunological and microbiological skills and infrastructure of the laboratory network6.
Laboratory monitoring cost
A critical component in determining the value of laboratory tests is their cost, including the cost of the test kits; test administration; specimen transport; purchase or rental of laboratory equipment; laboratory reagents; personnel time, training, and retention; specimen processing; laboratory information systems; and ongoing quality assurance. In most resource-limited settings, the cost of CD4 cell count at 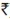 1,200 per test7 and an HIV RNA assay by polymerase chain reaction (PCR) costs approximately
1,200 per test7 and an HIV RNA assay by polymerase chain reaction (PCR) costs approximately 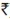 1,500-4,500 per test8. However, test cost alone does not convey a complete picture of the costs and/or savings associated with the use of these assays9–11.
1,500-4,500 per test8. However, test cost alone does not convey a complete picture of the costs and/or savings associated with the use of these assays9–11.
Although the use of clinical monitoring alone to guide ART initiation or switching is often considered to be “free” of cost, this assumption ignores the costs associated with the increased likelihood of developing an opportunistic infection, which confers substantial morbidity and mortality, prompting the use of costly health care services. A more comprehensive assessment of the value of laboratory tests takes into account both economic and health outcomes and incorporates test costs and costs of care required or avoided by their use12,13.
Ratio of cost-effectiveness in resource-limited settings
To assert that an intervention is cost-effective does not mean that it is cheap or that it saves money. Most interventions that improve health and extend survival add costs to care. By standard definition, a strategy of care may be considered “cost- effective” if its additional clinical benefit, relative to another strategy, is felt to be worth its additional cost14. Cost-effectiveness analysis is a formal methodology that includes both costs (current and future) and effectiveness (short- and long-term), either per person or as a total amount for a defined population. Costs are measured in a specific currency, and effectiveness is most often quantified in either years of life saved (YLS) or quality-adjusted life-years saved (QALY). The latter outcome assigns quality-of-life “weights” to health conditions and values each year lived in imperfect health as worth less than one year in perfect health14.
In comparing interventions, any strategy that costs more but produces fewer YLS or QALYs than a competing strategy is said to be “strongly dominated,” or an economically irrational choice, and is removed from consideration14. Incremental cost-effectiveness ratios are then calculated for all remaining strategies, comparing each to the next less expensive strategy. Strategies that represent economically inefficient uses of resources (i.e., have higher cost-effectiveness ratios than less expensive but less effective interventions) are described as “weakly dominated” and are also eliminated from consideration15.
The cost-effectiveness on CD4 cell count and HIV RNA level monitoring
Although the discussion above describes the results from each of the individual studies, it is important to recognize that the incremental cost-effectiveness ratios in each of the above studies depend not only on the costs of the laboratory monitoring interventions but also on the costs of ART and clinical care. In addition, such costs vary depending not only on the year and the country in which the analysis takes place but also on how the cost is estimated. The costs associated with the CD4 cell count and HIV RNA, per test, in each of the above studies; the costs range from $5 to $31 per CD4 cell count test and from $26 to $92 per HIV RNA test. Annual costs for ART regimens vary even more widely ($130–$429 for first-line regimens and $640–$1,432 for second-line regimens)14,15.
The WHO and other health-governing agencies rely on cost-effectiveness analyses among their guiding principles. Current published studies on the cost-effectiveness of CD4 cell count and HIV RNA laboratory monitoring differ in design, setting, test cost, and specific strategies compared. Although it may be desirable for cost-effectiveness analyses to be individualized to specific settings, relying on this approach may not be practical; some results may be generalizable across countries16. Future cost-effectiveness analyses would be more comparable and generalizable if these clearly state the time horizon and year of currency, include critical components of test costs (personnel training, laboratory infrastructure, specimen transportation, and quality assurance programmes), and analyze strategies that are most reflective of current in-country clinical practice. Many, although not all studies, suggest that CD4 cell count monitoring is cost-effective and maybe cost-saving in at least some resource-limited settings. The cost-effectiveness of HIV RNA level monitoring, however, ranges widely. The lowest published values suggest that biannual HIV RNA monitoring may be considered cost-effective17,18.
HIV laboratory monitoring guidelines
While several pilot programmes and studies show that successful anti retroviral (ARV) therapy is feasible in resource-limited countries (RLC), insufficient political, physical, and human resources limit the capacity of most RLC to offer sustainable HIV health care and ARV access. The costs of HIV monitoring laboratory tests significantly restrict their access, which may jeopardize the quality of care. Acknowledging the value these tests bring to patient care, assessing alternative methods, and developing resource-appropriate ARV monitoring tests have rapidly become a high priority19.
The US National Institutes of Health and the WHO, the ARV guidelines include a section on ARV monitoring tests, which are categorized and priority ranked on a resource availability continuum. The ‘absolute minimum’ tests: HIV antibody and haemoglobin/haematocrit are built upon as resources allow. ‘Basic recommended’ tests for pregnancy, co-infections, glucose, and organ and immune function make up the next tier. CD4 cell counts, serum lipids, bilirubin, and amylase are considered ‘desirable’ but not essential. ‘Optional’ tests: viral load and resistance assays are resource prohibitive in most areas20.
HIV laboratory monitoring tests
A wide variety of tests are available to monitor HIV disease progression and the state of overall health. HIV viral load tests provide a picture of viral activity, while CD4 cell counts shed light on the status of the immune system and can help physicians to predict and prevent the development of opportunistic infections. These tests can help guide treatment decisions and indicate whether treatment is working. Viral load tests and CD4 cell counts offer a more accurate representation of HIV activity and disease progression than older, indirect surrogate markers like β-2 microglobulin and neopterin21,22.
In addition, general tests such as the complete blood count, the blood chemistry panel, and blood sugar and lipid tests can help keep track of side effects such as low blood cell counts, liver toxicity, and elevated triglyceride and cholesterol levels. Other tests, such as genotypic and phenotypic resistance assays and therapeutic drug monitoring, can help optimize anti-HIV therapy23.
Almost all the laboratory tests require a blood sample; fortunately, a single sample often can be used for several assays. In general, most monitoring tests should be performed every 3-6 months or so. Results obtained by different laboratories and different test methods can vary greatly, and results may even vary from day to day at the same laboratory tests should preferably be done at the same laboratory using the same procedure each time, to allow for more accurate comparisons.
Many factors such as time of day, recent vaccinations, and concurrent infections may influence test results. A test with an unexplained or unexpected result should be repeated. A single abnormal laboratory result is not always cause for concern; upward and downward trends over time are usually more important.
Complete blood count
The complete blood count (CBC) is important because people with HIV may have low blood cell counts (cytopenias) due to chronic HIV infection or as a side effect of medications, particularly drugs that damage the bone marrow, where all blood cells are produced. Blood cell counts are typically reported as the number of cells per μl of blood (cells/μl) or as a percentage of all blood cells. People with HIV infection should receive a CBC about every six months, and more often if they are experiencing symptoms or taking drugs associated with low blood cell counts24,25.
Red and white blood cells: Anemia is common in HIV positives. HIV itself and various OIs such as Mycobacterium avium complex (MAC) can affect red blood cells and their oxygen-carrying capacity26,27. People with HIV infection should be especially concerned with neutrophil and lymphocyte levels, in particular CD4 and CD8 cell counts. Neutrophils normally make up about 50-70 per cent of all white blood cells. Various anti-HIV drugs, OI medications [including ganciclovir (Cytovene), used to treat cytomegalovirus, or CMV], and cancer chemotherapies that suppress the bone marrow may lead to low neutrophil levels (neutropenia)28.
Platelets: Platelets (thrombocytes) are necessary for blood clotting. A normal platelet count is about 130,000-440,000 cells/μl. Low platelet counts (thrombocytopenia) - which can lead to easy bruising and excessive bleeding may be caused by certain drugs, autoimmune reactions, accelerated destruction by the spleen, or HIV disease itself29.
CD4 and CD8 Cell tests
CD4 cell count: HIV primarily targets CD4 cells. As HIV disease progresses, CD4 cell counts decline, typically by about 30-100 cells/μl per year (depending on viral load), leaving a person increasingly vulnerable to infections and cancers. People with CD4 cell counts above 500 cells/μl generally have relatively normal immune function and are at low risk for OIs30,31.
Current U.S. HIV treatment guidelines32 recommend that people should consider starting anti-HIV therapy when their CD4 cell count falls below 350 cells/μl. In 2001, this level was reduced from 500 cells/μl after research showed little benefit of starting treatment in asymptomatic people with 350-500 cells/μl especially given the adverse effects, inconvenience, and cost of therapy. Studies are underway of assess the value of treatment in people with 200-350 cells/μl. Many people who have started combination anti-HIV therapy have seen dramatic increases in their CD4 cell counts and have been able to safely stop OI prophylaxis (preventive treatment). Most experts recommend that the CD4 cell count should be measured when HIV infection is diagnosed, then every 3-6 months or closer to three months if the count is low or falling or a person is starting or changing treatment and closer to six months if the count is high or has been stable for several months. An individual CD4 cell measurement is not as informative as downward or upward trends over time; any large or unexpected change should be confirmed with a repeat test.
Along with viral load, it provides information about when anti-HIV therapy is indicated and how well it is working; effective treatment can halt HIV replication and restore CD4 cell levels. Assays with specificity and cost-effectiveness are needed for the measurement of HIV-1 burden to monitor disease progression or response to anti-retroviral therapy. We have developed an affordable one step real-time RT-PCR assay with high specificity and sensitivity to measure plasma HIV-1 loads in HIV-1 subtype C infected patients33. An inverse correlation was found between plasma viral loads (PVL) and CD4+ T-cell numbers at all CDC stages. Significant correlations were found between CD8+ T-cell activation and PVL, as well as with the clinical and immunological status of the patients33.
Low CD4+ T-cell counts: A variety of causes and their implications to HIV and AIDS: Low CD4+ T-cell counts (CD4 counts) are associated with a variety of conditions, including many viral infections, bacterial infections, parasitic infections, sepsis, tuberculosis, coccidioidomycosis, burns, trauma, intravenous injections of foreign proteins, malnutrition, over-exercising, pregnancy, normal daily variation, psychological stress, and social isolation34. The low CD4 counts caused by some of these conditions often fall below 200 cells/μl which is the level needed to diagnose AIDS in someone who was previously HIV-positive. In addition to the diagnosis of AIDS, CD4 counts are regularly used to make treatment decisions, such as when to start antiretroviral medications and when to begin preventative antibiotics32,33,35.
Because many of the conditions that cause low CD4 counts are common in HIV positive, caution is advised on the use of CD4 counts to make treatment and diagnostic decisions. Some like psychological stress are greatly increased when people are told that their CD4 counts are low, which may compound the problem and cause the CD4 count to fall even further. It appears that low CD4 counts are a common reaction to many kinds of physical and psychological stressors. When several of these factors are combined, as is often the case in HIV and AIDS, extremely low CD4 counts may be a natural result36,37.
