Abstract
Human immunodeficiency virus (HIV), causative agent of acquired immunodeficiency syndrome (AIDS), is a global health concern. To control its transmission, safe sex has been proposed as one of the strategies. Microbicides- intravaginal/intrarectal topical formulations of anti-HIV agents have also been proposed to prevent HIV transmission. Microbicides would provide protection by directly inactivating HIV or preventing the attachment, entry or replication of HIV in susceptible target cells as well as their dissemination from target cells present in semen or the host cells lining the vaginal/rectal wall to other migratory cells. Microbicides must be safe, effective following vaginal or rectal administration, and should cause minimal or no genital symptoms or inflammations following long-term repeated usage. However, a safe and efficacious anti-HIV microbicide is not yet available despite the fact that more than 60 candidate agents have been identified to have in vitro activity against HIV, several of which have advanced to clinical testing. Nonetheless, proof-of-concept of microbicides has been established based on the results of recent CAPRISA 004 clinical trials. In this article, the trends and challenges in the development of effective and safe microbicides to combat HIV transmission are reviewed.
Keywords: HIV, intravaginal, microbicides, prevention
Introduction
Globally there are approximately 39.4 million adults and children suffering from HIV infection and, approximately 7,000 new cases of HIV infection are reported worldwide everyday, among whom 50 per cent are women. The surveillance programme run by National AIDS Control Organization (NACO) and latest UNAIDS estimate revealed that approximately 2.5 million people in India are seropositive for HIV, which accounts for roughly half of Asia's HIV prevalence1. In majority of the cases, the HIV infection occurs through heterosexual route, however, the transmission of HIV from the infected mother to the child is also on rise. To prevent HIV transmission through heterosexual route, use of the condoms and limiting the numbers of sexual partners are endorsed by WHO/UNAIDS. Male and female condoms provide an effective means of preventing HIV infection, however, women fail to negotiate their use. Male circumcision is suggested to have an important role in reducing HIV transmission through sexual route2,3. Treatment option for HIV infection with highly active anti-retroviral drugs has expanded during the past few years4. In patients treated with a highly active antiretroviral therapy (HAART)-triple-drug cocktail of two nucleoside inhibitors and one protease inhibitor, blood levels of virus can be reduced below the detectable level (< 50 copies of viral RNA per milliliter of plasma)5. Study of pre-exposure prophylaxis (PrEP) to prevent HIV infection shows an estimated efficacy of combination drug, emtricitabine and tenofovir disoproxil fumarate (TDF), to be 44 per cent against HIV infection6.
Several groups are engaged in developing vaccines to prevent HIV transmission, which are at different stages of development and clinical trials. It has been shown that HIV specific T-cell responses, both CD4 helper and CD8 killer T cells, are important in controlling HIV infection7. Several vaccines using a variety of vectors such as canarypox, adenovirus serotype 5 (Ad5); adeno-associated virus, and modified vaccinia virus Ankara strain, incorporating variety of HIV proteins or multiple mapped CD8 T-cell epitopes have been evaluated in humans8,9. Unfortunately, the HIV-specific CD8 T-cell responses are generated only in small percentage of the vaccine recipients. Ad5 based vaccine generated CD8 T-cell responses in higher percentage (60-70%) of the recipients, who otherwise did not have circulating neutralizing antibody titres against Ad510. However, in developing world a high percentage (~90%) of population has circulating antibodies against Ad5, and hence its immunogenicity in this context needs to be established. DNA vaccines for HIV have also been proposed, primarily as prime-boost strategy, whereby priming is done with DNA vaccine followed by one of the vector based vaccine11. Two large efficacy trials with AIDSVAX (made by VaxGen, San Francisco, USA) showed failure to protect against HIV infection12. A combination of the above vaccine with the Sanofi-Pasteur's ALVAC canarypox/HIV vaccine elicited protective response for a short period13.
One of the promising options for prevention of HIV transmission through hetero- or homo-sexual routes is to apply topically to the vaginal or rectal surface, a cream, gel, lubricant or even insert a tablet, which have incorporated anti-HIV compound(s) before sex14. These are generically termed as microbicides and are self-administered prophylactic agents. Due to the greater vulnerability of women who often are unable to adjudicate use of condoms by their male partners, microbicides may help to decrease the risk of sexual acquisition of HIV. Even after a safe and effective vaccine is discovered, vaccines and microbicides will have different, complementary roles to play in an integrated, multi-faceted global HIV prevention strategy.
