Abstract
Human immunodeficiency virus (HIV) associated tuberculosis (TB) remains a major global public health challenge, with an estimated 1.4 million patients worldwide. Co-infection with HIV leads to challenges in both the diagnosis and treatment of tuberculosis. Further, there has been an increase in rates of drug resistant tuberculosis, including multi-drug (MDR-TB) and extensively drug resistant TB (XDRTB), which are difficult to treat and contribute to increased mortality. Because of the poor performance of sputum smear microscopy in HIV-infected patients, newer diagnostic tests are urgently required that are not only sensitive and specific but easy to use in remote and resource-constrained settings. The treatment of co-infected patients requires antituberculosis and antiretroviral drugs to be administered concomitantly; challenges include pill burden and patient compliance, drug interactions, overlapping toxic effects, and immune reconstitution inflammatory syndrome. Also important questions about the duration and schedule of anti-TB drug regimens and timing of antiretroviral therapy remain unanswered. From a programmatic point of view, screening of all HIV-infected persons for TB and vice-versa requires good co-ordination and communication between the TB and AIDS control programmes. Linkage of co-infected patients to antiretroviral treatment centres is critical if early mortality is to be prevented. We present here an overview of existing diagnostic strategies, new tests in the pipeline and recommendations for treatment of patients with HIV-TB dual infection.
Keywords: Co-infection, diagnosis, drug resistance, IRIS, treatment, tuberculosis
Introduction
Human immunodeficiency virus (HIV) associated tuberculosis (TB) remains a major global public health challenge. By the end of 2009, an estimated 33.3 million people were living with HIV, the vast majority in sub-Saharan Africa and Asia. An estimated 2.6 million individuals had become newly infected with HIV and 1.8 million had died of AIDS in that year alone1. TB is the most common opportunistic infection (OI) among HIV-infected individuals, and co-infected individuals are at high risk of death2,3. The estimates of the global burden of disease caused by TB in 2009 were as follows: 9.4 million incident cases (range 8.9-9.9 million), 1.3 million deaths among HIV-negative TB patients (range 1.2-1.5 million) and 0.38 million deaths among HIV-positive TB patients (range 0.32-0.45 million). Most TB cases were in the South-East Asia, African and Western Pacific regions (35, 30 and 20%, respectively). An estimated 11-13 per cent of incident cases were HIV-positive4. TB may occur at any stage of HIV disease and is frequently the first recognized presentation of underlying HIV infection5,6. As compared to people without HIV, people living with HIV (PLWH) have a 20-fold higher risk of developing TB7 and the risk continues to increase as CD4 cell counts progressively decline5.
As a result of WHO's 3 by 5 campaign, >6 million HIV-infected individuals in resource limited settings have had access to antiretroviral therapy (ART) since 20048, though this is still far short of the actual need. Although ART can reduce the incidence of TB both at the individual and population level, PLWH on ART still have higher TB incidence rates and a higher risk of dying from TB9. This may be due to delayed initiation of ART or the fact that patients present with advanced TB or both10. Routine TB screening among PLWH offers the opportunity to identify those without TB, prevent TB by chemoprophylaxis as well as to diagnose and promptly treat TB. However, co-administration of ART along with anti-TB therapy presents several management challenges, including drug-drug interactions, overlapping drug toxicities and immune reconstitution syndrome.
In this review, we summarize and update information on the screening, diagnosis and management of TB in HIV infected adults.
Diagnosis of TB in HIV-infected individuals
Clinical screening algorithms: The WHO recommends TB screening at the time that HIV infection is diagnosed, before the initiation of antiretroviral therapy and at regular intervals during follow up11. Currently there is no internationally accepted evidence-based tool to screen for TB in PLWH. Multiple studies have been conducted to develop a simple method for ruling out TB in people with HIV infection, but methodological issues preclude the use of any of these as the basis for global health policy12–14. In 2007, a WHO International Expert Committee issued new guidelines to improve the diagnosis of TB in HIV infected individuals15. The feasibility, accuracy and operational performance of these guidelines were tested in various settings and were found to be acceptable16. It was recommended that screening for TB should include asking questions about a combination of symptoms rather than only about chronic cough. A recent meta-analysis evaluated the performance of individual and combinations of symptoms as screening rules for TB among 8,148 participants from 12 studies17. The best performing rule was the presence of any one of current cough, fever, night sweats or weight loss. The overall sensitivity of this rule was 79 per cent, increasing to 90 per cent in clinical settings but the specificity was only 50 per cent. The negative predictive value of the rule was high across a range of TB disease prevalence estimates as well as across high and low CD4 counts. The major change to existing practice would be the replacement of chronic cough with current cough as a screening question and the addition of other symptoms to standard screening17. While a screening tool needs to have high sensitivity and negative predictive value, a diagnostic strategy should ideally have both high sensitivity and specificity. The screening tool could be used in ART clinics to identify patients eligible for chemoprophylaxis as well as to identify those who need further investigations for TB.
Radiographic features: The spectrum of radiographic manifestation of pulmonary TB is dependent on the relative level of HIV-related immunodeficiency18. During the early phase of HIV when individuals are not immunosuppressed, the radiographic pattern is similar to HIV uninfected individuals with more typical lesions - upper lobe infiltrates with or without cavities. With advancing immunosuppression, extra pulmonary involvement, intra-thoracic/mediastinal lymphadenopathy, lower lobe infiltrate and miliary TB become more common19.
Adding chest X-ray to symptom screening increases the number of TB cases detected but is non-specific and adds to the cost of screening. Chest X-ray can still miss a substantial proportion of individuals with sub-clinical disease, often seen in advanced HIV immunosuppression20. Moreover, chest radiographs may appear normal in 7-14% of patients with HIV/TB18,19. This sub-population of co-infected individuals is particularly likely to benefit from sputum culture or nucleic acid amplification tests for TB diagnosis.
Sputum smear microscopy: The most frequent method of TB detection involves microscopic examination of sputum for acid-fast bacilli (AFB)21. Microscopy has the advantage of being inexpensive, relatively rapid to perform, and specific in most settings. However, to be considered smear positive a specimen needs to contain approximately 105 mycobacteria per milliliter. The sensitivity of sputum microscopy in HIV infection ranges from 43 to 51 per cent22, and in many resource-limited settings with high rates of co-infection, the sensitivity may be much lower23. Methods that improve speed or sensitivity include fluorescence microscopy24 and alternative specimen processing methods, such as concentration, bleach sedimentation and same-day sputum collection (so-called front loading) strategies25–27. Any procedure for digestion or liquefaction followed by centrifugation, prolonged gravity sedimentation, or filtration increases sensitivity by 13 to 33 per cent over direct microscopy, when culture is used as the reference standard26.