Factors influencing CD4+ and CD8+ T-cells: We have previously determined the lymphocyte subset reference range in HIV seronegative north Indian adults. The reference ranges for CD4 count [304 CD4% (17.5-50.6% with the median of 35%], CD8% (14-53% with the median of 32.3%), CD3% (43-89% with the median of 70.5%), and CD4/CD8 ratio (0.04-3.5 with the median of 1.04) were calculated. Significant variations were observed for normal reference intervals for T lymphocyte subsets according to the race, ethnic origin, age group, and gender. Our findings on T-lymphocyte subsets reference ranges of normal healthy north Indians validate the utility of determination of CD4 cell count as a useful predictor of AIDS in Indian conditions38. Another study indicated that HIV infected Indian patients, who require effective initiation of ART on the basis of their clinical category and percentage of CD4, are deprived of it in lieu of adopting a staging criterion for HIV infection based on their clinical category and absolute CD4 counts39.
(i) Influence of sex: Two studies on Asian adults40,41, one from Britain42, and one from the United States43 have reported that males have higher CD8 percentages than females and that, conversely, females have higher CD4 percentages and counts than males. Females have significantly higher CD4 counts, percentage of CD4 cells, and CD4:CD8 ratio, whereas males had significantly higher percentages of CD8 cells.
(ii) Influence of age: In a study of age-dependent changes with respect to the expression of the major lymphocyte surface receptors in healthy elderly subjects, T lymphocytes from elderly individuals were found to express lower levels of CD3 when compared with levels in young subjects44. Reduced T cells and lower CD4:CD8 ratios have been described in the frail elderly45.
(iii) Effects of smoking, alcohol, and time since last meal: In a study on U.S. air force personnel, alcohol consumption showed a significant correlation with suppressed counts and functions for nearly all variables, whereas tobacco use was associated with stimulation of T-cell number and function46. The absolute CD4 counts were reported to be significantly higher in smokers than in non smokers in two other studies47. Smoking increased the number of T cells and mainly CD4+ peripheral blood lymphocytes48. However, researchers found no differences related to smoking or alcohol but noted that all but one of the smokers and alcohol consumers in the study were males. Also, there were no differences in the study49 with regard to the time interval between taking the last meal and drawing of the blood sample.
Methods for enumerating absolute numbers of CD4+ T-cells: To date, both automated and manual methods have been developed for determining the absolute number of CD4+ T-cells and evaluated in multicentric studies. These include both flow cytometric and non-flow cytometric technologies. Most of the technologies summarized here are commercially available as cell counting systems and/or kit packages.
Flow cytometric (automated) methodologies: Flow cytometry is the gold standard method for the estimation of CD4 counts due to its accuracy, precision and reproducibility and thus widely used. This technology is capable of high sample throughput and great versatility in its applications; CD4 counting is just one of its numerous uses in the biomedical field. However, flow cytometry based CD4 counting is relatively complex, and therefore, technically demanding, costly and needs regular maintenance. Additionally, it is essential that operators of the flow cytometer be sufficiently trained in the technical and biological aspects of CD4 counting. Although, the system has a high initial and running cost at present. Flow cytometry provides options like dual- and single-platform approach for estimation of absolute CD4+ T-cell counts.
(i) Dual-platform approach: The dual-platform flow cytometry requires a haematological analyser and a flow cytometer. The percentage of CD4 lymphocyte population (obtained from the flow cytometer) is multiplied by the total lymphocyte count obtained from the haematology analyser to provide an absolute CD4 count50. Traditionally, in a blood sample with a high proportion of lymphocytes, a lymphocyte gate for CD4+ T-cell testing is easily derived from a bivariate histogram or homogeneous gate that includes forward scatter (FSC, size of the cell populations) and right angle side scatter (SSC, granularity of the cell populations) patterns. However, when there is a high proportion of non- lymphocytes (monocytes, basophils and immature red blood cells), this traditional FSC/SSC lymphocyte gate tends to be unreliable as non-lymphocytes have been shown to contaminate the gate, thus this morphological gating is now considered as unacceptable51,52. A more reliable method for assessing lymphocyte gate purity and lymphoid cell recovery on the basis of differential CD45 marker density expression has been developed. This method also known as CD45 gating and uses two markers (CD45 and CD14)53. Based on this CD45 gating strategy together with the advent of the flow cytometry, the more practical and accurate three-color (CD3/CD4/CD45) or four-color (CD3/CD4/CD8/CD45) immunophenotyping assays of CD4+ T-cells have become available54–56.
The dual platform approach requires the results of three separate laboratory tests: percentage CD4+ T-cells from the flow cytometer, total white blood counts and percentage of lymphocytes from the haematological analyzer. This undoubtedly leads to wide variability. Moreover, the patient samples contain cell numbers beyond the sensitivity and linearity range of haematological analyzer (e.g., severely leucopenic patients), the lymphocyte population defined by flow cytometer may not match exactly to that of the haematological analyzer. Further, total white blood cell counts are not subjected to regular internal quality control and external quality assurance. In spite of that, this dual- platform technology is still widely practiced and recommended in a number of institutional guidelines57–59.
(ii) Single-platform approach: The single-platform systems are dedicated flow cytometers designed to provide absolute CD4 counts without the need for a haematology analyser. Single platform systems may be volumetric flow cytometers, which count absolute number of cells in a given volume, or bead based systems which require the addition of a known concentration of fluorochrome labelled reference beads enabling the absolute number of CD4 cells to be calculated based on the ratio of beads to cells. Importantly, the precision of this technology depends on the flow cytometric measurement and the methodology of pipetting in the staining process. The use of reverse pipetting technique is the most reliable dispensing method for absolute CD4+ T-cell count assay60,61. To date, single-platform technologies have two options of microbead-based technologies and the volumetric technologies.
(a) FACScan or FACSCount microbead-based system: FACSCount system is the only available microbead-based single-platform instrument that is designed specifically for enumerating the absolute CD4+, CD8+ and CD3+ T-cell counts in no-lyse, no-wash whole blood. The system has been approved by many international organizations as one of the predicate method. This system requires ready-to- use twin-tube reagent tubes. One tube determines the absolute number of helper/inducer T-cells (CD4+ /CD3+) by using a combination of two-colour monoclonal anti- human CD3 antibody conjugated to the tandem dye phycoerythrin Cy5 (PECy5) and a monoclonal anti-human CD4 antibody conjugated to phycoerythrin (PE). The other tube determines the absolute number of cytotoxic/suppressor T-cells (CD8-+ /CD3+). Both tubes also give the absolute number of total T-cells (CD3) or CD3+ T-cells, as well as CD4/CD8 ratio. In addition to the antibody reagents, the reagent tubes also contain a known number of fluorochrome-labelled reference beads. These beads function as fluorescence standard for locating the lymphocytes and also as a quantitation standard for enumerating the cells62.
(b) Guava Technologies (EasyCD4 CD8) volumetric system: Guava Technologies (EasyCD4 CD8) volumetric system is based on a micorcapillary cytometry technology that enables the enormous analytical and diagnostic power of conventional flow cytometry in a highly miniaturized single platform. The system contains two-colour, direct immunofluroscence reagents for enumeration of mature CD4+ T-cells in human blood. The kit consists of two monoclonal antibodies directly conjugated to PECy5 and PE for CD3 and CD4 T-cell antigens. The CD3+ cells are gated and the CD3+ and CD4+ cells are identified in the gate. The system showed good correlation with conventional flow cytometry and is easy to operate. It uses smaller blood volume. However, there is a need for experienced technical personnel for operating the system63.
(c) Partec CyFlow and Cyflow SL-3 volumetric system: CyFlow, a volumetric software, is another desktop single-platform technology, controlled absolute count system equipped with either a single 532nm green solid-state laser used for one fluorescence parameter or two lasers with a mercury arc lamp applicable for 2 or 3-colour analyses. Data acquisition and analysis are performed in real time with FlowMax software. The system showed good correlation with the CD4 counts obtained by conventional flow cytometry64.
Non-flow cytometric methods: Although flow cytometry is the accepted standard method for the determination of absolute counts of CD4+ and CD8+ T-cells, the methodology involved the use of flow cytometer and haematology analyzer, which is expensive both in term of initial investment and maintenance as well as requiring highly, trained personnel. Thus it is unsuitable for routine use in most laboratories with limited facilities such as district hospitals. An ideal CD4 testing in a resource-poor setting would be an assay which is simple, uses inexpensive instrument and minimum training period that would reliably identify CD4+ T-cells without sacrificing much accuracy and precision65,66. There are several alternative non-flow cytometric technologies available for determination of absolute CD4+ and CD8+ T-cells. These technologies are cost-effective and thus might be suitable in the local situation in the resource-poor settings. Currently available manual assays rely on microscopes (optical or fluorescence). Additionally, these require the use of other small equipment such as centrifuges and magnets. These tests are designed to operate with low sample throughput in resource-limited laboratories but their running costs are not always lower when compared to the automated methods67.
(i) Coulter manual CD4 count assay: This manual CD4 count kit contains CD4+ cytospheres reagent, inert latex spheres coated with monoclonal antibody, used to identify and manually enumerate the absolute number of CD4+ T-cells by visible light microscopy in fresh whole blood. The coulter assay uses small latex anti-CD14 beads (0.6 μm diameter) to bind monocytes thus blocking subsequent binding of larger anti-CD4 coated beads (2.0 μm diameter) to these cells. The red blood cells are lysed and white blood cells ± 3 large beads attached (CD4+ T-lymphocytes) are counted in a chamber using light microscopy68. The Cytosphere system has advantage for those haematology technicians who are familiar with the shapes of cells. Here the monocytes are not removed but appear different under the microscope, so the bead-covered CD4+ T cells can be counted. The Cytosphere system has been found to be a useful alternative to flow cytometry for the estimation of CD4 T-lymphocyte counts, in resource-poor settings like Indian laboratories, for monitoring HIV progression and response to therapy69.
(ii) Dynal T4-T8 quantitative assay: This non-flow cytometric technology is based on the use of anti CD14- labelled magnetic beads and magnet to deplete the blood of CD14 expressing monocytes followed by anti CD4 T-lymphocytes. The nuclei of the CD4 positive T-cells are then stained and counted in a haemocytometer under a microscope light or fluorescence microscopy. The method has been validated in resource-poor settings in Africa. The method showed significant correlation with that obtained by flow cytometry and found to be cost effective with US$ 3/test70. However, these methods are manual and labour intensive. The automation by addition of image analysis software would increase the potential of its use in ART monitoring. The system could be cheaper than other alternatives and will be useful in small settings if it is backed up with flow cytometry for quality assurance71.
(iii) ELISA based counting systems (Capcellia ELISA and TRAx and Zymmune): The ELISA systems measure CD4 protein in the lysed whole blood. The system could be used in 96 well formats, thus could be automated easily and can be used on a large number of samples. The Capcellia immunocapture assay has been used to estimate CD4 counts in HIV seropositives and compared with the CD4 counts obtained using flow cytometry and the plasma viral load. Capcellia for CD4+ and CD8+ T- cell counts was found to be a cost-effective, user- friendly assay, which provides counts that correlate well with HIV-1 load measurements71. Another study done by the same group showed that the test can be a useful alternative for flow-based method, however, the assay system has certain limitations inherent to ELISA techniques. Hence, these systems have not found favour with the investigators.
CD4 count estimation from dried blood spots uses an antibody ‘sandwich’ to capture and detect CD4 proteins in the sample. One study in Zambia has shown promise in the methodology showing good correlation with the conventional flow cytometry72,73. Detailed descriptions of the above explained commercial assays are provided in Table I.