Modes and Mechanisms of HIV Transmission
To develop microbicides, understanding the physiology of mucosal tissue underlining the female and male reproductive tracts is essential. Stratified epithelial cells cover the vagina, outer cervix, and foreskin of penis, whereas the upper cervix and rectum are lined with a single layer of columnar epithelium. In stratified epithelial lining of vagina, HIV may spread through the dendritic cells (DCs) present in the sub-mucosal layer15,16. These dendritic cells capture viruses via mannose-dependent C-type lectin receptors (CLRs), such as mannose receptor (CD206), dendritic-cell C-specific intercellular adhesion molecular-3-grabbing non-integrin DC-SIGN (CD209) and langerin (CD207). In addition, CCR5-dependent pathway that binds HIV through gp120 is also involved17. Later these dendritic cells move to nearby lymph nodes and transmit HIV to other cells including CD4+ T cells. Abrasions in the mucosal epithelium due to physical injury or infections such as chlamydia or herpes may lead to immigration of the infected donor cells or free virions to mucosal stroma and then transported to local lymph nodes or into the blood circulation leading to direct transmission of virus to stromal DCs, T cells and macrophages18. However, in upper cervix, HIV directly attaches to the columnar epithelium, internalizes and passes to other side of mucosal lining by a process known as ‘transcytosis’19.
The establishment of infection at the portal of entry and timing of dissemination might also be affected by the number and types of cells that are initially infected. During male-to-female sexual transmission of HIV, following ejaculation, HIV is believed to remain infectious in semen for several hours, although the precise duration is not known20. During this time, diffusion is likely to be a principal mechanism of HIV transport from semen to vaginal epithelial surface. HIV infected cells present in the semen can also cross epithelial barrier leading to transmission of HIV infection (transmigration). The susceptibility to HIV can be intensified in the presence of an ulcerative sexually transmitted infection (STI), either through mucosal disruption or through an increase in the presence or activation of cells susceptible to HIV21. Non-ulcerative infections have also been linked to increase susceptibility to HIV infection by triggering the pro-inflammatory responses that enhance viral replication or by proliferation of HIV susceptible cells22.
In addition to CD4+ T cells, DCs and macrophages, small Ki67 negative T cells (activated cells returning to resting state) which greatly outnumber these cells in the healthy mucosa, can also be infected with HIV23. The Ki67 negative T cells maintain low level of viral replication and may play a role in sustaining infection, whereas infected activated CD4+ T cells that have high viral replication may be more efficient in dissemination of viral infection.
Rationale for Development of Microbicides
Based on the recent epidemiological data, women comprise more than half of new HIV infections and accordingly, there is a need for a woman-controlled method to prevent sexually transmitted HIV infection1. Topical microbicides that can be self-administered, are being developed as a subset of pre-exposure prophylaxis strategy that together with vaccines might significantly reduces the HIV infection. Microbicides will also be useful for prevention of HIV infection in women having multiple sex partners. It will be challenging to develop user-friendly delivery methods and slow release devices such as intravaginal rings to make microbicide effective for a longer period. These microbicides can be formulated as semi-solid gels, creams, vaginal films and tablets. For microbicide development, biodegradable nanoparticle drug delivery system is also being investigated24. Acceptability studies in several countries such as Brazil, India, South Africa, Thailand, USA, and Zimbabwe have revealed that women have expressed in general very positive attitude towards the concept and use of microbicide products in both contraceptive and non-contraceptive formulations25–27. Interestingly, initial indications suggest that the men are also supportive of the idea of microbicides28. A practical microbicide must be not only effective, safe, and user-friendly but also economically affordable in the developing world. Topical microbicides are grouped into five classes of agents, based on their mode and site of action29.
1. Surfactants/membrane disruptors based microbicides
Earliest compounds that have been clinically evaluated as topical microbicides are the surfactants. These agents non-specifically disrupt the membranes, offering contraceptive properties and activity against a wide range of potential STI pathogens. Nonoxynol-9 (N9), an anionic surfactant initially developed in the 1960s as a spermicide, was the first vaginal microbicide to be studied30. In vitro antiviral activity of N9 was first recognized in 198531. It has a virucidal action through disrupting the viral envelope. In a vaginal challenge macaque model, administration of N9 led to reduction in the transmission of simian immunodeficiency virus32. However, initial experience in placebo-controlled field studies by Kreiss et al33 in Kenya and Roddy et al34 among commercial sex workers in Cameroon suggested that N9 may be associated with local vaginal toxicity, including ulcerations, without apparent evidence of efficacy against HIV transmission. Later on, Phase III multi-centric randomized placebo-controlled trial of N9 (COL 1492), undertaken by the United Nations Joint Programme on HIV/AIDS, showed that N9 had no efficacy in preventing HIV transmission35. Indeed, the transmission rate was marginally higher in the N9 treated group, and it was considered that this might be related to the local vaginal toxicity, including ulcerations and increased CD4+ T lymphocytes and macrophages trafficking at the site of application34,36. The experience with N-9 led to a greater scrutiny of safety studies of microbicides before the commencement of larger clinical trials. Another candidate in this class, C31G (Savvy, Cellegy Pharmaceuticals, Quakertown, PA, USA), consisting of cetylbetaine and myristamine oxide, has shown in vitro safety and broad-spectrum activity against bacteria including Chlamydia trachomatis, and viruses HSV, and HIV37,38. However, C31G failed to demonstrate efficacy and confirmed this surfactant might not be a good microbicide candidate39. Sodium lauryl sulphate (Invisible Condom, Universite Laval, Quebec, Canada) is another surfactant compound that has been shown to disrupt both non-enveloped and enveloped viruses40. The extended safety study showed that the Invisible Condom gel formulations were well tolerated and acceptable and hence further phases of its clinical development as a potential microbicide to prevent sexual transmission of HIV are warranted41.