Equipment costs limit the wider use of fluorescence microscopes in resource-limited settings. Alternative technologies using light-emitting diode bulbs allow fluorescence microscopes at a much lower cost; field-level evaluation showed promising results and this technology is now being widely scaled up28,29. Nevertheless, because sputum smear is the primary mode of TB detection in many resource constrained settings, a sizable number of smear-negative individuals often remain undiagnosed or receive delayed anti-TB therapy30. It is also important to note that drug susceptibility cannot be ascertained by smear microscopy, so treatment for drug resistant TB is invariably empirical.
Growth based detection: Culture of Mycobacterium tuberculosis is much more sensitive than smear microscopy and has been recommended to assist in the diagnosis of TB in HIV-infected individuals31. Culture also allows subsequent strain characterization and drug susceptibility tests. The traditional method of inoculating solid medium such as the Lowenstein-Jenson (L-J) medium or Middlebrook medium is sensitive but slow, as growth may not be visible until after 6-8 wk of incubation. This results in delay in initiation of therapy, with detrimental effects on outcome of HIV-TB co-infected patients. Automated liquid culture systems detect growth of mycobacteria within 1-2 wk by bacterial carbon dioxide production or oxygen consumption with radiometric sensors (BACTEC 460 TB; Becton Dickinson Diagnostic Instruments Systems, USA), fluorescent sensors [BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960; Becton Dickinson Diagnostic Instruments Systems], colorimetric sensors (MB/ BacT system; Organon Teknika), pressure sensors (ESP culture system II; Difco Laboratories, USA), or redox reagents, such as Alamar blue32–35.
Microscopic observation drug susceptibility (MODS) assay is a low cost non-commercial method that can be used for detection of microcolonies, cord formation and for early detection of drug resistance. It appears to have higher sensitivity, shorter time to culture positivity and is more cost effective than regular L-J medium36.
Bacteriophage based assays have been used for TB diagnostics (FASTPlaqueTB; Biotech Laboratories, UK). The FAST Plaque TB assay can detect mycobacteria in 50-65 per cent of smear negative specimens with a specificity of 98 per cent. These assays have relatively high accuracy when performed on culture isolates. However, their sensitivity in HIV-TB co-infection is low with a higher risk of contamination37.
There are currently multiple rapid diagnostic technologies under evaluation, such as recombinant mycobacteriophages (Luciferase reporter phage-based test “Bronx-box”)38, and colorimetric culture system using TK medium culture system (Salubris, Inc, MA, USA)39. The introduction of these rapid and automated systems has increased the sensitivity of isolation of mycobacteria from clinical samples and has brought down the time required for positive culture substantially (9-10 days). Faster culture results in HIV-infected patients can result in faster implementation of evidence-based therapy.
Molecular techniques: Nucleic acid amplification testing (NAAT) provides a reliable way of increasing the specificity of diagnosis (ruling in disease), but sensitivity is variable, especially in paucibacillary disease. Commercial kits have the advantage of being well standardized and reproducible. However, concerns about their accuracy, reliability, their high cost, requirement for proper laboratory infrastructure and strict quality control procedures limit their applicability in resource-limited settings. A few modified or simplified versions of NAAT kits include loop-mediated isothermal amplification (LAMP), fluorescence in-situ hybridization (FISH) and line probe assays (LPA)40. A recent meta-analysis showed high sensitivity (>95%) and specificity (100%) for LPA when culture isolates were used41. The WHO has endorsed the use of line probe assays, which can detect both M. tuberculosis complex as well as isoniazid and rifampicin resistance on smear-positive sputum or on early positive growth on culture42. Line probe assays are being used in conjunction with culture in the Intermediate Reference Laboratories set up by the Revised National TB Control Programme (RNTCP) in India43.
GeneXpert-Rif: Recently, the WHO endorsed the use of GeneXpert-Rif for the rapid diagnosis of TB as well as rifampicin resistance among HIV-infected individuals with clinical suspicion of TB44. GeneXpert is a TB-specific automated, cartridge-based nucleic acid amplification assay, having fully integrated and automated sample preparation, amplification and detection using real-time PCR, providing results within 100 minutes. Clinical validation trials done in four distinctly diverse settings showed that 92.2 per cent of culture-positive patients were detected by a single direct Xpert MTB/RIF test (in comparison to the sensitivity of a single direct smear of 59.5%)45. Sensitivity of a single Xpert MTB/RIF test in smear-negative/culture-positive patients was 72.5 per cent which increased to 90.2 per cent when three samples were tested. Xpert MTB/RIF specificity was 99 per cent. HIV co-infection substantially decreased the sensitivity of microscopy (to 47%), but did not significantly affect Xpert MTB/RIF performance46. Xpert MTB/RIF detected rifampicin resistance with 99.1% sensitivity and excluded resistance with 100 per cent specificity47,48. Mean time to detection was <1 day for Xpert MTB/RIF, 1 day for microscopy, 17 days for liquid culture and >30 days for solid culture45,46. Thus this test seems to have the potential to complement the current reference standard of TB diagnostics and increase its overall sensitivity and speed. Further implementation research is required to determine the optimal level of the health care system where this system can be cost-effectively utilized.
Serological diagnosis of TB
(i) Detection of antibodies: Performance of various immune based tests to detect antibodies to M. tuberculosis antigens has been reviewed extensively40,49–51. None of the existing commercial serological tests show adequate sensitivity and specificity to be recommended for diagnostic use. Interestingly, the WHO recently made a negative recommendation against the use of serological tests for TB, based on data suggesting that these tests could neither replace sputum microscopy nor be used as an add-on test to rule out TB52. This has been endorsed by the RNTCP and is particularly relevant in India, where it is estimated that millions of these tests are performed in the private sector leading to a huge waste of resources53.
(ii) Detection of antigen: Attempts have been made to detect M. tuberculosis MPB-64 (TAUNS) antigens in peripheral blood, early secreted antigenic target 6 in the cerebrospinal fluid, lipoarabinomannan (LAM) in the urine, etc. by ELISA–based commercial assays54–56. Urine LAM assays tend to perform better in HIV-infected compared to HIV uninfected TB patients. The combination of urine lipoarabinomannan testing and sputum smear microscopy needs further evaluation for use in settings with a high HIV burden57.
Tuberculin skin test: Tuberculin skin test if positive provides evidence of TB infection. Many HIV infected patients will have a negative skin test despite TB infection or disease, due to anergy. “Two stage or booster test” is not a substitute to anergy testing; however, it may have some utility in detecting M.tuberculosis infection in anergic HIV-TB co-infected patients51. Tuberculin skin test underestimates the prevalence of latent tuberculosis in endemic countries; it requires trained health care staff to correctly perform the tests and accurately read the results, and also requires a second patient visit58. The test is neither useful to rule in disease nor in high TB prevalence settings to identify eligible individuals for prophylaxis.