Table I.
Summary of CD4+ T- Lymphocyte enumeration assays
Quality assurance in CD4 testing: Since the use of CD4+ T-cell measurement has critical implications for the effective management of individuals at risk or infected with HIV or progressing into AIDS, it is crucial that external quality control is put in place to ensure that the test results from individual laboratories are equivalent. The aim of quality assurance is to ensure that doctors and patients are getting the same information from the laboratory tests, no matter which laboratory they use or the methods used there. For a good quality control, the diurnal variation in the CD4 counts should be considered as an important factor. To avoid such variations it is recommended that the sample should be collected at a specified time from an individual. Another point that has to be taken into account is the storage of sample before assessment. This period varies with the methodology used and location of the testing laboratory. Several sets of guidelines addressing quality control of flow cytometric CD4+ testing have been developed.
Though there is at present no external quality control on non-flow cytometric CD4 testing, it is important that accurate daily within laboratory (internal quality control) and proficiency test or external quality assurance programmes (EQA) be employed to ensure the reliability of CD4 data. Satisfactory performance in CD4 testing EQA is recommended for HIV research and clinical trial programmes in many parts of the world. For the most parts, internal control samples from the manufacturer are used to monitor both sample processing and instrument performance, however, internal quality control practice is not without merit, it does not provide full-process quality control. There are very few laboratories, particularly in the developing world participating in international EQA programmes such as United Kingdom National External Quality Assessment Schemes or UKNEQAS73. Proper transportation of specimens and reagents is necessary for quality assessment management and availability of good quality reagent at remote places. A technology that is being developed and refined at present is a fixative that allows blood samples to be stored and transported over 5 days (or even slightly longer) without loss of accuracy in test results. The high cost of these programmes has been a major burden, making their implementation in resource-poor countries difficult. However, there are free EQA programmes such as Quality Assessment and Standardization for Immunological Measures Relevant to HIV/AIDS (QASI)74–76, in spite of their irregular schedules; these are useful and cost saving.
CD8 cell count: Two types of T cells carry the CD8 surface molecule: T-suppressor cells, which inhibit immune responses, and killer T cells (also known as cytotoxic T lymphocytes, or CTLs), which target and kill infected or cancerous cells. As with CD4 cells, a variety of factors can cause CD8 cell counts to fluctuate. CD8 cell counts typically rise over time in people with HIV, but (unlike CD4 cells) CD8 cell numbers do not independently predict disease progression, and their relation to immune status is not well understood.
CD4 and CD8 Cell percentage: Because absolute CD4 and CD8 cell counts are so variable, some physicians prefer to look at CD4 or CD8 cell percentages, the proportion of all lymphocytes that are CD4 or CD8 cells. Percentages are usually more stable over time than absolute counts. A normal CD4 cell percentage in a healthy person is about 30-60 per cent, while a normal CD8 cell percentage is 15-40 per cent77.
CD4/CD8 cell ratio: The CD4/CD8 cell ratio will also be reported. This is calculated by dividing the CD4 cell count by the CD8 cell count. A normal CD4/CD8 cell ratio is about 0.9-3.0 or higher, there are at least 1-3 CD4 cells for every CD8 cell. In people with HIV this ratio may be much lower, with many more CD8 cells than CD4 cells78.
HIV viral load tests
Viral load tests measure the amount of HIV RNA in the blood. The presence of RNA indicates that the virus is actively replicating (multiplying). Along with the CD4 cell count, viral load is one of the most valuable measures for predicting HIV disease progression and gauging when anti-HIV treatment is indicated and how well it is working. Viral load is expressed either as copies of RNA per milliliter of blood (copies/ml) or in terms of logs. A log change is an exponential or 10-fold change. For example, a change from 100 to 1,000 is a 1 log (10-fold) increase, while a change from 1,000,000 to 10,000 is a 2 log (100-fold) decrease. If the level of HIV is too low to be measured, viral load is said to be undetectable, or below the limit of quantification. However, undetectable viral load does not mean that HIV has been eradicated; people with undetectable viral load maintain a very low level of virus. Even when HIV is not detectable in the blood, it may be detectable in the semen, female genital secretions, cerebrospinal fluid, tissues, and lymph nodes79–81.
Primary HIV infection (PHI) can include a self-limited period of flu-like symptoms occurring during the first weeks of HIV-1 infection. It is associated with peak levels of HIV-1 RNA viremia, which subsequently declines until reaching a set point, where levels remain for months to years. Initial studies suggested that those with more symptomatic acute infection and longer duration of illness have faster rates of progression to AIDS82. The presence of symptoms has conversely been correlated with faster clearance of the virus, however, suggesting that symptoms may indicate a beneficial immunologic response. In addition, data from a cohort of acutely infected African women showed association of more symptomatic disease with higher pre-seroconversion viral loads and increase in mortality independent of viral load set point82.
A viral load of 100,000 copies/ml or greater is considered high, while levels below 10,000 copies/ml are considered low. Research has consistently shown that higher viral loads are associated with more rapid HIV disease progression and an increased risk of death. Current U.S. HIV treatment guidelines82 recommend that people should consider starting treatment if their viral load is above 55,000 copies/ml (revised upward from 10,000 copies/ml in the previous guidelines). Importantly, most studies that have correlated viral load and HIV disease progression have been done in men; more recent research indicates that women may progress to AIDS at lower viral load levels, suggesting that the treatment threshold should perhaps be revised downward for women83.
Effective anti-HIV treatment can often reduce viral load to low or undetectable levels. Therapy that does not produce an undetectable viral load is often said to be failing. Another warning sign is viral breakthrough, an increase in viral load following earlier suppression. Inability to achieve an undetectable viral load may mean that a person's HIV is resistant to the drugs being used. Providers often take this as an indication to change or add new drugs to a person's anti-HIV regimen. However, research suggests that therapy that substantially decreases HIV levels (especially if it reduces them to below 10,000 copies/ml) is likely to be beneficial even if it does not achieve the “gold standard” of undetectability79,80.
Combination therapy with antiretroviral drugs suppresses virus replication, delays disease progression, and reduces mortality. In industrialized settings, plasma viral load assays are used in combination with CD4 cell counts to determine when to initiate therapy and when a regimen is failing. In addition, unlike serologic assays, these assays may be used to diagnose perinatal or acute HIV-1 infection. Unfortunately, the full benefits of antiretroviral drugs and monitoring tests have not yet reached the majority of HIV-1-infected patients who live in countries with limited resources84,85.
Viral load measurement in high-income countries: In high-income countries, determination of the CD4 cell count and viral load is used to determine whether antiretroviral treatment is indicated, and viral load data are used to find out whether antiretroviral treatment is successful83,84. When viral replication is suppressed to low levels, resistance mutations cannot emerge, and a durable treatment response ensues. Viral replication in the presence of antiretroviral treatment favours selection of resistance mutations and treatment failure. Viral load assays and drug resistance tests are used routinely in high-income settings to guide most treatment decisions. Viral load measurements are optimally used to guide treatment when two conditions are met. First, effective plasma drug levels must be assured. It would be erroneous to conclude that treatment has failed if the patient's adherence is poor or if the regimen has not been taken as prescribed. Second, alternative drugs must be available. In the absence of alternatives to a failing regimen, the use of resources for viral load testing is ill advised. In high-income countries, 120 antiretrovirals are now available, providing options for second-, third-, and even fourth-line (“salvage”) regimens, and new molecules, such as third-generation protease inhibitors (PIs), integrase inhibitors (IIs), and CCR5 inhibitors, will further expand the possibilities for salvage therapy. However, only a few second-line drugs are available in resource-limited settings. Although there is a consensus on the triggers for initiation of treatment (CD4cell count, 200-350 cells/ml) and on the viral load targets during treatment (the viral load should be “undetectable,” meaning that the HIV RNA level is 150 to 400 copies/ml, depending on the assay's sensitivity), opinions diverge on the critical issue of the appropriate response to low-level viremia in patients who are receiving treatment86,87. In high-income settings, some clinicians interpret any sustained, detectable viremia (viral load, 150 copies/ml) as treatment failure that necessitates a change in the regimen, because some resistance mutations are likely to emerge even with low-level replication. Others take a more flexible approach, weighing clinical and immunologic measures of treatment success, especially in heavily treatment-experienced patients whose options for active antiretrovirals are limited. Also, for any given viral load, the decrease in the CD4 cell count is slower in patients who are infected with drug-resistant HIV than in those who are infected with wild- type HIV88. In some triple-class-experienced patients, CD4 cell counts may remain stable for months or years, provided that the viral load does not exceed 10,000 copies/ml89–91.
Viral load assays currently used in developed countries: Currently there are three HIV-1 RNA assays licensed by the United States Food and Drug Administration: Roche Amplicor HIV-1 Monitor Test, version 1.5, bioMérieux NucliSens HIV-1 QT Assay, and Versant HIV-1 RNA 3.0 Assay (bDNA). Other viral load tests -- Organon Teknika's nucleic acid sequence-based assay (NASBA) and Digene's DNA hybridization test are newer and less widely used92–98. While first-generation tests measured viral load down to about 400 copies/ml, the ultrasensitive second-generation tests in widespread clinical use today have a lower limit of detection of about 50 copies/ml; some tests can measure as few as 5 copies/ml. The older-generation tests are better at measuring high viral loads, while the newer tests more accurately measure low viral loads. However, these kits are expensive (kit cost of $50-$100/test in the US), and rely on expensive, often dedicated equipment that can only be used for that assay. Although the manufacturers are decreasing kit costs and in some instances equipment costs for resource-limited countries, these assays are still technologically complex and require physical resources, such as uninterrupted electricity, air conditioning, and access to clean water, that may not be available in less-developed countries99. Newer assays making use of real-time polymerase chain reaction (Roche TaqMan, Abbott RealTime) or molecular beacon technology (Retina Rainbow, NucliSens EasyQ) are also available, but have not yet been approved by the Food and Drug Administration, and very few reports have been published describing their use.
Barriers to using current viral load assays in resource-limited settings: Human resources and infrastructure that might be readily available in major cities, such as trained personnel, clean water, and electricity, may not be found in rural areas. Reference laboratories that have the personnel, equipment, and infrastructure to perform CD4 cell counts and viral load testing may lack the resources to purchase the kits. Thus, what works for one country, or even for one city within a country, may not apply in all resource-limited settings. Options available to developing countries include performing the laboratory testing at the local site or transporting the specimens to the reference laboratories for testing. A well-defined infrastructure is needed to ensure proper specimen handling and efficient results reporting. While viral load kit prices have been decreasing for resource-poor countries, the cost of assay disposables and the establishment of infrastructure still limit the utility of HIV-1 RNA for monitoring ARV therapy in most resource-limited settings. Inexpensive, technologically simpler assays are still needed100–102.