2. Vaginal milieu protector based microbicides
Vaginal milieu protectors that work to maintain, restore, or enhance the natural protective mechanisms within the vaginal canal are the second broad class of microbicides under development. A pH between 4·0 to 5·8 has been shown to inactivate HIV. A polyacrylic acid Carbopol 974P (BufferGel, ReProtect, Baltimore, MD, USA) that buffers twice its volume of semen to a pH of 5.0 or less has been shown to be spermicidal42, virucidal in vitro to HIV43, HSV, C. trachomatis44 and human papilloma virus (HPV)45. However, during clinical trials, BufferGel was found to have no effect on preventing HIV infection46,47.
Acidform (Amphora, Instead Inc, Dallas, TX, USA) is currently approved as a sexual lubricant gel. Its acid-buffering and bioadhesive properties make it a suitable candidate for microbicide development. The phase-I study revealed that Acidform was well tolerated when used alone, but produced vaginal irritation when combined with N-948. Naturally occurring acidic compounds such as lime juice have been used in certain societies for contraception and have also been found to be effective against HIV infection. However, clinical trials of formulations based on lime juice have shown toxicity49.
Microbicide formulations based on "probiotic" strategy are also being developed to protect the vaginal milieu. Colonization of exogenous lactobacilli has been shown to correlate with decreased HIV proliferation50,51. Natural human vaginal isolates of Lactobacillus have also been bioengineered (live microbicides), to express proteins that bind to HIV and block either viral-host cell fusion or viral entry into the host cells. Some of the proteins expressed through this are CD452, a derivative of gp4153, cyanovirin54, RANTES and a CCR5 antagonist analogue55. These live microbicides are in preclinical development stage and seem to be a promising approach.
3. Microbicides based on inhibition of HIV entry in the host cell
This class of microbicide agents, block the attachment of HIV-1 to the host cells, the fusion of virus and host-cell membranes, or the entry of HIV-1 into the host cells. A variety of anionic polymers that target the adsorption and fusion processes of the virus infection are under investigation56. Through their negative charge, anionic polymers interact with HIV's viral envelope proteins and interfere with the attachment of HIV to target cells57. CCR5 is the most important co-receptor for macrophage-tropic viral strains, and predominates in the early stages of viral transmission58. The CCR5 inhibitor, PSC-RANTES (recombinant chemokines analogues), exhibits in vitro antiviral activity against all HIV clades and inhibits HIV-1 infection of Langerhans cells59. CCR5 inhibitors protect against infection in the rhesus vaginal challenge model and are amenable to low-cost production, represent promising new additions to the microbicides pipeline60. CMPD167, a cyclopentane-based compound has been shown to protect macaques from vaginal challenge by the CCR5-using virus SHIV-162P3, and act synergistically with other cell-entry inhibitors61. Maraviroc (MVC), a small-molecule drug that binds the CCR5 co-receptor and impedes HIV-1 entry into cells, has been evaluated as a vaginal microbicide with a stringent model that involves challenge of rhesus macaques with a high-dose of a CCR5-using virus, SHIV-162P3 and provided a dose-dependent protection62.
It is likely that compounds, which only block co-receptors, may provide incomplete protection and that infection via migratory DCs may still proceed63. For example, it was observed that AMD3100 and TAK779 (both CCR5-inhibitors) together inhibited infection by an R5X4 tropic virus in the phytohaemaglutinin-stimulated cervical explants tissue culture system. However, when cells migrating from these explants were cultured with indicator cells (PM1), HIV could be found. Thus, TAK-779 and AMP3100 provide incomplete protection, and infection by migratory DCs may still take place. Inclusion of MAb b12 (inhibit most HIV-1 strains tested in vitro)64 and CD4-IgG265 both of which target gp120, reduced infection of T cells and migratory DCs by more than 95 per cent in activated cervical explant tissues. Hence, the more potent microbicides need to simultaneously block the pathways that lead to localized infection as well as viral dissemination.