Other diagnostic techniques
(i). Interferon-γ release assay (IGRA): This test can be used to diagnose latent TB infection and is particularly useful in profoundly ill patients and those with severe malnutrition. There are two in vitro tests to detect latent tuberculosis: QuantiFERON- TB Gold (Cellestis, USA) and the T SPOT-TB test (Oxford Immunotec, USA). Both use an enzyme- linked immunospot assay to quantify the number of peripheral blood mononuclear cells producing IFN- γ in response to tuberculosis-specific antigen stimulation (ESAT-6 and CFP10). Both assays give objective results, with sensitivity (as measured in patients with active tuberculosis) comparable to that of the tuberculin skin test, but are significantly more expensive59. IFN-γ assays do not differentiate between latent and active tuberculosis or between immune reconstitution inflammatory syndrome (IRIS) and failure. Studies suggest that IGRAs are ideal for serial testing because these can be repeated without boosting60–62. These are also unaffected by previous BCG vaccination and require fewer patient visits. However, WHO recommended against the use of IGRAs for diagnosis of active or latent TB, in resource-limited settings63.
(ii) Sensing volatile organic compounds (VOCs): from tuberculosis bacteria in exhaled air or urine or headspace gas over sputum or bacterial culture, measured using sensors or gas chromatography–mass spectroscopy is a promising new technique64,65. A study from India compared the VOCs present in the urine of TB patients with VOCs in the urine of healthy subjects, and found that infection with TB produces a distinct pattern of certain VOCs in much the same way that distinct fingerprint patterns can identify individuals65. Identification of these patterns sets the stage for developing a portable “electronic nose” that can quickly sniff urine samples to detect TB.
(iii) Electronic nose devices: Electronic nose (EN) devices are an array of chemical sensors combined with some sort of pattern recognition system, which are being investigated to differentiate between sputum samples from TB patients and non-TB patients66. The function of an EN is to mimic the mammalian olfactory system and produce a unique classification based on the volatile organic compounds in sputum.
Screening for HIV among individuals with active TB
With regard to detecting HIV among individuals with active TB, provider initiated HIV testing is recommended for all TB patients, as standard of care67. The rapid expansion of HIV testing for TB patients has been particularly encouraging in Africa, where only 4 per cent of TB patients were tested for HIV in 2004, but by 2008 that number had increased to 45 per cent4. In a pilot study of implementation of provider initiated HIV testing and counselling in India, HIV status was successfully ascertained for 70 per cent of TB patients and this was found to be feasible and acceptable68. The policy has been rapidly scaled up with over 60 per cent of TB patients being aware of their HIV status in 2011.
Preventing TB among HIV-infected individuals
The WHO currently recommends that all HIV-infected persons be screened for TB, and HIV-infected persons without active TB disease be evaluated for treatment of latent TB infection69. Two meta-analyses have shown that isoniazid (INH) taken daily for six months (6H) reduces the incidence of TB by over two-thirds among HIV-infected individuals70,71. The most widely recommended regimen for TB preventive therapy is isoniazid 300 mg daily for 6 months. WHO guidelines (2010) strongly recommend the use of 6H regimen, with 36H (3 years of isoniazid) being a conditional recommendation for countries to adopt depending on local needs and resources72. However, very few high-burden TB countries have routinely implemented isoniazid preventive therapy (IPT) for PLWH, because of concerns about how to exclude TB disease, fears about selection for INH-resistant M. tuberculosis (MTB) strains, and the absence of public health models for how to deliver this treatment73. Symptom screening can detect culture-confirmed TB disease with greater than 90 per cent sensitivity and 97 per cent negative predictive value. None of the studies of IPT have documented higher rates of drug-resistance solely attributable to IPT. Studies from India and South Africa found the 6-month isoniazid regimen to be effective, well tolerated with low rates of emergence of drug resistance74,75. The South African cohort study, which used three new prophylactic regimens, did not find any superiority over the control regimen of 6 months of isoniazid75. In contrast, a randomized double-blind, placebo-controlled trial in Botswana found that 36 months isoniazid prophylaxis was more effective for prevention of TB than was 6-month prophylaxis, chiefly benefitting those who were tuberculin skin test positive and those initiating ART76. The National AIDS Control Organization (NACO) intends to test the effectiveness and feasibility of the WHO IPT guidelines in ART clinics as a precursor for adopting this recommendation77.
Treatment of TB and HIV in co-infected individuals
The basic principles of treatment for HIV-associated TB are the same as for HIV uninfected individuals. Certain areas of uncertainty remain, including the regimen duration, dosage and frequency of administration of anti-TB drugs, optimal timing of initiation of ART and optimal anti-TB drug combination for patients on second line treatment.
(i) Anti-TB therapy: Currently, standard therapy consists of four drugs in the intensive phase for 2 months namely isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E) followed by H and R in the continuation phase of four months. In India, under RNTCP, a fully intermittent thrice-weekly regimen Category I (2EHRZ3/4HR3) is recommended for newly diagnosed TB. This regimen is reinforced with streptomycin (Sm) in the intensive phase and the total duration increased to eight months for retreatment cases - Category II (2EHRZS3/1EHRZ3/5EHR3)78. Rifampicin plays a key role in the treatment of HIV-associated TB because of its ability to destroy both intracellular and intermittently and slowly growing TB bacilli. Non-rifampicin containing regimens are associated with inferior cure rates and prolong the period of treatment79. A meta-analysis on the duration of rifampicin showed that recurrences were 2-3 times higher if rifampicin use was restricted to 2 months80.
For a long time, it was believed that longer regimens could potentially improve TB outcomes in HIV infected individuals. To determine the optimal duration of treatment, we conducted a randomized controlled clinical trial in the pre-HAART era, comparing the standard RNTCP 6 months regimen (2EHRZ3/4HR3) with a 9 month extended continuation phase regimen (2EHRZ3/7HR3). It was found that extension to 9 months did not improve the outcome at the end of treatment but bacteriological recurrences were significantly reduced during follow up. Irrespective of the length of the regimen, acquired rifampicin resistance was high among failures in the absence of ART81. Various studies have shown that there is an increased risk of failure with high probability of acquired rifampicin resistance, especially in ART naïve individuals receiving intermittent regimens80,82,83. This in addition to high recurrence among HIV-infected TB patients led WHO to recommend that daily TB regimens (at least in the initial intensive phase) should be preferred to intermittent regimens among HIV-infected TB patients84. Review of the primary evidence indicates very limited, low-quality information on intermittency, mostly from observational studies in the pre-antiretroviral era. DNA fingerprinting studies in India indicate that most of the recurrences and many of the failures resulted from exogenous re-infection, indicating poor infection control and high transmission, and not poor regimen efficacy85. Concurrent ART during TB treatment can turn the tide with high treatment success rates and low fatality, failure and recurrence rates. A subsequent trial conducted at the Tuberculosis Research Centre, Chennai, India (now National Institute for Research in Tuberculosis) compared the efficacy of two different once-daily ART regimens co-administered with ATT and found that the favourable outcome to TB treatment had increased to 93 from 83 per cent supporting the fact that ART is important for a favourable response to ATT86. Treatment outcomes among HIV-infected TB patients treated in the programme show low failure rates, but high case-fatality associated with lack of access to ART.