Viral load assays
Ultrasensitive p24 antigen assay (PerkinElmer Life and analytical sciences): The ultrasensitive p24 (Ultra p24) antigen assay uses a standard ELISA format for the capture and detection of HIV-1 p24 antigens coupled with a specific amplification process to increase the assay sensitivity. Heat denaturation of the plasma prior to binding of p24 antigen in the ELISA step helps dissociate immune complexes and denature the antibodies so that these no longer compete for binding to the p24 antigen. The dynamic range of the assay can be increased by using a kinetic read-out with the Quanti-Kin Detection System Software103. Optimization of the standard p24 antigen assay includes an external reagent that improves the antigen detection sensitivity, perhaps by greater dissociation of the immune complexes104.
p24 antigen detection is a significant inverse correlate of CD4 cell changes in virally suppressed patients as well as in patients studied longitudinally who were either treatment naïve (85% of the population) or treated with dual nucleoside reverse transcriptase inhibitors (15%) or were on a structured treatment interruption study105. In one study researchers have found that the p24 antigen results are seemed to mirror of the RNA results. Use of the external buffer has improved the sensitivity of the assay 2- to 5-fold as well as the correlation with HIV-1 RNA106. Diagnosis of perinatal HIV infection is hindered by the presence of maternal immunoglobulins, which can persist in the infant for as long as 15-18 months. Thus, routine antibody assays cannot be used to diagnose infection until after maternal antibodies have waned107–111.
Cavidi ExaVir load reverse transcriptase assay: The reverse transcriptase (RT) enzyme is extracted from the virus particle in the Cavidi RT assay using a solid phase extraction manifold, and is quantified in a functional assay whereby RT synthesizes BrdU-DNA from a poly-A template bound to a 96-well plate112. Synthesized DNA is then quantified using anti-BrdU conjugated to alkaline phosphatase followed by the addition of its substrate. The RT activity in the unknown sample is compared to that of a recombinant RT enzyme standard with a known concentration. The extrapolated result is reported as fg RT/ml of plasma or as HIV-1 RNA equivalents/ml using a conversion factor supplied by the manufacturer.
The assay has undergone revisions to improve sensitivity113. Since the assay measures a virion-associated enzyme, results are usually more comparable to plasma RNA114. Additionally, since this is a functional assay for RT and does not rely on specific protein or nucleic acid sequences, it performs well when quantifying any HIV-1 subtype115–117.
Since this assay is newer than the Ultra p24 antigen assay, fewer data are available and most studies have been conducted either by or in collaboration with the manufacturer. Scientists have compared the Roche RNA assay with both Ultra p24 antigen (external buffer) and the earlier version (version 1.0) of the Cavidi RT assay and found excellent correlation between RNA and RT results.
Real-time PCR/molecular beacon assays: Real-time PCR such as TaqMan and Abbott RealTime, or molecular beacon assays such as Retina Rainbow or NucliSens EasyQ, might be useful for measuring viral load in resource-limited countries118,119. Real-time PCR detects amplicon production in real time with each PCR cycle, and thus does not rely on post-amplification detection of amplicons, which helps reduce the possibility of contamination and improves turn around time120,121. However, commercially available real-time PCR assays are just as expensive as the more standard nucleic acid viral load tests and also use expensive equipment. The use of in-house versions of these assays can help to reduce kit costs, but laboratories must provide their own reagents such as primers and probes, and optimize their methods. In settings with multiple HIV-1 subtypes, care must be taken in selecting reagents and amplification conditions, such as annealing temperatures. Quality assurance of each batch of such reagents remains problematic.
The Abbott RealTime HIV-1 assay: The Abbott RealTime HIV-1 assay is an in vitro RT-PCR assay for the quantitation of HIV-1 on the automated m2000 System in human plasma from HIV-1 infected individuals over the range of 40 to 10,000,000 copies/ml. The Abbott RealTime HIV-1 assay is intended for use in conjunction with clinical presentation and other laboratory markers for disease prognosis and for use as an aid in assessing viral response to antiretroviral treatment as measured by changes in plasma HIV-1 RNA levels. This assay is not intended to be used as a donor screening test for HIV-1 or as a diagnostic test to confirm the presence of HIV-1 infection.
The NucliSENS EasyQ HIV-1 assay: For the NucliSENS EasyQ HIV-1 assay, primers and probes have been designed based on a well-conserved region of the gag gene. The assay is based on the nucleic acid sequence-based amplification (NASBA) technology, which is a sensitive, isothermal, transcription-based amplification system specially designed for the detection of RNA targets and, interestingly, cleans up the interference effect of any DNA background. The method uses the simultaneous enzymatic activities of avian RT, ribonuclease H and bacteriophage T7 RNA polymerase. The generated amplicons are detected by molecular beacons (hairpin probes with a fluorescent dye and a quencher at the end). During the amplification process there is a constant growth in the concentration of amplicons to which the beacons can bind while generating fluorescent signals. The overall fluorescence curve contains kinetic information on both amplicon formation and beacon binding. Quantification can be made by considering the amplicon formation rate from the viral RNA, taking as reference, the fixed amount of calibrator RNA. The linear dynamic range of the lastest version of the assay, the NucliSENS EasyQ HIV-1 v2.0, runs from 10 (1 log) to 10,000,000 (7 log) HIV-RNA copies/ml when 1 ml of plasma is examined.
Although the NucliSens EasyQ assay has been evaluated in South Africa122 and China123, and the Abbott RealTime assay in Brazil124,125, in general these assays do not provide a simple, less expensive alternative to viral load monitoring in resource-limited settings. Low-level contamination and a relatively high frequency of invalid results requiring repeat testing were some of the problems cited with these studies.
The COBAS TaqMan HIV-1 assay: The COBAS TaqMan HIV-1 assay was the first RT-PCR detection method available. The targeted viral genome is a highly conserved region of the gag gene. Quantification of HIV-RNA is made using a second target sequence, the HIV-1 quantitation standard (QS), a known concentration of which is added to each test specimen. The QS amplicons have the same length and base composition as HIV-1 target amplicons. Detection of the QS binding region has been modified to discriminate from the target. The use of dual-labelled fluorescent probes allows a real-time detection of the accumulation of PCR products by monitoring the emission intensity of fluorescent reporter dyes released during the amplification process. The amplification of HIV-RNA and QS are measured independently at different wavelengths. This process is repeated for a designated number of cycles, each one effectively increasing the emission intensity of the individual reporter dyes, allowing a separate recognition of HIV-1 and QS. The exponential growth decay in the curve of the PCR amplification directly correlates with the baseline amount of genetic material. The test may quantify HIV-RNA over a dynamic range of 48-10,000,000 copies/ml.
TaqMan real-time PCR is being routinely used for infant diagnosis and patient monitoring126. The limit of quantitation for this assay was 300 copies/ml and RNA results were highly correlated with both the Versant and the Monitor HIV-1 RNA assay. The assay showed 100 per cent sensitivity and specificity when used in the early diagnosis of infants compared with the Versant assay127–129.
In-house real time PCR assay: The In-house real time PCR assays quantify HIV RNA and have a published dynamic ranging from 1-300 to 10,000,000 copies or IU/ml using 0.2-7 ml plasma. Viral RNA is extracted using kits or in-house methods. The RNA is amplified and detected using real time PCR technology.
Real time PCR assays provide faster detection and increased linear range to end point PCR reagents, equipment, technical complexity, and lack of quality control of these assays make these unsuitable for many laboratories in resource-limited countries. Similar to other nucleic acid based tests, laboratory infrastructure, staff expertise and contamination issues limit the utility of the real-time viral nucleic acid based tests in many regions. Detailed descriptions of the above explained commercial assays are provided in Table II.
Table II.
Summary of viral load assays
Steps to reduce the cost of viral load testing: First, each country must determine if the assay will quantify subtypes common in the region and is appropriate for the technical staff and laboratory equipment available. Infrastructure limitations impose significant barriers to implementation. Infrastructure includes both physical resources (water, reliable electricity, air conditioning, refrigeration, other equipment) and human resources (trained technologists). Training tools, trainers, guidelines, and consensus protocols, as well as monitoring and evaluation tools, including proficiency testing programmes, all need to be established. Costs must be taken into consideration - not only the costs of the kits, reagents, and supplies, but also the cost of equipment purchase, maintenance, kit procurement and the cost of labour. Once the assay is deemed acceptable, clinical validation studies in relevant populations are required to prove comparability to the gold standard with respect to sensitivity, specificity, precision, reproducibility, dynamic range, and linearity. It is imperative that the laboratory performing the gold standard assay is participating in national or international quality assurance programmes for the comparisons to be meaningful. Lastly, there must be acceptance and understanding by the clinicians who will use the new assay. This will require involvement and education of clinicians with results from the clinical validation studies. Communication at a high level between those implementing laboratory monitoring tests and the clinicians advising government on the use of ARV therapy has been lacking130.
HIV diversity vs viral load: HIV diversity is a critical point to be considered for the development of genomic amplification techniques, particularly for plasma viral load assays. On the one hand, from an epidemiological point of view, in the United States and Europe there is an increasing number of patients newly infected by non-B subtypes, especially by CRF02_AG strains131. In France, the proportion of non-B subtypes increased dramatically between 1995 and 2002 and has remained stable since 2003132. Indeed, almost 40 per cent of the newly diagnosed patients are infected by a non-B subtype. The increasing diversity of HIV-1 viruses in France, even in Caucasian patients diagnosed at the time of primary infection, was recently described in the French ANRS CO06 primo cohort study132. Moreover, in resource-limited countries, where 90 per cent of new infections occur, there is increasing access to ART for HIV-infected people133.
It is important to use appropriate viral load assays able to span this genetic diversity for the management of naïve and also ARV-treated patients. To verify this critical point, there is a need of comparative studies for the determination of the sensitivity of existing assays and their ability to amplify RNA, especially for non-B subtypes134.
This point is a major criterion to be considered for the development of assays able to amplify the different HIV-1 subtypes. Indeed, recent data have suggested viral load discrepancies between commercial quantitative assays, especially for the quantification of non-B sub-type strains. To achieve a high sensitivity threshold, a judicious choice of HIV-1 gene targets, primers, and probes is critical. Updated versions of the NucliSens EasyQ and Roche Cobas Ampliprep/Cobas TaqMan HIV-1 tests were recently introduced in order to improve the ability of their former versions to amplify non-B subtypes135,136.
A prospective study was conducted to compare 4 real-time commercial tests, namely the Abbott m2000 RealTime, bioMérieux NucliSens EasyQ, version 1.2 (v1.2), and Roche Cobas AmpliPrep-Cobas TaqMan v1.0 and v2.0 assays, for HIV-1 RNA quantification in blood plasma from a cohort of patients that tested positive for HIV-1 for the first time137. The study is focused on samples taken from a French cohort, including B- and CRF02_AG-infected patients. Globally, NucliSens EasyQ v1.2 seems to differ from CAP/CTM v1.0, CAP/CTM v2.0, and m2000 RealTime. However, results also underline the limitation of automated real-time protocols for non-B HIV-1 subtypes, despite a recent improvement of the sensitivity of these assays with these strains, with the possibility of wide discrepancies and the misestimating of the viral load. As a whole, these observations speak in favour of using the same assay for monitoring treatment of HIV-1-infected patients, for resistance studies, and for clinical trials, eventually, after testing for the most appropriate assay for non-B subtypes.