Interaction with the co-receptor triggers a rearrangement of the transmembrane subunit of the envelope glycoprotein, gp41, which leads to fusion between the virus and cell membrane. Hence, inhibiting the gp41 mediated viral-cell fusion is also one of the promising approaches. A proof of concept for this approach has been established with the use of T20 peptide, which possesses doubtless potent antiviral activity, but has two critical drawbacks: high cost of production and short half-life in vivo66. C52L, a peptide, which also inhibits gp41-mediated viral-cell fusion67, is a potent and broad inhibitor of viral infection and remains fully active against T-20-resistant HIV-168 and its efficacy was confirmed against simian immunodeficiency virus69. Another fusion inhibitor is cyanovirin-N, a lectin purified from cyanobacterium Nostoc ellipsosporum, which irreversibly inactivates diverse HIV strains and has undergone early clinical testing, as a topical microbicide. Different formulations of cyanovirin-N, including those expressed by lactobacilli, are under development70. Carrageenan, a sulphated polysaccharide formulation (PC-515; FMC Biopolymer, Rockland, ME; Carraguard/R515, Population Council, New York, NY, USA), a vaginal microbicide, is basically derived from a red seaweed, Gigartina skottsbergii71. It blocks HIV-1 infection of cervical epithelial cells and trafficking of HIV-infected macrophages from the vagina to lymph nodes by binding the HIV-1 envelope72. Results suggested that Carraguard gel was safe but HIV infections occurred at a similar rate in the Carraguard and placebo groups73,74. Ushercell (Cellulose sulphate) developed by Polydex Pharmaceuticals (Toronto, Canada and Topical Prevention of Conception and Disease, Chicago, IL, USA) is a contraceptive compound possessing in vitro activity against Niesseria gonorrhoeae, C. trachomatis, HPV, and Gardnerella vaginalis75–77. Cellulose sulphate acts by binding to the V3 loop of the gp120 HIV-1 envelope, and can inhibit both CXCR4 and CCR5-tropic virus78. Clinical trials indicated that it has no beneficial effect in curtailing the risk of HIV transmission, gonorrhoea or chlamydial infection but may have an increased risk of HIV infection, possibly owing to toxicity of the active ingredient or the hyperosmolar gel vehicle (iso-osmolar placebo)79,80.
Cellulose acetate phthalate (CAP) blocks gp120 and gp41 binding sites and has shown virucidal activity against HIV-1, HSV-1 and HSV-281. CAP blocks infection by both cell free and cell associated HIV as well as blocks CXCR4 and CCR5-tropic virus types in tissue explant82. Its preclinical evaluation showed neither any increase in the production of proinflammatory mediators during or after exposure, nor did it modify the epithelial resistance to leukocyte83. The micronised form of CAP (~1μm diameter) leads to disintegration and loss of infectivity of HIV-1 and its lack of systemic absorption increases its bioavailability to the topical surface84. However, due to heavy vaginal discharge in all the recipients of CAP based microbicide, the clinical trials were halted85. PRO2000 (Naphthalene sulphonate; Indevus Pharmaceuticals, Lexington, MA, USA) is a sulphonated polymer that interacts not only with viral gp120 but also with CD4 and CXCR4 receptors on the cell surface and interferes with virus attachment to and/or fusion with CD4+ T cells86. It possesses in vitro activity against both X4 and R5 strains of HIV, C. trachomatis, N. gonorrhoeae, and HSV. The HPTN035 study found that PRO 2000 gel (0.5% dose) reduced a woman's risk of HIV infection by 30 per cent over the course of two years, but this effect did not reach the level of statistical significance87. However, the MDP-301 trials demonstrated conclusively that PRO2000 was not effective in preventing HIV infection46,88.
Another promising approach is the use of dendrimers entry inhibitors as microbicides. Dendrimers are highly branched macromolecules synthesized from a polyfunctional core, with interior branches and terminal surface groups adapted to specific targets. In vitro and in vivo studies on selected compounds have shown that dendrimers are potent inhibitors of a range of sexually transmitted infections. The first dendrimer to be formulated as a microbicide gel and tested clinically, SPL7013 (Vivagel, Starpharma Holdings Ltd, Melbourne, Australia), a lysine-based dendrimer with naphthalene disulphonic acid surface groups, can be engineered with optimized potency against HIV and HSV89. In phase-I clinical trial, it was found to be safe and well tolerated in healthy women, with no evidence of systemic toxicity or absorption90.
4. Microbicides that act after entry of HIV in the host cells
Once in the intracellular environment, entry inhibitors cannot block the virus. It can only be stopped from productive replication and release through inhibition of the virus-encoded reverse transcriptase (RT) or integrase (IN). The HIV RT is a well-exploited target for therapeutic intervention. With the success of anti-retroviral therapy in the treatment of HIV infection, as well as in the prevention of mother-to-child HIV transmission, interest has grown in using these targeted drugs for prevention of the sexual transmission of HIV. RT inhibitors bind the HIV-1 reverse transcriptase enzyme and block the conversion of viral RNA into DNA-effectively halting viral replication. Tenofovir (nucleoside reverse transcriptase inhibitor, NRTI), is the first anti-retroviral drug to safely demonstrate in animal models both pre-exposure and post-exposure prophylaxis as proof-of-concept against the sexual transmission of simian immunodeficiency virus (SIV)91. Based on the in vitro and in vivo efficacy studies, this compound became the first antiretroviral drug to be assessed as a vaginal microbicide in clinical trials92. CAPRISA 004 study, conducted by Centre for the AIDS Programme of Research in South Africa for the first time indicated that pre-exposure prophylaxis with tenofovir has been found to be successful in prevention against HIV infection93. The success of this study has buoyed the microbicide field, providing the first proof of principle that vaginal microbicide gels can successfully function, in a clinical trial setting, to reduce the rate of HIV transmission. Specifically the study found that tenofovir gel users were 39 per cent less likely to become infected with HIV than women who received a placebo gel. For women who used the tenofovir gel correctly more than 80 per cent of the time, HIV infection was 54 per cent less likely than the placebo group94. Researchers found that the tenofovir gel also reduced the rate of new genital herpes infections. These results, combined with another ongoing trial called the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study MTN-003, which is testing daily tenofovir gel use, regardless of when participants have sex, may enable development of new strategies for microbicide application95.