A recent meta-analysis on the treatment of HIV-associated TB, addressing the three key issues of dosing schedule, duration of therapy and influence of ART concluded that relapses were more common with regimens using rifampicin for less than 2 months, thrice-weekly regimens were associated with more failures and greater relapses and that ART reduced failures and relapses considerably. The main limitation of this meta- analysis was the paucity of adequately powered randomized trials in HIV-TB addressing the issue of dosing schedule87. Given the poor evidence for change and operational advantages of an intermittent regimen, this recommendation has not yet been implemented by large Asian countries including India and China until more evidence is generated through randomized controlled trials (RCT) to answer basic questions of schedule and duration of TB treatment among PLWH88. The National Institute for Research in Tuberculosis, Chennai, is currently addressing this issue through a RCT comparing daily vs. intermittent ATT in HIV-associated TB.
(ii) Anti-retroviral therapy: The WHO guidelines for management of HIV-infected TB patients in resource- limited settings recommend a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) along with one non-nucleoside reverse transcriptase inhibitor (NNRTI) for first line therapy89. In India, the NACO recommends a regimen containing zidovudine or stavudine along with lamivudine and efavirenz90. Rifamycins induce the cytochrome CYP-450 enzyme system in the liver and intestinal wall, thereby increasing the metabolism of protease inhibitors (PIs) and NNRTIs91. The effect is weaker with rifabutin than with rifampin. Rifampin is metabolized through deacetylation and is not itself affected by the CYP-3A system. When rifampicin and some antiretroviral drugs are given together, decreased trough levels of the latter may result, leading to therapeutic failure. Nevirapine levels are reduced by about 40–55 per cent, efavirenz by 18-25 per cent, delavaridine by 96 per cent and most PIs by 80-90 per cent92. It has been suggested that the dose of efavirenz be increased to 800 mg when administered along with rifampicin, but this may not be necessary in subjects weighing <50 kg93. Many studies have shown excellent virological and clinical outcomes with the use of efavirenz 600 mg along with ATT. In India, efavirenz is the preferred NNRTI for use in HIV-TB co-infected individuals at the standard dose of 600 mg once-daily90. However, in patients who cannot tolerate or have contraindications to efavirenz (e.g. psychiatric disturbances, pregnancy), a triple NRTI regimen or a combination of two NRTIs and nevirapine can be used. While once-daily nevirapine was shown to be inferior to efavirenz, with higher virological failure and mortality rates, this was probably due to the sub-therapeutic levels achieved during the lead-in period, in a situation of induced liver enzymes leading to faster metabolism of nevirapine86. Manosuthi et al94 demonstrated comparable efficacy with ATT and concomitantly administered twice-daily NVP and efavirenz. In their study comparing plasma levels of NVP and treatment outcomes between patients treated with rifampicin based and non-rifampicin based regimens, the level of NVP was low in the former compared to non-rifampicin containing regimens but the virological and immunological outcomes were similar95. An alternate strategy is to modify the anti-TB regimen with rifabutin replacing rifampicin - the dose of rifabutin recommended is 300 mg OD twice/thrice-weekly with nevirapine based ART91.
Many countries are now rolling out PI-based second line regimens for patients with first line therapy failure89. Rifampicin markedly reduces the level of unboosted PIs and hence is not recommended with nelfinavir, indinavir and atazanavir without boosting with ritonavir. High doses of ritonavir can be used with rifampicin but at the expense of increased hepatotoxicity. Recommended doses of PIs to be used with rifampicin include lopinavir/ritonavir at 400/400mg or saquinavir/ritonavir at 1000/100 mg BID. Alternatively, rifabutin which has less interaction with PIs can be used with dose modification. Rifabutin is usually given at a dose of 300 mg daily and this remains the same with NRTIs and saquinavir. The dose needs to be increased to 450-600 mg daily with EFV while it should be decreased to 150 mg thrice-weekly with amprenavir, ritonavir and lopinavir/ritonavir.87 Rifabutin is contraindicated in leucopenia and thrombocytopenia while high doses are known to cause uveitis. The PI currently recommended with rifabutin based ATT is lopinavir/ritonavir at the standard dose of 400/100 mg BID while the dosage of atazanavir/ritonavir is currently unknown.
(iii) Timing of ART & concomitant administration with ATT: It is currently recommended that HIV-infected individuals with TB receive prompt treatment for both diseases, irrespective of CD4+ T cell count, but the optimal /ideal timing of ART is still under debate89. The advantages of early ART include reduction in early mortality, improvement in cure rates, reduction in relapses, reduction in malabsorption secondarily preventing drug resistance to ATT and reduction in incidence of HIV-associated opportunistic infections other than TB. The disadvantages include cumulative toxicity, drug interactions of ART with rifampicin, limiting the choice of combinations and immune reconstitution inflammatory syndrome (IRIS). These can have an adverse effect on the long term adherence required for the lifelong therapy of ART. The significant toxicities of the two classes of drugs are mentioned in Table I.
Table I.
Adverse drug reactions with anti-TB (ATT) and antiretroviral (ART) drugs

Evidence from randomized controlled trials shows that early initiation of ART during TB treatment is associated with reduced mortality rates, especially in patients with profound immunosuppression (CD4<50 cells/μl). The CAMELIA trial conducted in Cambodia (median CD4 count 25 cells/μl) showed that mortality was reduced by 34 per cent when ART was initiated two weeks vs. eight weeks after onset of TB treatment96. The STRIDE and SAPIT trials similarly observed lower deaths and AIDS-related events with combined and earlier ART and TB treatment, especially among people with CD4 count <50 cells/μl97,98. Based on these three trials, it is believed that ART should be started as a matter of emergency in TB patients with CD4 less than 50 cells/μl and as early as possible in the remaining cases. Caution is needed in people living with HIV with TB meningitis as immediate ART was significantly associated with more severe adverse events when compared to initiation of ART two months after the start of TB treatment without survival benefit99. Our approach is to initiate ART within the first few weeks as soon as TB treatment is tolerated and the patient is stable, after treatment of active opportunistic infections. Table II gives the results of the available studies on timing of ART.
Table II.
Studies on timing of ART in HIV-infected TB patients on antituberculosis therapy
Tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS)
Transient worsening of symptoms and signs of tuberculosis or radiological deterioration after the initiation of ART, despite a reduction in HIV load (>1 log10 copies/μl) and immunological recovery, is known as IRIS. Consensus case definitions for TB-IRIS have recently been published by the International Network for the Study of HIV-associated IRIS (INSHI)102. Drug resistance and other opportunistic infections need to be ruled out before a diagnosis of IRIS is made. Hypercalcaemia is a unique feature of tuberculosis IRIS103. There are two types of IRIS presentation: unmasking of undiagnosed tuberculosis and a paradoxical deterioration of existing tuberculosis lesions or appearance of new lesions after initial improvement (Fig.A–F). Manifestations of IRIS include fever, lymph node enlargement, worsening respiratory symptoms and signs, cold abscess, psoas abscesses, and worsening central nervous system lesions (tuberculoma and meningitis)103,104. The incidence of tuberculosis IRIS ranges from 8 to 43 per cent and it can usually be managed by anti-inflammatory drugs and steroids, with death being a rare outcome and associated mostly with CNS IRIS105,110. Rarely, termination of ART is required. Risk factors for IRIS include lower CD4 cell count, higher viral load at start of treatment, rapidity of viral load decline; bacillary and antigen load (disseminated tuberculosis) at initiation, starting highly active ART closer to starting ATT, and genetic predisposition (HLA B-44)111–113. Although the pathophysiology of IRIS is incompletely understood, it is associated with an exuberant production of cytokines, such as IFN-γ or a lack of inhibitory immune responses114.