Surrogate markers for HIV monitoring
The majority of individuals with HIV-1 infection present with no signs or symptoms, or only lymphadenopathy. To initiate prophylactic measures in time it is necessary to establish risk criteria. CD4+ cell counts are significant predictors. Supplementary methods to improve the predictive information of CD4+ cell counts are still required. In addition, CD4+ cell counting is labourious, expensive, and restricted to specialized laboratories. Thus, there is also a place for more easily performed laboratory tests with similar predictive value as CD4+ cell counts. Neopterin and β2-microglobulin levels proved to be significant predictors of AIDS risk in HIV-1 seropositives136. The predictive value of both parameters is equal to CD4+ cell counts and both markers are significant joint predictors in addition to CD4+ cell counts. Measurement of the parameters is done in serum (neopterin and β2-microglobulin) or urine (neopterin) specimens which reduces the risk of HIV-1 transmission compared to handling of whole-blood samples which is required for cell counting. Although more studies are needed, especially in developing countries and in persons receiving zidovudine, it can be recommended to use neopterin and β2-microglobulin as additional markers to estimate AIDS risk in HIV-1 seropositive individuals138,139. Moreover, both markers may be useful for this purpose without CD4+ cell counts if cell counting is not available140,141.
β2-microglobin: β2-microglobin is an 11kD protein expressed on the surface of most nucleated cells. It forms a heterodimer with class I major histocompatibility complex molecules present on the surface of most nucleated cells and exhibit amino acid homology with the constant region of immunoglobins142–145. Free β2-microglobin can be measured in both serum and urine and levels of urine β2-microglobin correlate with the degree of progression of HIV disease. It spikes in acute infection, declines and then rises during the infection. Levels of β2-microglobin are elevated in a variety of conditions characterized by lymphocyte activation and/ or lymphocyte destruction; e.g. lymphoproliferative syndromes, autoimmune diseases, viral infection and in patients with renal diseases. It can be measured in serum or plasma by using radio immunoassay radio immunoassay (RIA) or competivive ELISA based tests. β2-microglobin measurement has several advantages as a laboratory assay to help determine prognosis. By contrast with CD4 cell count which require special procedures for specimen handling and processing, β2-microglobin can be measured with a serological assay and equipment available in many laboratories.
Neopterin: Neopterin (6-D- erythrohydroxy-propylpterin) is a low molecular weight compound derived from an intermediate product of the de novo biosynthesis of tetrahydrobiopterin from guanosine triphosphate (GTP)142–145. It is an early marker of HIV infection. The levels rise further on progression from pre AIDS to clinical AIDS. Since neopterin levels are stimulated by HIV infection, measurement of neopterin levels can be useful in monitoring progression and evaluating antiviral therapy.
Serum IgA levels: Generally elevated in HIV infected persons, serum IgA can be measured by simple immunodiffussion method using reference anti IgA146.
Soluble IL-2 receptor levels: Levels are generally elevated in AIDS and data suggest a good correlation with the disease stage as indicated by CD4+ T-cell level147. Assay can be done by EIA which uses standard anti IL-2 receptor capture antibody and a peroxidase coupled secondary anti soluble IL-2 receptor antibody.
Levels of soluble receptor for TNF-α and IFN-γ: Whether these levels have some value as a marker of disease activity has not been evaluated carefully and at present these do not play a major role in laboratory monitoring of patients with HIV-1 infection148.
Pap smears: Research has shown that women and men with HIV are at greater risk of developing genital and anal cancer than HIV negative people. Cervical, penile, and anal cancer are associated with certain strains of the human papillomavirus (HPV), and HPV disease progression appears to be more aggressive in people with HIV. In March 2003, the FDA approved a new test to detect high-risk strains of HPV; the Digene HC2 HPV DNA assay is used in conjunction with a Pap smear, using the same sample of cervical cells149.
Treatment monitoring tests: Increasing viral load (viral breakthrough) and decreasing CD4 cell counts in a person on anti-HIV therapy are indications that adherence is not adequate or that the drugs being used are not effective and may need to be changed. In addition, today there are other tests that can help physicians determine whether current therapy is working or whether a treatment option under consideration is likely to work.
Resistance testing: HIV can develop drug resistance by mutating in such a way that the virus can continue to replicate despite the drug. This usually occurs when a drug is not completely effective, allowing HIV to multiply and mutate in the presence of the drug. Also, an increasing number of people are becoming infected with HIV that is already resistant to one or more drugs150.
Several studies have shown that use of resistance testing to guide treatment decisions leads to more sustained viral load reductions151. The ability to tell which drugs are no longer working provides the opportunity to change a specific drug, rather than tossing out an entire failing regimen. Resistance tests are increasingly being used in clinical practice, but are not yet standardized and should be interpreted by an experienced physician. The tests work best when a person has a viral load of at least 1,000 copies/ml and is currently on a failing anti-HIV drug regimen. Three types of tests are used to measure drug resistance.
Genotypic tests: These tests examine the genetic sequence of HIV's reverse transcriptase and/or protease enzymes to look for mutations that are known to be associated with resistance to particular drugs. For example, the K103N mutation confers resistance to current non-nucleoside reverse transcriptase inhibitors (NNRTIs)152.
Phenotypic tests: These tests are done by adding a medication to an HIV culture in the laboratory to determine how much drug is needed to inhibit viral replication. Resistance levels are usually reported in terms of how much drug is needed to inhibit viral replication by 50 or 90 per cent (the IC50 or IC90, respectively), compared with wild-type virus. Phenotypic tests provide a more direct measure of resistance, but are more difficult, time-consuming, and expensive153.
Virtual phenotype: This is a new approach to estimate the viral phenotype using a large database of more than 18,000 pairs of genotypic and phenotypic data. HIV with a similar genotype is identified in the database, and the corresponding phenotypic information is used to estimate resistance. Preliminary research suggests that the virtual phenotype predicts treatment response about as well as a true phenotypic test154.
Therapeutic drug monitoring: Therapeutic drug monitoring (TDM) is used to help individualize anti-HIV therapy by measuring the amount of drug in an individual's blood. This is important because different people absorb, process, and eliminate drugs at different rates, and blood levels may vary considerably among individuals taking the same doses of the same medications. Ideally, the lowest plasma drug concentration between doses (the trough level, or Cmin) should still be high enough to inhibit HIV, but the highest concentration (the peak level, or Cmax) should not cause intolerable side effects.
Some, but not all, studies have shown that using TDM to guide treatment decisions increases the chance of successful viral suppression. However, drug level monitoring is not appropriate for all anti-HIV drugs. TDM has grown in popularity, especially for guiding salvage therapy, but it remains controversial and is still not widely used in the U.S155.
There has been a dramatic and commendable rapid scale-up of human immunodeficiency virus (HIV) prevention and care services in resource-limited settings, including sub-Saharan Africa in the past 5 years; thousands of lives have been saved, and 13 million people are now receiving antiretroviral therapy (ART). Although much remains to be done to continue the scale-up of HIV prevention, care, and treatment, we must strive to hold onto gains we have achieved. More efficacious drugs, which are less toxic, are easier to take, and have greater genetic barriers to the emergence of resistance, as well as improved models on how to best deliver HIV services, are needed. A critical area of need in resource-limited settings is improved access to appropriate laboratory tests for initiating and monitoring ART. To sustain the benefits of ART, maximal suppression of HIV is required. This is true both for the long-term prognosis of the individual and for potential community-level benefits, such as reduced risk of transmission. Currently, the only reliable way to ensure that treatment is achieving viral suppression is to measure HIV levels.
Although ART can sometimes be initiated on clinical criteria without CD4 cell count or viral load testing, these are necessary for optimal monitoring of ART. The World Health Organization recommends use of clinical and/or immunological monitoring (CD4 cell count) if viral load testing is not available. This situation applies to most ART settings in resource- limited countries, where HIV load testing is most often unaffordable or unavailable. An important goal in ART is ensuring maximum durability of current drug regimens, first through continued support of health care systems to ensure uninterrupted access to ART services and improved ART adherence support, but also through the identification of the early warning signs of potential virologic failure, before the development of multiple HIV drug resistance, which will limit the response to future ARTs and this has aided greatly in the scale-up of ART, and continued success of first-line regimens in resource-limited settings.
References
- 1. UNAIDS Epidemic update 2007.
- 2.2.5 million people in India living with HIV, according to new estimates. UNAids , NACO. [Retrieved July 6, 2007]. http://data.unaids.org/pub/PressRelease/2007/070706_indiapressrelease_en.pdf .
- 3. http://www.nacoonline.org/upload/HomePage/NACO , Press Release on HIV Estimates.pdf .
- 4.Fiona Samuels and Sanju Wagle. London: Overseas Development Institute; 2011. Population mobility and HIV and AIDS: review of laws, policies and treaties between Bangladesh, Nepal and India. [Google Scholar]
- 5. UNGASS (2010, March 31st) India - Country Progress Report.
- 6.World Health Organization. Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. 2009. [accessed December 3, 2009]. Available at: http://www.who.int/entity/hiv/pub/arv/rapid_advice_art.pdf .