The HIV-1-specific non-nucleoside reverse transcriptase inhibitors (NNRTIs) as compared to NRTIs have the advantage of a very high therapeutic index and acting directly (without metabolisation) against the virus replication. Two NNRTIs, TMC120 and UC781 are most advanced in clinical trials as potential topical microbicides and usually require at least two mutations before viral resistance occurs96,97. These small molecules with low solubility in water or physiological fluids have the potential to form a long-lasting “depot” at sites susceptible to cervico-vaginal HIV infection. This could allow application of the microbicide well before sexual intercourse98. However, extremely poor water solubility of UC781 leads to a great challenge for its formulation development. A beta-cyclodextrin (beta-CD) based drug delivery system is being developed to enhance the aqueous solubility of UC78199.
5. Microbicides based on inhibitors with unknown mechanism of action
Praneem is a combination of extracts prepared from the neem tree (Azadirachta indica), saponins from Sapindus mukorossi, and menthe citrate oil, has shown wide-spectrum antimicrobial activity against reproductive tract infections, including anti-retroviral properties with an unknown mechanism of action100. It has undergone phase-I and -II safety and acceptability studies101–103. Another formulation in this category is a polyherbal cream (Basant) proposed by Talwar's group104, which has diferuloylmethane (curcumin), purified extracts of Emblica officinalis (Amla), purified saponins from Sapindus mukorossi, Aloe vera and rose water along with pharmacopoeially approved excipients and preservatives. Basant has the potential of regressing vulvovaginal candidiasis and preventing N. gonorrhoeae, HIV and HPV infections104.
Current status and challenges in microbicide development
Development of microbicides against HIV infection is on an incremental progression path. Different candidate microbicides at various stages of clinical trials as per the AIDS Vaccine Advocacy Coalition are summarized in the Table105. There are number of obstacles to overcome for the successful development of microbicides. These includes but not limited to (i) understanding of various responses of the host as well as the pathogen when HIV comes in contact with mucosal surface, (ii) developing compounds that can thwart HIV entry and infection, and (iii) preparation of the formulation based on these compounds in ways that promote their extensive and regular use. Understanding the likely toxicity of the ingredients of the microbicide on mucosal lining of the reproductive tract with respect to proinflammatory cytokines secretion and other non favourable responses has become relevant as these may directly impact the HIV infection106. Several microbicide trials, which have been initiated on the basis of the successful in vitro anti-HIV efficacy of the microbicide candidates, however, failed to demonstrate in vivo efficacy presenting the discrepancies between the in vitro and in vivo data. This necessitates a re-evaluation of the current microbicide development paradigm and prompts a renewed search for preclinical testing systems that can predict negative outcomes of microbicide trials. Lack of a validated animal model for testing the safety and efficacy of microbicide candidates is also a major obstacle, as the animal models used currently (the mouse HSV-2 model, the rabbit vaginal irritation index, and the macaque SIV model) have substantial differences from humans. However, the recent advances include the development of humanized murine models, which allow better vaginal and rectal HIV efficacy challenge studies107. Absence of appropriate markers that correlates with the protective efficacy of microbicides also poses hindrance in the rapid development of successful microbicide candidates. Since most of the ongoing/planned microbicide clinical trials involved the participation of more than a single country, these multi-country trials have their own unique challenges. The trials need to have both ethics and regulatory approval not only in the country of the trial sponsor but also in each country where the trial will be held. Recruiting the required number of women and ensuring their stay in the trial can be challenging. A difficult but important issue in HIV trials is the care of people who seroconvert during the study. A meeting convened by the Bill & Melinda Gates Foundation and the Alliance for Microbicide Development (AMD) has recommended the formation of a co-ordination body that would help facilitate harmonizing across a number of areas including protocol design, monitoring, and decision-making for next-generation candidates108.
Table.