Fig.
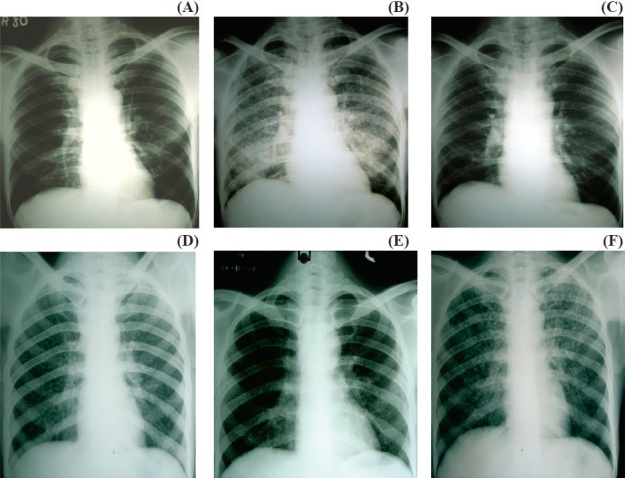
Types of IRIS: A, B and C shows unmasking IRIS; D, E, F shows paradoxical IRIS. (A) Asymptomatic patient when started on ART; (B) developed miliary TB after ART –unmasking reaction; (C) After ATT showing resolution; (D) Patient with miliary TB at baseline; (E) After 1 month of ATT treatment; (F) After ART showing flare up of lesion (paradoxical reaction).
Anti-TB drug resistance in HIV
There are limited data on TB drug resistance from India. In a study conducted among HIV/TB patients in Tamil Nadu, the prevalence of drug resistance among patients with no history of previous treatment was 13.2 per cent to INH, 2.4 per cent to EMB, 7.8 per cent to SM and 4.2 per cent to RMP, either alone or in combination with other anti-tuberculosis drugs115. A smaller cohort study revealed that the prevalence of drug resistant M. tuberculosis isolates among HIV seropositive tuberculosis patients was similar to that of HIV seronegative TB patients, indicating that HIV infection may not be associated with drug resistant tuberculosis116. The data from most HIV-endemic countries show that the prevalence of multidrug-resistant tuberculosis in HIV is similar to that in the general population; however, localized mini-epidemics tend to occur in settings where there is close congregation of HIV-infected persons. As individuals with HIV infection are more susceptible to new infections, the higher prevalence of MDR-TB in HIV co-infected persons in some settings could indicate more recent transmission of drug-resistant strains, compared to reactivation of infection acquired in the distant past in the non-HIV infected population. Although multidrug-resistant TB appears not to cause infection or disease more readily than drug-susceptible TB in HIV infected persons, delayed diagnosis, inadequate initial treatment, and prolonged infectiousness contribute to increased attack rates among contacts and high case fatality rates among patients117.
At least four effective drugs - including a fluoroquinolone, an injectable agent (capreomycin, kanamycin, or amikacin) and at least two agents from the remaining second-line anti-tuberculosis drug classes (cycloserine, thioamides like ethionamide or prothionamide, and p-aminosalicyclic acid)- along with pyrazinamide and EMB, if still sensitive, should be used. Therapy may be individualized on the basis of drug susceptibility test results; however, many countries use standardized regimens that are based on surveillance of antituberculosis drug resistance in the community117. DOTS plus regimen is currently followed in India comprising of kanamycin, levofloxacin, ethionamide, cycloserine, ethambutol, and pyrazinamide given for a period of 6-9 months daily in the intensive phase followed by all drugs except kanamycin and pyrazinamide during the continuation phase of 18 months, with dosages prescribed for 3 weight bands115. Shorter regimens and newer drugs are being tested but conclusive evidence is still to emerge. Extensively drug-resistant tuberculosis (XDRTB) is defined as multidrug-resistant TB plus resistance to any fluoroquinolone and one of the second-line antituberculosis injectable agents (kanamycin, amikacin, or capreomycin). Treatment options are extremely limited and challenging, with high frequencies of adverse events and death118.
TB-HIV co-ordination activities
In 2007, approximately 5 per cent of all diagnosed TB cases in India came from Integrated Counselling and Testing Centres (ICTCs), demonstrating that these are excellent sites for active TB case finding1. Further, the yield of cases was similar (approximately 20%) from HIV infected and uninfected clients. One of the programmatic limitations encountered is the lack of investigations for extrapulmonary TB at peripheral health facilities; another is the distance between Designated Microscopy Centres (DMCs) and ICTCs in some districts. The system works much better when both are located at the same site. In a pilot study on 4000 TB patients in two districts of Tamil Nadu, India, it was demonstrated that over two-thirds were willing to undergo an HIV test and the major barrier to acceptance was patients not perceiving themselves to be at risk119. If patients are counselled and explained the importance of having an HIV test, when they are diagnosed with TB, most will accept the test. Provider-initiated HIV testing and counselling (PITC) is internationally recommended for TB patients; the feasibility, effectiveness, and impact of this policy on the TB programme were evaluated in a study across two districts in south India considered to have generalized HIV epidemics, Tiruchirappalli (population 2.5 million)71 and Mysore (population 2.8 million). With implementation of PITC, HIV status was successfully ascertained for 70 per cent of TB patients. Previously undiagnosed HIV-infection was detected in 6.4 per cent of those TB patients newly tested, enabling referral for lifesaving anti-retroviral treatment. ART uptake, however, was poor, suggesting that PITC implementation should include measures to strengthen and support ART referral, evaluation, and initiation68. With increasing availability of ART across the country, diagnosis of HIV is beneficial to the individual as he/she can be referred to the nearest ART centre for evaluation and initiation of antiretroviral treatment, if indicated. TB clinics, therefore, form an important entry point for HIV diagnosis, care and support. Co-ordination and cross-talk between these two government health programmes are crucial not only to improve the outcome of HIV-infected TB patients but also to control the burden of tuberculosis in India.
It is concluded that the HIV pandemic presents a massive challenge to global TB control. The prevention of HIV and TB, the extension of WHO DOTS programs, and a focused effort to control HIV-related TB in areas of high HIV prevalence are matters of great urgency.
References
- 1.Global Report. UNAIDS repot on the global AIDS epidemic 2010. [accessed on September 20, 2011]. Available from: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf .
- 2.Corbett E, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Lawn S, Churchyard G. Epidemiology of HIV associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO report 2010. Global tuberculosis control. [accessed on September 20, 2011]. Available from: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf .
- 5.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2007;191:150–8. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 6.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300:423–30. doi: 10.1001/jama.300.4.423. [DOI] [PubMed] [Google Scholar]
- 7.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50:S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 8.Geneva: WHO; 2007. [accessed on 20 September 28, 2011]. Towards universal access by 2010: How WHO is working with countries to scale-up HIV prevention, treatment, care and support. Available from: https://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf . [Google Scholar]
- 9.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 10.Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol. 2005;5:819–26. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- 11.Geneva: World Health Organization; 2004. Interim policy on collaborative TB/HIV activities. (Report no: WHO/HTM/TB/ 2004.330) [Google Scholar]
- 12.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis. 2006;10:523–9. [PubMed] [Google Scholar]
- 13.Mohammed A, Ehrlich R, Wood R, Cilliers F, Maartens G. Screening for tuberculosis in adults with advanced HIV infection prior to preventive therapy. Int J Tuberc Lung Dis. 2004;8:792–5. [PubMed] [Google Scholar]
- 14.Shah S, Demissie M, Lambert L, Ahmed J, Leulseged S, Kebede T, et al. Intensified tuberculosis case finding among HIV-infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50:537–45. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 15.WHO (2007) Improving the diagnosis and treatment of smearnegative pulmonary and extrapulmonary tuberculosis among adults and adolescents: Recommendations for HIV-prevalent and resource-constrained settings. [accessed on September 20, 2011]. Available from: http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf .
- 16.Koole O, Thai S, Khun KE, Pe R, van Griensven J, Apers L, et al. Evaluation of the 2007 WHO guideline to improve the diagnosis of tuberculosis in ambulatory HIV-positive adults. PLoS ONE. 2011;6(4):e18502. doi: 10.1371/journal.pone.0018502. doi:10.1371/journal.pone.0018502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Getahun H, Kittikraisak W, Heiling CM, Corbett EL, Ayles H, Cain KP, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource constrained settings: Individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+T-lymphocytes count. Tuberc Lung Dis. 1995;76:518–21. doi: 10.1016/0962-8479(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 19.Swaminathan S, Narendran G, Menon PA, Padmapriyadarsini C, Arunkumar N, Sudharshanam NM, et al. Impact of HIV infection on radiographic features in patients with pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2007;49:133–6. [Google Scholar]
- 20.Churchyard G, Fielding KL, Lewis JJ, Chihota VN, Hanifa Y, Grant AD. Symptom and chest radiographic screening for infectious tuberculosis prior to starting isoniazid preventive therapy: yield and proportion missed at screening. AIDS. 2010;24:S19–27. doi: 10.1097/01.aids.0000391018.72542.46. 49. [DOI] [PubMed] [Google Scholar]
- 21.Hopewell P, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–25. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 22.Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, et al. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis. 2009;9:53. doi: 10.1186/1471-2334-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliot AM, Namaambo K, Allen BW, Luo N, Hayes RJ, Pobee JO, et al. Negative sputum smear results in HIV positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tubercle Lung Dis. 1993;74:191–4. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- 24.Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 25.Cattamanchi A, Davis JL, Pai M, Huang L, Hopewell PC, Steingart KR, et al. Does bleach processing increase the accuracy of sputum smear microscopy for diagnosing pulmonary tuberculosis? J Clin Microbiol. 2010;48:2433–9. doi: 10.1128/JCM.00208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664–74. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 27.Yassin MA, Cuevas LE. How many sputum smears are necessary for case finding in pulmonary tuberculosis? Trop Med Int Health. 2003;8:927–32. doi: 10.1046/j.1365-3156.2003.01107.x. [DOI] [PubMed] [Google Scholar]
- 28.Dorman S. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis. 2010;50(Suppl 3):173–7. doi: 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 29.FIND study trials at a glance: Primo Star iLED microscope. [accessed on September 10, 2011]. Available from: http:// www.finddiagnostics.org .
- 30.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–7. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 31.Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. Geneva: World Health Organization; 2007. World Health Organization. [Google Scholar]
- 32.Williams-Bouyer N, Yorke R, Lee HI, Woods GL. Comparison of the BACTEC MGIT 960 and ESP culture system II for growth and detection of mycobacteria. J Clin Microbiol. 2000;38:4167–70. doi: 10.1128/jcm.38.11.4167-4170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gil-Setas A, Torroba L, Fernandez JL, Martinez-Artola V, Olite J. Evaluation of the MB/BacT system compared with Middlebrook 7H11 and Lowenstein-Jensen media for detection and recovery of mycobacteria from clinical specimens. Clin Microbiol Infect. 2004;10:224–8. doi: 10.1111/j.1198-743x.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee JJ, Suo J, Lin CB, Wang JD, Lin TY, Tsai YC. Comparative evaluation of the BACTEC MGIT 960 system with solid medium for isolation of mycobacteria. Int J Tuberc Lung Dis. 2003;7:569–74. [PubMed] [Google Scholar]
- 35.Farnia P, Mohammadi F, Mirsaedi M, Zarife AZ, Tabatabee J, Bahadori K, et al. Application of oxidation reduction assay for monitoring treatment of patients with pulmonary tuberculosis. J Clin Microbiol. 2004;42:3324–5. doi: 10.1128/JCM.42.7.3324-3325.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore DA, Mendoza D, Gilman RH, Evans CA, Hollm Delgado MG, Guerra J, et al. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug resistant tuberculosis suitable for use in resource poor settings. J Clin Microbiol. 2004;42:4432–7. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalantri S, Pai M, Pascopella L, Riley L, Reingold A. Bacteriophage based tests for the detection of Mycobacterium tuberculosis in clinical specimens: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:59. doi: 10.1186/1471-2334-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai M, Minion J, Steingart K, Ramsay A. New and improved tuberculosis diagnostics: evidence, policy, practice, and impact. Curr Opin Pulm Med. 2010;16:271–84. doi: 10.1097/MCP.0b013e328338094f. [DOI] [PubMed] [Google Scholar]
- 39.Baylan O, Kisa O, Albay A, Doganci L. Evaluation of a new automated, rapid, colorimetric culture system using solid medium for laboratory diagnosis of tuberculosis and determination of anti-tuberculosis drug susceptibility. Int J Tuberc Lung Dis. 2004;8:772–7. [PubMed] [Google Scholar]
- 40.Chaudahary M, Gupta S, Khare S, Lal S. Diagnosis of tuberculosis in an era of HIV pandemic: A review of current status and future prospects. Indian J Med Micro. 2010;28:281–9. doi: 10.4103/0255-0857.71805. [DOI] [PubMed] [Google Scholar]
- 41.Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in mycobacterium tuberculosis: A systematic review and meta analysis. BMC Infect Dis. 2005;5:62. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Molecular line probe assays for rapid screening of patients at risk of multidrug resistant tuberculosis. [accessed on September 15, 2011]. Available from: http://www.who.int/tb/laboratory/lpa_policy.pdf .