- 7.Financing issues in Providing Anti-retroviral Drugs for HIV/AIDS Treatment in India, Ramesh B, Somen S. Economic and Political Weekly. 2005 Apr;:16, 1640–6. [Google Scholar]
- 8.Wilson LS, Basu R, Christenson M, Hensic L, Paoli C, Wara D, et al. Pediatric HIV costs across three treatment eras from 1986 to 2007. Pediatrics. 2010;126:e541–9. doi: 10.1542/peds.2009-3485. [DOI] [PubMed] [Google Scholar]
- 9.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein ML, Auglarget X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings - the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 10.Bishai D, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21:1333–40. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 11.Vijayaraghavan A, Efrusy MB, Mazonson PD, Ebrahim O, Sanne IM, Santas CC. Cost-effectiveness of alternative strategies for initiating and monitoring highly active antiretroviral therapy in the developing world. J Acquir Immune Defic Syndr. 2007;46:91–100. doi: 10.1097/QAI.0b013e3181342564. [DOI] [PubMed] [Google Scholar]
- 12.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371:1443–51. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 13.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Arch Intern Med. 2008;168:1910–8. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14:259–65. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 16.Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, Kityo C, et al. DART Trial Team. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina Lara A, Kigozi J, Amurwon J, Muchabaiwa L, Nyanzi WB, Walker S, et al. The 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (Cape Town, South Africa) Geneva: International AIDS Society; 2009. Cost effectiveness analysis of routine laboratory or clinically driven strategies for monitoring anti- retroviral therapy in Uganda and Zimbabwe (DART Trial) [Google Scholar]
- 18.Gilks CF. The 5th International AIDS Soctiety Conference on HIV Pathogenesis, Treatment and Prevention (Cape Town, South Africa) Geneva: International AIDS Society; [accessed on November 18, 2009]. Cost effectiveness analysis of routine laboratory or clinically driven strategies for monitoring anti-retroviral therapy in Uganda and Zimbabwe. http://www.ias2009.org/PAGMaterial/TUSS104_Gilks_1.ppt . [Google Scholar]
- 19.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendation for public health approach. 2006. [accessed on November 10, 2009]. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf . [PubMed]
- 20.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents - A Working Group of the Office of AIDS Research Advisory Council (OARAC) [accessed on November 18, 2009];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf . [Google Scholar]
- 21.Bogner JR, Matuschke A, Heinrich B, Eberle E, Goebel FD. Serum neopterin levels as predictor of AIDS. Klin Wochenschr. 1988;66:1015–8. doi: 10.1007/BF01733445. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 23.Gerber JG. Therapeutic drug monitoring in the treatment of HIV-infection. J Clin Virol. 2003;27:117–28. doi: 10.1016/s1386-6532(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 24.De Santis GC, Brunetta DM, Vilar FC, Brandão RA, de Albernaz Muniz RZ, de Lima GM, et al. Hematological abnormalities in HIV-infected patients. Int J Infect Dis. 2011;15:e808–11. doi: 10.1016/j.ijid.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Olayemi E, Awodu OA, Bazuaye GN. Autoimmune hemolytic anemia in HIV-infected patients: a hospital based study. Ann Afr Med. 2008;7:72–6. doi: 10.4103/1596-3519.55677. [DOI] [PubMed] [Google Scholar]
- 26.Volberding PA, Baker KR, Levine AM. Human immunodeficiency virus hematology. Haematol Am Soc Hematol Educ Program. 2003;2003:294–313. doi: 10.1182/asheducation-2003.1.294. [DOI] [PubMed] [Google Scholar]
- 27.Owiredu WK, Quaye L, Amidu N, Addai-Mensah O. Prevalence of anaemia and immunological markers among ghanaian HAART-naïve HIV-patients and those on HAART. Afr Health Sci. 2011;11:2–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Firnhaber C, Smeaton L, Saukila N, Flanigan T, Gangakhedkar R, Kumwenda J, et al. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis. 2010;14:e1088–92. doi: 10.1016/j.ijid.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torre D, Pugliese A. Platelets and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6:411–8. doi: 10.2174/157016208785861140. [DOI] [PubMed] [Google Scholar]
- 30.Hammer SM, Saag MS, Schechter M, Montaner Julio SG, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 Recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–43. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 31.Kannangai R, Kandathil AJ, Ebenezer DL, Nithyanandam G, Samuel P, Abraham OC, et al. Evidence for lower CD4+ T cell and higher viral load in asymptomatic HIV-1 infected individuals of India: implications for therapy initiation. Indian J Med Microbiol. 2008;26:217–21. doi: 10.4103/0255-0857.42031. [DOI] [PubMed] [Google Scholar]
- 32.Walensky RP, Wood R, Ciaranello AL, Paltiel AD, Lorenzana SB, Anglaret X, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A, Sankaran S, Vajpayee M, Sreenivas V, Seth P, Dandekar S. Correlation of immune activation with HIV-1 RNA levels assayed by real-time RT-PCR in HIV-1 subtype C infected patients in Northern India. J Clin Virol. 2007;40:301–6. doi: 10.1016/j.jcv.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickler HD, Palefsky JM, Shah KV, Anastos K, Klein RS, Minkoff H, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95:1062–71. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 35.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. NA-ACCORD Investigators. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghate MV, Mehendale SM, Mahajan BA, Yadav R, Brahme RG, Divekar AD, et al. Relationship between clinical conditions and CD4 counts in HIV-infected persons in Pune, Maharashtra, India. Natl Med J India. 2000;13:183–7. [PubMed] [Google Scholar]
- 37.Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan SP, Mayer KH. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 38.Amatya R, Vajpayee M, Kaushik S, Kanswal S, Pandey RM, Seth P. Lymphocyte immunophenotype reference ranges in healthy Indian adults: implications for management of HIV/AIDS in India. Clin Immunol. 2004;112:290–5. doi: 10.1016/j.clim.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Vajpayee M, Kaushik S, Sreenivas V, Wig N, Seth P. CDC staging based on absolute CD4 count and CD4 percentage in an HIV-1-infected Indian population: treatment implications. Cl Exp Immunol. 2005;141:485–90. doi: 10.1111/j.1365-2249.2005.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS, et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26:8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Choong ML, Ton SH, Cheong SK. Influence of race, age and sex on the lymphocyte subsets in peripheral blood of healthy Malaysian adults. Ann Clin Biochem. 1995;32:532–9. doi: 10.1177/000456329503200603. [DOI] [PubMed] [Google Scholar]
- 42.Maini MK, Gilson RJ, Chavda N, Gill S, Fakoya A, Ross EJ, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med. 1996;72:27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tollerud DJ, Clark JW, Brown LM, Neuland CY, Pankiw-Trost LK, Blattner WA, et al. The influence of age, race, and gender on peripheral blood mononuclear-cell subsets in healthy nonsmokers. J Clin Immunol. 1989;9:214–22. doi: 10.1007/BF00916817. [DOI] [PubMed] [Google Scholar]
- 44.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto F, Modesti M, et al. Changes in the expression of surface receptors on lymphocyte subsets in the elderly: quantitative flow cytometric analysis. Am J Hematol. 2001;67:63–72. doi: 10.1002/ajh.1082. [DOI] [PubMed] [Google Scholar]
- 45.Fahey JL, Schnelle JF, Boscardin J, Thomas JK, Gorre ME, Aziz N, et al. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;115:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe WH, Miner JC, Michalek JE. Immunological parameters in current and former US Air Force personnel. Vaccine. 1993;11:545–7. doi: 10.1016/0264-410x(93)90228-p. [DOI] [PubMed] [Google Scholar]
- 47.Mark DW, Philip TD, Mary EW, Melissa PL, Haikady NNN, Thomas LC. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998;158:1543–9. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]
- 48.Schaberg T, Theilacker C, Nitschke OT, Lode H. Lymphocyte subsets in peripheral blood and smoking habits. Lung. 1997;175:387–94. doi: 10.1007/pl00007585. [DOI] [PubMed] [Google Scholar]
- 49.Uppal SS, Verma S, Dhot PS. Normal values of CD4 and CD8 lymphocyte subsets in healthy Indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry Part B (Clinical Cytometry) 2003;52B:32–6. doi: 10.1002/cyto.b.10011. [DOI] [PubMed] [Google Scholar]
- 50.Mandy FF, Bergeron M, Minkus T. Evolution of leukocyte immunophenotyping as influenced by the HIV/AIDS pandemic: a short history of the development of gating strategies for CD4+ T-cell enumeration. Cytometry. 1997;30:157–65. [PubMed] [Google Scholar]
- 51.Gelman R, Wilkening C. Analyses of quality assessment studies using CD45 for gating lymphocytes for CD3+CD4+% Cytometry. 2000;42:1–4. doi: 10.1002/(sici)1097-0320(20000215)42:1<1::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 52.Thompson JM, Gralow JR, Levy R, Miller RA. The optimal application of forward and ninety-degree light scatter in flow cytometry for the gating of mononuclear cells. Cytometry. 1985;6:401–6. doi: 10.1002/cyto.990060503. [DOI] [PubMed] [Google Scholar]
- 53.Loken MR, Brosnan JM, Bach BA, Ault KA. Establishing optimal lymphocyte gates for immunophenotyping by flow cytometry. Cytometry. 1990;11:453–9. doi: 10.1002/cyto.990110402. [DOI] [PubMed] [Google Scholar]
- 54.Nicholson JKA, Jones BM, Hubbard M. CD4 T-lymphocyte determinations on whole blood specimens using a single-tube three-color assay. Cytometry. 1993;14:685–9. doi: 10.1002/cyto.990140614. [DOI] [PubMed] [Google Scholar]
- 55.Pattanapanyasat K, Kyle DE, Tongtawe P, Yongvanitchit K, Fucharoen S. Flow cytometric immunophenotyping of lymphocyte subsets in samples that contain a high proportion of non-lymphoid cells. Cytometry. 1994;18:199–208. doi: 10.1002/cyto.990180403. [DOI] [PubMed] [Google Scholar]
- 56.Bergeron M, Nicholson JK, Phaneuf S, Ding T, Soucy N, Badley AD, et al. Selection of lymphocyte gating protocol has an impact on the level of reliability of T-cell subsets in aging specimens. Cytometry. 2002;50:53–61. doi: 10.1002/cyto.10092. [DOI] [PubMed] [Google Scholar]
- 57.Aboulker JP, Autran B, Beldjord K, Touraine F, Debre P. Consistency of routine measurements of CD4+, CD8+ peripheral blood lymphocytes. J Immunol Methods. 1992;154:155–61. doi: 10.1016/0022-1759(92)90187-x. [DOI] [PubMed] [Google Scholar]
- 58.Cohen AJ, Peerschke EI, Steigbigel RT. A comparison of the Coulter STKS, Coulter S+IV, and manual analysis of white blood cell differential counts in a human immunodeficiency virus-infected population. Am J Clin Pathol. 1993;100:611–7. doi: 10.1093/ajcp/100.6.611. [DOI] [PubMed] [Google Scholar]
- 59.Malone JL, Simms TE, Gray GC, Wagner KF, Burge JR, Burke DS. Sources of variability in repeated T-helper lymphocyte counts from human immunodeficiency virus type 1 infected patients: total lymphocyte count fluctuations and diurnal cycle are important. J Acquir Immune Defic Syndr. 1990;3:144–51. [PubMed] [Google Scholar]
- 60.Janossy G, Jani IV, Goehde W. Affordable CD4+ T cell counts on “single-platform” flow cytometers I. Primary CD4 gating. Br J Haematol. 2000;111:1198–208. doi: 10.1046/j.1365-2141.2000.02433.x. [DOI] [PubMed] [Google Scholar]
- 61.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate cost-effective dual- platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 62. [accessed on December 19, 2011]. Available from: http://www.bd.com .
- 63. [accessed on June 25, 2007]. Available from: http://www.guavatechnologies.com/ main/index.cfm .
- 64. [accessed on June 20, 2011]. Available from: http://www.partec.de .
- 65.[No authors listed] WHO Global Programme on AIDS Report of a WHO workshop on flow cytometry and alternative methodologies for CD4 lymphocyte determinations: Applications for developing countries. 1992 Apr 16; AIDS 8, WHO1-WHO4, Geneva. [PubMed] [Google Scholar]
- 66.Carella AV, Moss MW, Provost V, Quinn TC. A manual bead assay for the determination of absolute CD4+ and CD8+ lymphocyte counts in human immunodeficiency virus-infected individuals. Clin Diagn Lab Immunol. 1995;2:623–5. doi: 10.1128/cdli.2.5.623-625.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicholson JK, Velleca WM, Jubert S, Green TA, Bryan L. Evaluation of alternative CD4 technologies for the enumeration of CD4 lymphocytes. J Immunol Methods. 1994;177:43–54. doi: 10.1016/0022-1759(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 68. [accessed on March 23, 2011]. Available from: http://www.beckmancoulter.com .
- 69.Balakrishnan P, Dunne M, Kumarasamy N, Crowe S, Subbulakshmi G, Ganesh AK, et al. An inexpensive, simple, and manual method of CD4 T-cell quantitation in HIV-infected individuals for use in developing countries. J Acquir Immune Defic Syndr. 2004;36:1006–10. doi: 10.1097/00126334-200408150-00002. [DOI] [PubMed] [Google Scholar]
- 70.Suzanne Crowe, Shannon Turnbull1, Robert Oelrichs1, Dunne Amanda. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin Infect Dis. 2003;37(Suppl 1):S25–S35. doi: 10.1086/375369. [DOI] [PubMed] [Google Scholar]
- 71. [accessed on November 30, 2011]. Available from: http://www.dynalbiotech.com .