Status of clinical trials of topical microbicide candidates*
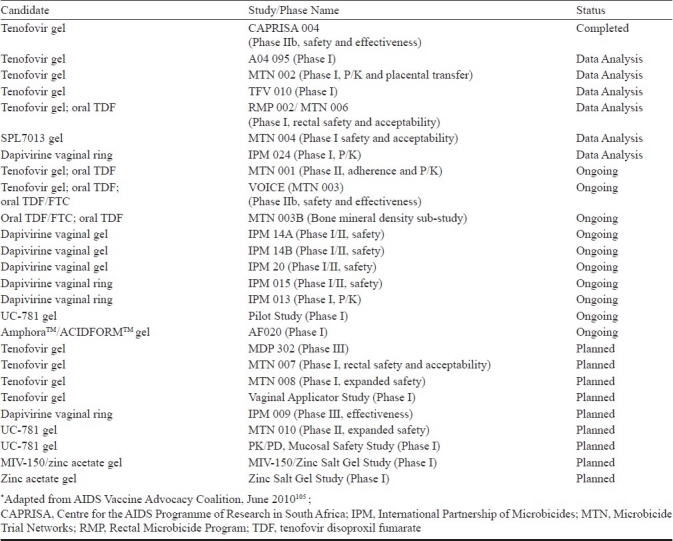
However, as a result of each of these challenges, new information and essential lessons have emerged in this field. These lessons also have resulted in a momentous increase in microbicide development efforts focusing on compounds with highly potent and HIV-specific mechanisms of action, combination products, novel formulations, and carefully designed pharmacokinetic and pharmacodynamic evaluations, which may lead towards a safe and effective microbicide in future.
Acknowledgments
The authors acknowledge National Institute of Immunology, New Delhi, Department of Biotechnology, Government of India and Indian Council of Medical Research, Government of India, New Delhi for financial support.
References
- 1.Geneva, Switzerland: UNAIDS/World Health Organization; 2010. [accessed on September 10, 2010]. UNAIDS, WHO. AIDS epidemic update 2009. Available from: http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive /2007/ [Google Scholar]
- 2.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bruyn G, Martinson NA, Gray GE. Male circumcision for HIV prevention: developments from sub-Saharan Africa. Expert Rev Anti Infect Ther. 2010;8:23–31. doi: 10.1586/eri.09.120. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. The history of antiretrovirals: key discoveries over the past 25 years. Rev Med Virol. 2009;19:287–99. doi: 10.1002/rmv.624. [DOI] [PubMed] [Google Scholar]
- 5.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Panel on Clinical Practices for Treatment of HIV.Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10:806–10. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 8.Spearman P. HIV vaccine development: lessons from the past and promise for the future. Curr HIV Res. 2003;1:101–20. doi: 10.2174/1570162033352093. [DOI] [PubMed] [Google Scholar]
- 9.Excler JL. AIDS vaccine development: perspectives, challenges & hopes. Indian J Med Res. 2005;121:568–81. [PubMed] [Google Scholar]
- 10.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, et al. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol. 2010;84:10522–32. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri M, Ugen KE, Weiner DB. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin Microbiol Rev. 2004;17:370–89. doi: 10.1128/CMR.17.2.370-389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. The rpg120 HIV Vaccine Study Group.Placebo-controlled phase 3 trials of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 13.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 14.Doncel G, Mauck C. Vaginal microbicides: a novel approach to preventing sexual transmission of HIV. Curr HIV/AIDS Rep. 2004;1:25–32. doi: 10.1007/s11904-004-0004-0. [DOI] [PubMed] [Google Scholar]
- 15.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 16.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–52. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 17.Turville SG, Arthos J, Donald KM, Lynch G, Naif H, Clark G, et al. HIV gp120 receptors on human dendritic cells. Blood. 2001;98:2482–8. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- 18.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–57. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4 + cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–82. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 20.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie BL, Kiarie JN, Morrison S, John-Stewart GC, Kinuthia J, Whittington WL, et al. Sexually transmitted infections among HIV-1-discordant couples. PLoS One. 2009;4:e8276. doi: 10.1371/journal.pone.0008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci USA. 2004;101:5640–5. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26:502–11. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coggins C, Elias CJ, Atisook R, Bassett MT, Ettiegne-Traore V, Ghys PD, et al. A study of women's preferences regarding the formulation of over-the-counter vaginal spermicides. New York: Population Council; 1998. [DOI] [PubMed] [Google Scholar]
- 26.Han L, Lv F, Xu P, Zhang G, Juniper NS, Wu Z. Microbicide acceptability among female sex workers in Beijing, China: results from a pilot study. J Womens Health (Larchmt) 2009;18:1377–84. doi: 10.1089/jwh.2008.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darroch J, Frost J. Women's interest in vaginal microbicides. Fam Plann Perspect. 1999;31:16–23. [PubMed] [Google Scholar]
- 28.Ramjee G, Morar NS, Braunstein S, Friedland B, Jones H, van de Wijgert J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res Ther. 2007;4:20. doi: 10.1186/1742-6405-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–97. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourinbaiar AS, Fruhstorfer EC. The efficacy of nonoxynol-9 from an in vitro point of view. AIDS. 1996;10:558–9. doi: 10.1097/00002030-199605000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Hicks DR, Martin LS, Getchell JP, Heath JL, Francis DP, McDougal JS, et al. Inactivation of HTLV-III/LAV-infected cultures of normal human lymphocytes by nonoxynol-9 in vitro. Lancet. 1985;2:1422–3. doi: 10.1016/s0140-6736(85)92584-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller CJ, Alexander NJ, Gettie A, Hendrickx AG, Marx PA. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil Steril. 1992;57:1126–8. [PubMed] [Google Scholar]