- 43.Chauhan L.S. Status Report on RNTCP. Indian J Tuberc. 2009;56:151–3. [PubMed] [Google Scholar]
- 44.World Health Organization and STOP TB department. Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB. [accessed on October 5, 2011]. Available from: http://www.who.int/tb/laboratory/roadmap_xpert_mtb-rif.pdf .
- 45.Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay - a clinical validation study. PLoS One. 2011;6:e20458. doi: 10.1371/journal.pone.0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Rie A, Page-Shipp L, Scott L, Sanne I, Stevens W. Xpert(®) MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: hype or hope? Expert Rev Mol Diagn. 2010;10:937–46. doi: 10.1586/erm.10.67. [DOI] [PubMed] [Google Scholar]
- 47.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF Assay for the Rapid Diagnosis of Tuberculosis and detection of RIF-resistance in Pulmonary and Extra pulmonary Specimens. J Clin Microbiol. 2011;49:4138–41. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Eng J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gennaro ML. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000;30:S243–6. doi: 10.1086/313868. [DOI] [PubMed] [Google Scholar]
- 50.Chan ED, Heifets L, Iseman MD. Immunologic diagnosis of tuberculosis: A review. Tuberc Lung Dis. 2000;80:131–40. doi: 10.1054/tuld.2000.0243. [DOI] [PubMed] [Google Scholar]
- 51.Wanchu A. Advances in serology for diagnosing TB in the HIV infected. Indian J Chet Dis Allied Sci. 2005;47:31–7. [PubMed] [Google Scholar]
- 52.Morris K. WHO recommends against inaccurate tuberculosis tests. Lancet. 2011;377:113–4. doi: 10.1016/s0140-6736(11)60005-6. [DOI] [PubMed] [Google Scholar]
- 53.Dowdy DW, Steingart KR, Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001074. doi: 10.1371/journal.pmed.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khosla R, Dwivedi A, Sarin BC, Sehajpal PK. Peripheral blood based C-PCR assay for diagnosing extra-pulmonary tuberculosis. Indian J Exp Biol. 2009;47:447–53. [PubMed] [Google Scholar]
- 55.Kashyap RS, Ramteke SS, Morey SH, Purohit HJ, Taori GM, Daginawala HF. Diagnostic value of early secreted antigenic target-6 for the diagnosis of tuberculous meningitis patients. Infection. 2009;37:508–13. doi: 10.1007/s15010-009-8261-x. [DOI] [PubMed] [Google Scholar]
- 56.Mutetwa R, Boehme C, Dimairo M, Bandason T, Munyati SS, Mangwanya D, et al. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int J Tuberc Lung Dis. 2009;13:1253–9. [PMC free article] [PubMed] [Google Scholar]
- 57.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, McCallum J, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a high HIV prevalence setting. J Acquir Immune Defic Syndr. 2009;52:145–51. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swaminathan S, Subbaraman R, Venkatesan P, Subramanyam S, Kumar SR, Mayer KH, et al. Tuberculin skin test results in HIV-infected patients in India: Implications for latent tuberculosis treatment. Int J Tuberc Lung Dis. 2008;12:168–73. [PubMed] [Google Scholar]
- 59.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–76`. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 60.Liebeschuets S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with T-cell based assay: A prospective cohort study. Lancet. 2004;364:2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 61.Rangaka MX, Wilkinson KA, Seldone R, Van Cutsem G, Meintjes GA, Morroni C, et al. The effect of HIV-1 infection on T-cell based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 62.Pai M. Alternatives to the tuberculin skin test: interferon γ assays in the diagnosis of Mycobacterium tuberculosis infection. Indian J Med Micro. 2005;23:151–8. doi: 10.4103/0255-0857.16585. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. Strategic and Technical Advisory Group (STAG-TB) Report of the 10th Meeting. 2010. [accessed on September 30, 2011]. Available from: http:// www.who.int/tb/advisory_bodies/stag_tb_report_2010.pdf .
- 64.Philips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MPR, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis. 2010;90:145–51. doi: 10.1016/j.tube.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Banday KM, Pasikanti KK, Chan EC, Singla R, Rao KV, Chauhan VS, et al. Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal Chem. 2011;83:5526–34. doi: 10.1021/ac200265g. [DOI] [PubMed] [Google Scholar]
- 66.Arend Kolk, Michael Hoelscher, Leonard Maboko, Jung J, Kuijper S, Cauchi M, et al. Electronic-nose technology using sputum samples in diagnosis of patients with tuberculosis. J Clin Microbiol. 2010;48:4235–8. doi: 10.1128/JCM.00569-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guidance on provider initiated HIV testing and counseling in health facilities. Geneva: World Health Organization; 2007. World Health Organization UNJPoHA. [Google Scholar]
- 68.Vijay S, Swaminathan S, Vaidyanathan P, Thomas A, Chauhan LS, Kumar P, et al. Feasibility of Provider-Initiated HIV Testing and Counseling of tuberculosis patients under the TB Control Programme in two districts of South India. PLoS One. 4(11):e7899. doi: 10.1371/journal.pone.0007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Essential Prevention and Care Interventions for adults and adolescents living with HIV in resource limited settings. Geneva: World Health Organization; 2008. World Health Organization. [Google Scholar]
- 70.Wilkinson D, Squire SB, Garner P. Effect of preventive treatment for tuberculosis in adults infected with HIV: systematic review of randomized placebo controlled trials. BMJ. 1998;317:625–9. doi: 10.1136/bmj.317.7159.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bucher H, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–7. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 72.Geneva: World Health Organization; 2011. [accessed on August 2, 2011]. Guidelines for intensified tuberculosis case finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. Stop TB department. Available from: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf . [Google Scholar]
- 73.Churchyard GJ, Scano F, Geant AD, Chaisson RE. Tuberculosis preventive therapy in the era of HIV infection: overview and research priorities. J Infect Dis. 2007;196:S52–62. doi: 10.1086/518662. [DOI] [PubMed] [Google Scholar]
- 74.Swaminathan S, Menon PA, Narendran G, Venkatesan P, Ramesh Kumar S, Ranjani Ramachandran, et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: A Randomized clinical trial. Unpublished Data. doi: 10.1371/journal.pone.0047400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomized, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 77.NACO Annual Report 2010-2011. [accessed on September 30, 2011]. Available from: http://www.nacoonline.org/upload/REPORTS/NACO%20Annual%20Report%202010-11.pdf .
- 78.Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. TB India 2008 - RNTCP status report. [accessed on October 2, 2011]. Available from; http://www.tbcindia.org/pdfs/TB-India-2008.pdf .