- 72.Kannangai R, Ramalingam S, Jesudason MV, Vijayakumar TS, Abraham OC, Zachariah A, et al. Correlation of CD4 + T-Cell counts estimated by an immunocapture technique (Capcellia) with viral loads in human immunodeficiency virus-seropositive individuals. Clin Diagn Lab Immunol. 2001;8:1286–8. doi: 10.1128/CDLI.8.6.1286-1288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mwaba P, Cassol S, Pilon R, Chintu C, Janes M, Nunn A, et al. Use of dried whole blood spots to measure CD4+ lymphocyte counts in HIV-infected patients. Lancet. 2003;362:1459–60. doi: 10.1016/S0140-6736(03)14693-4. [DOI] [PubMed] [Google Scholar]
- 74. [accessed on December 30, 2011]. Available from: http://www.ekneqas.org.uk .
- 75.Rickman WJ, Waxdal MT, Monical C, Damato JD, Burke DS. Department of Army lymphocyte immunophenotyping quality assurance program. Clin Immunol Immunopathol. 1989;52:85–95. doi: 10.1016/0090-1229(89)90195-5. [DOI] [PubMed] [Google Scholar]
- 76. [accessed on December 23, 2011]. Available from: http://www.phac-aspc.gc.ca/nlhi-1niv/activ2_e.html .
- 77.Taylor JMG, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: Which to choose and how to use. J Acquir Immune Defic Syndr. 1989;2:114–24. [PubMed] [Google Scholar]
- 78.Giorgi JV. Characterization of T lymphocyte subset alterations by flow cytometry in HIV disease. Ann NY Acad Sci. 1993;677:126–37. doi: 10.1111/j.1749-6632.1993.tb38771.x. [DOI] [PubMed] [Google Scholar]
- 79.Calmy A, Klement E, Teck R, Berman D, Pecoul B, Ferradini L. Simplifying and adapting antiretroviral treatment in resource-poor settings: a necessary step to scaling-up. AIDS. 2004;18:2353–60. [PubMed] [Google Scholar]
- 80.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–82. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 81.Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Geneva: World Health Organization; 2006. World Health Organization. [Google Scholar]
- 82.Koenig SP, Kuritzkes DR, Hirsch MS, Leandre F, Mukherjee JS, Farmer PE, et al. Monitoring HIV treatment in developing countries. BMJ. 2006;332:602–4. doi: 10.1136/bmj.332.7541.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gandhi Monica, Bacchetti Peter, Miotti Paolo, Quinn Thomas C, Veronese Fulvia, Greenblatt Ruth M. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 84.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load (VL), and viral load set point in primary HIV infection (PHI) J Acquir Immune Defic Syndr. 2007;45:445–8. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 85.Progress on global access to HIV antiretroviral therapy. Geneva: World Health Organization; 2006. World Health Organization. [Google Scholar]
- 86.Gazzard B. BHIVA Writing Committee. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2005) HIV Med. 2005;6(Suppl 2):1–61. doi: 10.1111/j.1468-1293.2005.0311b.x. [DOI] [PubMed] [Google Scholar]
- 87.Hammer SM, Saag M, Schechter MS, Montaner JS, Schooley RT, Jacobsen DM, et al. International AIDS Society. USA Panel. Treatment for HIV adult infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–43. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 88.Deeks SG, Hoh R, Grant RM, Wrin T, Barbour JD, Narvaez A, et al. CD4 + T cell kinetics and activation in human immunodeficiency virus-infected patients who remain viremic despite long-term treatment with protease inhibitor-based therapy. J Infect Dis. 2002;185:315–23. doi: 10.1086/338467. [DOI] [PubMed] [Google Scholar]
- 89.Kousignian I, Abgrall S, Duval X, Descamps D, Matheron S, Costagliola D. Modeling the time course of CD4 T-lymphocyte counts according to the level of virologic rebound in HIV-1-infected patients on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;34:50–7. doi: 10.1097/00126334-200309010-00007. [DOI] [PubMed] [Google Scholar]
- 90.Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, Reiss P, et al. PLATO Collaboration. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 91.Tomasoni LR, Patroni A, Torti C, Paraninfo G, Gargilulo F, Quiros-Roldan E, et al. Predictors of long-term immunological outcome in rebounding patients on protease inhibitor based HAART after initial successful virologic suppression: implications for timing to switch. HIV Clin Trials. 2003;4:311–23. doi: 10.1310/FGMF-Y0LY-DMX8-371V. [DOI] [PubMed] [Google Scholar]
- 92.Schuurman R, Descamps D, Weverling GJ, Kaye S, Tijnagel J, William I, et al. Multicenter comparison of three commercial methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:3016–22. doi: 10.1128/jcm.34.12.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holguin A, de Mendoza C, Soriano V. Comparison of three different commercial methods for measuring plasma viraemia in patients infected with non-B HIV-1 subtypes. Eur J Clin Microbiol Infect Dis. 1999;18:256–9. doi: 10.1007/s100960050273. [DOI] [PubMed] [Google Scholar]
- 94.Nolte FS, Boysza J, Thurmond C, Clark WS, Lennox JL. Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:716–20. doi: 10.1128/jcm.36.3.716-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nkengasong JN, Kalou M, Maurice C, Bile C, Borget MY, Koblavi S, et al. Comparison of NucliSens and Amplicor monitor assays for quantification of human immunodeficiency virus type 1 (HIV-1) RNA in plasma of persons with HIV-1 subtype A infection in Abidjan, Cote d’Ivoire. J Clin Microbiol. 1998;36:2495–8. doi: 10.1128/jcm.36.9.2495-2498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dyer JR, Pilcher CD, Shepard R, Schock J, Eron JJ, Fiscus SA, et al. Comparison of NucliSens and Roche Monitor assays for quantitation of levels of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1999;37:447–9. doi: 10.1128/jcm.37.2.447-449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elbeik T, Charlebois E, Nassos P, Kahn J, Hecht FM, Yajko D, et al. Quantitative and cost comparison of ultrasensitive human immunode.ciency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000;38:1113–20. doi: 10.1128/jcm.38.3.1113-1120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy DG, Cote L, Fauvel M, Rene P, Vincelette J. Multicenter comparison of Roche COBAS AMPLICOR MONITOR version 1.5, Organon Teknika NucliSens QT with Extractor, and Bayer Quantiplex version 3.0 for quanti. cation of human immunode.ciency virus type 1 RNA in plasma. J Clin Microbiol. 2000;38:4034–41. doi: 10.1128/jcm.38.11.4034-4041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elbeik T, Chen Y-MA, Soutchkov SV, Loftus RA, Beringer S, et al. Global cost modeling analysis of HIV-1 and HCV viral load assays. Expert Rev Pharmacoeconomics Outcomes Res. 2003;3:383–407. doi: 10.1586/14737167.3.4.383. [DOI] [PubMed] [Google Scholar]
- 100.Crowe S, Turnbull S, Oelrichs R, Dunne A. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin Infect Dis. 2003;37(Suppl 1):S25–S35. doi: 10.1086/375369. [DOI] [PubMed] [Google Scholar]
- 101.Majchrowicz M. Beyond antiretroviral access: low-cost laboratory tests needed for the developing world. AIDS. 2003;17(Suppl 4):S12–5. [PubMed] [Google Scholar]
- 102.Balakrishnan P, Solomon S, Kumarasamy N, Mayer KH. Low-cost monitoring of HIV infected individuals on highly active antiretroviral therapy (HAART) in developing countries. Indian J Med Res. 2005;121:345–55. [PubMed] [Google Scholar]
- 103.Giacomini M, McDermott JL, Giri AA, Martini I, Lillo FV, Varnier DE. A novel and innovative quantitative kinetic software for virological colorimetric assays. J Virol Methods. 1999;73:201–9. doi: 10.1016/s0166-0934(98)00059-7. [DOI] [PubMed] [Google Scholar]
- 104.Schupbach J, Boni J, Bisset LR, Tomasik Z, Fischer M, Günthard HF, et al. HIV-1 p24 antigen is a significant inverse correlate of CD4 T-cell change in patients with suppressed viremia under long-term antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;33:292–9. doi: 10.1097/00126334-200307010-00002. [DOI] [PubMed] [Google Scholar]
- 105.Sterling TR, Hoover DR, Astemborski J, Vlahov D, Bartlett JA, Schupbach J, et al. Heat-denatured HIV-1 protein p24 antigen: Prognostic value in adults with early stage disease. J Infect Dis. 2002;186:1181–5. doi: 10.1086/343807. [DOI] [PubMed] [Google Scholar]
- 106.Jennings C, Danilovic A, Burgess D, Morack R, Fiscus S, et al. American Society for Microbiology. Chicago, Illinois, United States of America: American Society for Microbiology; 2003. Sep 14-17, [accessed on September 7, 2006]. A comparison of two non-RNA-based assays for the quantitation of HIV [abstract]. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Available: http://gateway.nlm.nih.gov/robot_pages/MeetingAbstracts/102266490.html . [Google Scholar]
- 107.Simonds RJ, Brown TM, Thea DM, Orloff SL, Steketee RW, et al. Sensitivity and specificity of a qualitative RNA detection assay to diagnose HIV infection in young infants. Perinatal AIDS Collaborative Transmission Study. AIDS. 1998;12:1545–9. doi: 10.1097/00002030-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 108.Cunningham CK, Charbonneau TT, Song K, Patterson D, Sullivan T, Cummins T, et al. Comparison of human immunodeficiency virus 1 DNA polymerase chain reaction and qualitative and quantitative RNA polymerase chain reaction in human immunodeficiency virus 1-exposed infants. Pediatr Infect Dis J. 1999;18:30–5. doi: 10.1097/00006454-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 109.Young NL, Shaffer N, Chaowanachan T, Chotpitayasunondh T, Vanparapar N, Mock PA, et al. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assays sensitive to non-B subtypes. J Acquir Immune De.c Syndr. 2000;24:401–7. doi: 10.1097/00126334-200008150-00001. [DOI] [PubMed] [Google Scholar]
- 110.Benjamin DK, Jr, Miller WC, Fiscus SA, Benjamin DK, Morse M, et al. Rational testing of the HIV-exposed infant. Pediatrics. 2001;108:E3 1–5. doi: 10.1542/peds.108.1.e3. [DOI] [PubMed] [Google Scholar]
- 111.Lambert JS, Harris DR, Stiehm ER, Moye J, Jr, Fowler MG, et al. Performance characteristics of HIV-1 culture and HIV-1 DNA and RNA amplification assays for early diagnosis of perinatal HIV-1 infection. J Acquir Immune Defic Syndr. 2003;34:512–9. doi: 10.1097/00126334-200312150-00011. [DOI] [PubMed] [Google Scholar]
- 112.Malmsten A, Shao XW, Aperia K, Corrigan GE, Sandstrom E, Källander CF, et al. HIV-1 viral load determination based on reverse transcriptase activity recovered from human plasma. J Med Virol. 2003;71:347–59. doi: 10.1002/jmv.10492. [DOI] [PubMed] [Google Scholar]