- 33.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–82. [PubMed] [Google Scholar]
- 34.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled clinical trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 35.van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. COL-1492 Study Group.Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 36.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Wyrick PB, Knight ST, Gerbig DG, Jr, Raulston JE, Davis CH, Paul TR, et al. The microbicidal agent C31G inhibits Chlamydia trachomatis infectivity in vitro. Antimicrob Agents Chemother. 1997;41:1335–44. doi: 10.1128/aac.41.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krebs FC, Miller SR, Catalone BJ, Welsh PA, Malamud D, Howett MK, et al. Sodium dodecyl sulfate and C31G as microbicidal alternatives to nonoxynol 9: Comparative sensitivity of primary human vaginal keratinocytes. Antimicrob Agents Chemother. 2000;44:1954–60. doi: 10.1128/aac.44.7.1954-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piret J, Desormeaux A, Bergeron MG. Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr Drug Targets. 2002;3:17–30. doi: 10.2174/1389450023348037. [DOI] [PubMed] [Google Scholar]
- 41.Mbopi-Keou FX, Trottier S, Omar RF, Nkele NN, Fokoua S, Mbu ER, et al. A randomized, double-blind, placebo-controlled Phase II extended safety study of two invisible condom formulations in Cameroonian women. Contraception. 2010;81:79–85. doi: 10.1016/j.contraception.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Olmsted SS, Dubin NH, Cone RA, Moench TR. The rate at which human sperm are immobilized and killed by mild acidity. Fertil Steril. 2000;73:687–93. doi: 10.1016/s0015-0282(99)00640-8. [DOI] [PubMed] [Google Scholar]
- 43.Ongradi J, Ceccherini-Nelli L, Pistello M, Specter S, Bendinelli M. Acid sensitivity of cell-free and cell-associated HIV-1: clinical implications. AIDS Res Hum Retroviruses. 1990;6:1433–6. doi: 10.1089/aid.1990.6.1433. [DOI] [PubMed] [Google Scholar]
- 44.Achilles SL, Shete PB, Whaley KJ, Moench TR, Cone RA. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sex Transm Dis. 2002;29:655–64. doi: 10.1097/00007435-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Zeitlin L, Hoen TE, Achilles SL, Hegarty TA, Jerse AE, Kreider JW, et al. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28:417–23. doi: 10.1097/00007435-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–66. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minces LR, McGowan I. Advances in the development of microbicides for the prevention of HIV infection. Curr Infect Dis Rep. 2010;12:56–62. doi: 10.1007/s11908-009-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amaral E, Faundes A, Zaneveld L, Waller D, Garg S. Study of the vaginal tolerance to Acidform, an acid-buffering, bioadhesive gel. Contraception. 1999;60:361–6. doi: 10.1016/s0010-7824(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 49.Hemmerling A, Potts M, Walsh J, Young-Holt B, Whaley K, Stefanski DA. Lime juice as a candidate microbicide? An open-label safety trial of 10% and 20% lime juice used vaginally. J Womens Health (Larchmt) 2007;16:1041–51. doi: 10.1089/jwh.2006.0224. [DOI] [PubMed] [Google Scholar]
- 50.Klebanoff SJ, Coombs RW. Virucidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–92. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 52.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci USA. 2000;100:11672–7. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, et al. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA. 2005;102:11993–8. doi: 10.1073/pnas.0504881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lagenaur LA, Berger EA. An anti-HIV microbicide comes alive. Proc Natl Acad Sci USA. 2005;102:12294–5. doi: 10.1073/pnas.0505960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vangelista L, Secchi M, Liu X, Bachi A, Jia L, Xu Q, et al. Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob Agents Chemother. 2010;54:2994–3001. doi: 10.1128/AAC.01492-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balzarini J, van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–97. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 57.Schols D, Pauwels R, Desmyter J, De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology. 1990;175:556–61. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- 58.Maeda K, Nakata H, Ogata H, Koh Y, Miyakawa T, Mitsuya H. The current status of, and challenges in, the development of CCR5 inhibitors as therapeutics for HIV-1 infection. Curr Opin Pharmacol. 2004;4:447–52. doi: 10.1016/j.coph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Kawamura T, Gulden FO, Sugaya M, McNamara DT, Borris DL, Lederman MM, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci USA. 2003;100:8401–6. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veazey RS, Ling B, Green LC, Ribka EP, Lifson JD, Piatak M, Jr, et al. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J Infect Dis. 2009;199:1525–7. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 62.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–44. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, et al. Blockade of attachment and fusion receptors inhibit HIV-1 infection of human cervical tissue. J Exp Med. 2004;119:1065–75. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 65.Allaway GP, Davis-Bruno KL, Beaudry GA, Garcia EB, Wong EL, Ryder AM, et al. Expression and characterization of CD4-IgG2, a novel heterodimer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 1995;11:533–9. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 66.Kilby JM, Eron JJ. Novel therapies based on mechanisms of HIV-1 cell entry. N Engl J Med. 2003;348:2228–38. doi: 10.1056/NEJMra022812. [DOI] [PubMed] [Google Scholar]