- 79.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomized trial. Lancet. 2004;364:1244–51. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 80.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effect of human immunodeficiency virus infection on recurrences of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–12. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 81.Swaminathan S, Narendran G, Venkatesan P, Iliayas S, Santhanakrishnan R, Menon PA, et al. Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: a randomized clinical trial. Am J Respir Crit Care Med. 2010;181:743–51. doi: 10.1164/rccm.200903-0439OC. [DOI] [PubMed] [Google Scholar]
- 82.Burman W, Benator D, Vernon A, Khan A, Jones B, Silva C, et al. Tuberculosis Trials Consortium. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173:350–6. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 83.Nahid P, Gonzalez LC, Rudoy I, de Jong BC, Unger A, Kawamura LM, et al. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO Library Cataloguing-in-Publication Data: Treatment of tuberculosis: guidelines. 4th ed. accessed on September 28, 2011 from WHO/HTM/TB/2009.420. [Google Scholar]
- 85.Narayanan S, Swaminathan S, Supply P, Shanmugam S, Narendran G, Hari L, et al. Impact of HIV infection on the recurrence of Tuberculosis in South India. J Infect Dis. 2010;201:691–703. doi: 10.1086/650528. [DOI] [PubMed] [Google Scholar]
- 86.Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV associated tuberculosis: A randomized clinical trial. Clin Infect Dis. 2011;53:716–24. doi: 10.1093/cid/cir447. [DOI] [PubMed] [Google Scholar]
- 87.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, et al. Treatment of active tuberculosis in HIV-coinfected patients: A systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–99. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 88.Swaminathan S, Padmapriyadarsini C, Ponnuraja C, Ranjani R, Sriram S, Kumar RS, et al. Tuberculosis treatment outcomes among patients treated with a short-course intermittent anti-TB regimen and either once-daily Nevirapine or Efavirenz based antiretroviral therapy: a randomized clinical trial. Abstract presented in 41st Union World Conference on Lung Health, Berlin, November 2011. 2010 Clinical trials.gov number NCT00332306. [Google Scholar]
- 89.Antiretroviral therapy for HIV infection in adults and adolescents - World Health organization. 2010. [accessed on September 12, 2011]. Available from: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf . [PubMed]
- 90.Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents Including Post-exposure Prophylaxis –National Aids Control organization. 2007. [accessed on September 12, 2011]. Available from: http://upaidscontrol.up.nic.in/ART%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents%20Including%20Post-exposure.pdf .
- 91.de Jong BC, Israelski DM, Corbett EL, Small PM. Clinical management of tuberculosis in the context of HIV infection. Annu Rev Med. 2004;55:283–301. doi: 10.1146/annurev.med.55.091902.103753. [DOI] [PubMed] [Google Scholar]
- 92.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196:S63–S75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 93.Manosuthi W, Sungkanuparph S, Thakkinstian A, Vibhagool A, Kiertiburanakul S, Rattanasiri S, et al. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS. 2005;19:148. doi: 10.1097/01.aids.0000183630.27665.30. 1486. [DOI] [PubMed] [Google Scholar]
- 94.Manosuthi W, Mankatitham W, Lueangniyomkul A, Chimsuntorn S, Sungkanuparph S. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received Rifampicin. HIV Med. 2008;9:294–9. doi: 10.1111/j.1468-1293.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- 95.Manosuthi W, Sungkanuparph S, Thakkinstian A, Rattanasiri S, Chaovavanich A, Prasithsirikul W, et al. Plasma nevirapine levels and 24-week efficacy in HIV-infected patients receiving nevirapine-based highly active antiretroviral therapy with or without Rifampicin. Clin Infect Dis. 2006;43:253–5. doi: 10.1086/505210. [DOI] [PubMed] [Google Scholar]
- 96.Blanc F, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Havlir D, Ive P, Kendall M, Luetkemeyer A, Swindells S, Kumwenda J, et al. Boston (MA): 2011. A5521 Team International Randomized Trial of Immediate vs Early ART in HIV+ Patients Treated for TB: ACTG 5221 STRIDE Study. Abstract presented at the XVIII Conference on Retroviruses and Opportunistic Infections. Feb 27-Mar 2. [Google Scholar]
- 98.Abdool Karim S, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;25:697–70. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torok ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis. 2011;52:1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.World Health Organization. An evaluation of the impact of early initiation of Highly Active Anti-Retroviral Therapy (HAART) on Tuberculosis (TB) treatment outcomes for TB patients co-infected with Human Immunodeficiency Virus (HIV) [ISRCTN77861053] Current Controlled Trials [online] [accessed on October 9, 2011]. Available from: http://www.controlledtrials.com .
- 101.Shao HJ, Crump JA, Ramadhani HO, Uiso LO, Ole-Nguyaine S, Moon AM, et al. Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Res Hum Retroviruses. 2009;25:1277–85. doi: 10.1089/aid.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. International network for the study of HIV-associated IRIS.Tuberculosis associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 104.Robertson JC, Fichtenbaum CJ. Case on the Web: diagnosis and management of the immune reconstitution syndrome in HIV-infected patients. International AIDS Society -USA. [accessed on September 30, 2011]. Web site Available from: http://www.iasusa.org/cow . Presentation 37 .
- 105.Kumar RS, Narendran G, Patrawalla P, Menon PA, Mayer K, Swaminathan S. Immune reconstitution inflammatory syndrome in HIV infected patients with and without prior tuberculosis. Unpublished data. doi: 10.1258/ijsa.2009.009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawn SD, Myer L, Beckker LG, Wood R. Tuberculosis associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–73. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 107.Ratnam I, Chiu C, Kandal NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–27. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 108.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 109.Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo Mvondo D, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–12. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 110.Breen RA, Smith CJ, Bettinson H, Dart S, Bannister B, Johnson MA, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704–7. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robertson J, Meier M, Wall J, Ying J, Fitctenbaum CJ. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639–46. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]
- 112.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46:456–62. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 113.Price P, Morahan G, Huang D, Stone E, Cheong KY, Castley A, et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–7. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 114.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, et al. Explosion of tuberculin specificTh1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 115.Swaminathan S, Paramasivan CN, Ponnuraja C, Iliayas S, Rajasekaran S, Narayanan PR. Anti-tuberculosis drug resistance tuberculosis in South India. Int J Tuberc Lung Dis. 2005;9:896–900. [PubMed] [Google Scholar]
- 116.Pereira M, Tripathy S, Inamdar V, Ramesh K, Bhavsar M, Date A, et al. Drug resistance pattern of Mycobacterium tuberculosis in seropositive and seronegative HIV-TB patients in Pune, India. Indian Med J Res. 2005;121:235–9. [PubMed] [Google Scholar]
- 117.Wells CD, Cegielski P, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196:S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 118.Revised National Tuberculosis Control Programme-DOTS plus guidelines 2010. [accessed on October 13, 2011]. Available from: http://www.tbcindia.org/pdfs/DOTS_Plus_Guidelines_Jan2010.pdf .
- 119.Thomas BE, Ramachandran R, Anitha S, Swaminathan S. Feasibility of routine HIV testing among TB patients through a Voluntary, Counseling and Testing Centre (VCTC) Int J Tuberc Lung Dis. 2007;11:1296–301. [PubMed] [Google Scholar]