- 113.Malmsten A, Shao XW, Sjodahl S, Fredriksson EL, Pettersson I, et al. Improved HIV-1 viral load determination based on reverse transcriptase activity recovered from human plasma. J Med Virol. 2005;76:291–6. doi: 10.1002/jmv.20360. [DOI] [PubMed] [Google Scholar]
- 114.Braun J, Plantier JC, Hellot MF, Tuaillon E, Gueudin M, Damond F, et al. A new quantitative HIV load assay based on plasma virion reverse transcriptase activity for the different types, groups and subtypes. AIDS. 2003;17:331–6. doi: 10.1097/00002030-200302140-00006. [DOI] [PubMed] [Google Scholar]
- 115.Greengrass VL, Turnbull SP, Hocking J, Dunne AL, Tachedjian G, et al. Evaluation of a low cost reverse transcription assay for plasma HIV-1 viral load monitoring. Curr HIV Res. 2005;3:183–90. doi: 10.2174/1570162053506955. [DOI] [PubMed] [Google Scholar]
- 116.Seyoum E, Wolday D, Girma M, Malmsten A, Meselle T, Gronowitz JS, et al. Reverse transcriptase activity for quantitation of HIV-1 subtype C in plasma: Relation to RNA copy number and CD4 T-cell count. J Med Virol. 2006;78:161–8. doi: 10.1002/jmv.20523. [DOI] [PubMed] [Google Scholar]
- 117.Sivapalasingam S, Essajee S, Nyambi PN, Itri V, Hanna B, Holzman R, et al. Human immunodeficiency virus (HIV) reverse transcriptase activity correlates with HIV RNA load: Implications for resource-limited settings. J Clin Microbiol. 2005;43:3793–6. doi: 10.1128/JCM.43.8.3793-3796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewin SR, Vesanen M, Kostrikos L, Hurley A, Duran M, Zhang L, et al. Use of real-time PCR and molecular beacons to detect virus replication in HIV-1 infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73:6099–103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Baar MP, Timmermans EC, Bakker M, de Rooij E, van Gemen B, Goudsmit J, et al. One-tube real-time isothermal amplification assay to identify and distinguish human immunodeficiency virus type 1 subtypes A, B, and C and circulating recombinant forms AE and AG. J Clin Microbiol. 2001;39:1895–902. doi: 10.1128/JCM.39.5.1895-1902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Baar MP, Timmermans EC, Buitelaar M, Westrop M, Dekker JT, Bethell R, et al. Evaluation of the HIV-1 RNA RetinaTM Rainbow assay on plasma and dried plasma spots: Correlation with the Roche Amplicor HIV-1 Monitor v 1.5 assay. Antivir Ther. 2003;8(Suppl 1):S533. [Google Scholar]
- 121.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodefi- ciency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stevens W, Wiggill T, Horsfield P, Coetzee L, Scott LE. Evaluation of the NucliSens EasyQ assay in HIV-1 infected individuals in South Africa. J Virol Methods. 2005;124:105–10. doi: 10.1016/j.jviromet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 123.Yao J, Liu Z, Ko LS, Pan G, Jiang Y. Quantitative detection of HIV-1 RNA using NucliSens EasyQ HIV-1 assay. J Virol Methods. 2005;129:40–6. doi: 10.1016/j.jviromet.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 124.Rekhviashvii N, Stevens G, Scott L, Stevens W. Fluorogenic LUX primer for quantitation of HIV-1 by real-time RT-PCR. Mol Biotechnol. 2006;32:101–10. doi: 10.1385/MB:32:2:101. [DOI] [PubMed] [Google Scholar]
- 125.Swanson P, Huang S, Holzmayer V, Bodelle P, Yamaguchi J, Brennan C, et al. Performance of the automated Abbott RealTime HIV-1 assay on a genetically diverse panel of specimens from Brazil. J Virol Methods. 2006;134:237–43. doi: 10.1016/j.jviromet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 126.Rouet F, Ekouevi DK, Chaix ML, Burgard N, Inwoley A, Tony TD, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of HIV-1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gueudin M, Simon F. Plasma RNA viral load in HIV-1 group O infection by real-time PCR. Methods Mol Biol. 2005;304:221–8. doi: 10.1385/1-59259-907-9:221. [DOI] [PubMed] [Google Scholar]
- 128.Gueudin M, Plantier JC, Damond F, Roques P, Mauclere P, Simon F, et al. Plasma viral RNA assay in HIV-1 group O infection by real-time PCR. J Virol Methods. 2003;113:43–9. doi: 10.1016/s0166-0934(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 129.Candotti D, Temple J, Owusu-Ofori S, Allain JP. Multiplex real-time quantitative realtime PCR assay for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. J Virol Methods. 2004;118:39–47. doi: 10.1016/j.jviromet.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 130.Rouet F, Rouzioux C. HIV-1 viral load testing cost in developing countries: what's new? Expert Rev Mol Diagn. 2007;7:703–7. doi: 10.1586/14737159.7.6.703. [DOI] [PubMed] [Google Scholar]
- 131.James MM, Wang L, Musoke P, Donnell D, Fogel J, Towler WI, et al. Association of HIV diversity and survival in HIV-infected Ugandan infants. PLoS One. 2011;6:e18642. doi: 10.1371/journal.pone.0018642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frange P, Galimand J, Vidal N, Goujard C, Deveau C, Souala F, et al. New and old complex recombinant HIV-1 strains among patients with primary infection in 1996-2006 in France: the French ANRS CO06 primo cohort study. Retrovirology. 2008;5:69. doi: 10.1186/1742-4690-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;358:1590–602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swanson P, de Mendoza C, Joshi Y, Golden A, Hodinka RL, Soriano V, et al. Impact of human immunode.ciency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J Clin Microbiol. 2005;43:3860–8. doi: 10.1128/JCM.43.8.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scott L, Carmona S, Stevens W. The performance of the new Roche COBAS Ampli/COBAS TaqMan HIV-1 version 2.0 assay. J Clin Microbiol. 2009;47:3400–2. doi: 10.1128/JCM.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Planella T, Cortes M, Martinez-Bru C, Barrio J, Sambeat MA, Gonzalez-Sastre F. The predictive value of several markers in the progression to acquired immunodeficiency syndrome. Clin Chem Lab Med. 1998;36:169–73. doi: 10.1515/CCLM.1998.031. [DOI] [PubMed] [Google Scholar]
- 137.Thomas Bourlet, Anne Signori-Schmuck, Laurent Roche, Vinca Icard, Henia Saoudin, Mary-Anne Trabaud, et al. HIV-1 load comparison using four commercial real-time assays. J Clin Microbiol. 2011;49:292–7. doi: 10.1128/JCM.01688-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ortigao-de-Sampaio MB, Abreu TF, Linhares-de-Carvalho MI, Ponce de Leon A, Castello-Branco LR, et al. Surrogate markers of disease progression in HIV-infected children in Rio de Janeiro, Brazil. J Trop Pediatr. 1999;45:299–302. doi: 10.1093/tropej/45.5.299. [DOI] [PubMed] [Google Scholar]
- 139.Halota W, Jaruga B, Pawlowska M. Serum neopterin and beta2-microglobulin concentration as “prognostic markers” of AIDS. Pol Merkuriusz Lek. 2002;13:126–8. [PubMed] [Google Scholar]
- 140.Hosp M, Lisse IM, Quigley M, Mwinga AM, Godfrey- Faussett P, Porter JD, et al. An evaluation of low-cost progression markers in HIV-1 seropositive Zambians. HIV Med. 2000;1:125–7. doi: 10.1046/j.1468-1293.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 141.Phillips AN, Sabin CA, Elford J, Bofill M, Timms A, Janossy G, et al. Serum beta 2-microglobulin at HIV-1 seroconversion as a predictor of severe immunodeficiency during 10 years of followup. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:262–6. doi: 10.1097/00042560-199611010-00008. [DOI] [PubMed] [Google Scholar]
- 142.Dar L, Singh YGK. Laboratory tests for monitoring stage and progression of HIV infection. In HIV testing manual by NICD and NACO. New Delhi: CBS Publishers; 1999. pp. 114–25. [Google Scholar]
- 143.Fauci AS, Lane HC. HIV disease: AIDS and related disorders. Chapter 308. In: Fauci AS, Braunwald E, Wilson JD, Martin JB, Kaspar DL, Hauser SL, Longo DL, editors. Harrison's principles of internal medicine. 14th ed. New York: Mc Graw Hill; 1998. pp. 1816–8. [Google Scholar]
- 144.Quin JW, Benson EM. It is HIV: Immediate and long term plans. Chapter 24. In: Stewart G, editor. Could it be HIV? 2nd ed. Sydney, Australia: Australasian medical publishing company Ltd; 1994. pp. 66–9. [Google Scholar]
- 145.Laboratory diagnosis of HIV infection (NICD-AIDS) Vol. 1. New Delhi: CBS publishers DGHS; 1992. pp. 1–2. [Google Scholar]
- 146.Rai A, Kumari S. Current trends in the laboratory diagnosis of HIV infection. Trans Assoc AIDS Infect. 1990;8:34–43. [Google Scholar]
- 147.Priince HE, Kleinman S, Willaims AE. Soluble IL-2 receptor levels in blood donors seropositive for HIV. J Immunol. 1988;140:1139–41. [PubMed] [Google Scholar]
- 148.Easterbrook P. Surrogate markers in HIV infection. Venerology. 1993;6:126. [Google Scholar]
- 149.Lindsey K, DeCristofaro C, James J. Anal Pap smears: Should we be doing them? J Am Acad Nurse Pract. 2009;21:437–43. doi: 10.1111/j.1745-7599.2009.00433.x. [DOI] [PubMed] [Google Scholar]
- 150.Hirsch MS, Günthard HF, Schapiro JM, Brun-Vézinet F, Clotet B, Hammer SM, et al. International AIDS Society-USA. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Top HIV Med. 2008;16:266–85. [PubMed] [Google Scholar]
- 151.Alexandra Calmy, Nathan Ford, Bernard Hirschel, Reynolds Steven J, Lynen Lut, et al. HIV viral load monitoring in resource-limited regions: Optional or necessary? HIV/AIDS View Points. 2007;44:128–34. doi: 10.1086/510073. [DOI] [PubMed] [Google Scholar]
- 152.Elgalib A, Perry M, Aboud M, Mullen J, O’Shea S, Chrystie IL, et al. Clinical utility of genotypic resistance tests for HIV-1-infected patients with low-level virological failure. J Clin Microbiol. 2010;48:3358–9. doi: 10.1128/JCM.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bellosillo NA, Bacheler L, Villacian J. HIV drug resistance tests: an update on methods for calculating phenotypic fold change from a viral genotype. Clin Infect Dis. 2009;48:687. doi: 10.1086/597016. [DOI] [PubMed] [Google Scholar]
- 154.Puchhammer-Stöckl E, Steininger C, Geringer E, Heinz FX. Comparison of virtual phenotype and HIV-SEQ program (Stanford) interpretation for predicting drug resistance of HIV strains. HIV Med. 2002;3:200–6. doi: 10.1046/j.1468-1293.2002.00116.x. [DOI] [PubMed] [Google Scholar]
- 155.Pretorius E, Klinker H, Rosenkranz B. The role of therapeutic drug monitoring in the management of patients with human immunodeficiency virus infection. Ther Drug Monit. 2011;33:265–74. doi: 10.1097/FTD.0b013e31821b42d1. [DOI] [PubMed] [Google Scholar]