- 67.Lu M. Stabilizing peptides and their use in the preparation of stabilized HIV inhibitors. World Intellectual Property Organization Patent. 2004 WO-04/106364A1. [Google Scholar]
- 68.Ketas TJ, Schader SM, Zurita J, Teo E, Polonis V, Lu M, et al. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–40. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Deng Y, Zheng Q, Ketas TJ, Moore JP, Lu M. Protein design of a bacterially expressed HIV-1 gp41 fusion inhibitor. Biochemistry. 2007;46:4360–9. doi: 10.1021/bi7001289. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, et al. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50:3250–9. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaeffer DJ, Krylov VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf. 2000;45:208–27. doi: 10.1006/eesa.1999.1862. [DOI] [PubMed] [Google Scholar]
- 72.Perotti ME, Pirovano A, Phillips DM. Carrageenan formulation prevents macrophage trafficking from vagina: implications for microbicide development. Biol Reprod. 2003;69:933–9. doi: 10.1095/biolreprod.102.014555. [DOI] [PubMed] [Google Scholar]
- 73.Population Council. Trial shows anti-HIV microbicide is safe, but does not prove it effective. 2008. [accessed on September 12, 2010]. Available from: http://www.popcouncil.org/mediacenter/newsreleases/Carraguard .
- 74.Morris GC, Lacey CJ. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr Opin Infect Dis. 2010;23:57–63. doi: 10.1097/QCO.0b013e328334de6d. [DOI] [PubMed] [Google Scholar]
- 75.Christensen ND, Reed CA, Culp TD, Hermonat PL, Howett MK, Anderson RA, et al. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob Agents Chemother. 2001;45:3427–32. doi: 10.1128/AAC.45.12.3427-3432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson RA, Feathergill KA, Diao XH, Cooper MD, Kirkpatrick R, Herold BC, et al. Preclinical evaluation of sodium cellulose sulfate (Ushercell) as a contraceptive antimicrobial agent. J Androl. 2002;23:426–38. [PubMed] [Google Scholar]
- 77.Simoes JA, Citron DM, Aroutcheva A, Anderson RA, Jr, Chany CJ, 2nd, Waller DP, et al. Two novel vaginal microbicides (polystyrene sulfonate and cellulose sulfate) inhibit Gardnerella vaginalis and anaerobes commonly associated with bacterial vaginosis. Antimicrob Agents Chemother. 2002;46:2692–5. doi: 10.1128/AAC.46.8.2692-2695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scordi-Bello IA, Mosoian A, He C, Chen Y, Cheng Y, Jarvis GA, et al. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–15. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195:703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 80.Halpern V, Ogunsola F, Obunge O, Wang CH, Onyejepu N, Oduyebo O, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a Phase III trial in Nigeria. PLoS One. 2008;3:e3784. doi: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neurath AR, Strick N, Li YY. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect Dis. 2003;3:27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu H, Zhao Q, Wallace G, Liu S, He Y, Shattock R, et al. Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates. AIDS Res Hum Retroviruses. 2006;22:411–8. doi: 10.1089/aid.2006.22.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fichorova RN, Zhou F, Ratnam V, Atanassova V, Jiang S, Strick N, et al. Anti-human immunodeficiency virus type 1 microbicide cellulose acetate 1,2-benzenedicarboxylate in a human in vitro model of vaginal inflammation. Antimicrob Agents Chemother. 2005;49:323–35. doi: 10.1128/AAC.49.1.323-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neurath AR, Strick N, Li YY, Lin K, Jiang S. Design of a “microbicide” for prevention of sexually transmitted diseases using “inactive” pharmaceutical excipients. Biologicals. 1999;27:11–21. doi: 10.1006/biol.1998.0169. [DOI] [PubMed] [Google Scholar]
- 85.Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, et al. Unacceptable side effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int J STD AIDS. 2010;21:714–7. doi: 10.1258/ijsa.2010.010215. [DOI] [PubMed] [Google Scholar]
- 86.Huskens D, Vermeire K, Profy AT, Schols D. The candidate sulfonated microbicide, PRO 2000, has potential multiple mechanisms of action against HIV-1. Antiviral Res. 2009;84:38–47. doi: 10.1016/j.antiviral.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Keller MJ, Zerhouni-Layachi B, Cheshenko N, John M, Hogarty K, Kasowitz A, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 88.Abdool Karim SS. Results of effectiveness trials of PRO2000 gel: lessons for future microbicide trials. Future Microbiol. 2010;5:527–9. doi: 10.2217/fmb.10.29. [DOI] [PubMed] [Google Scholar]
- 89.Tyssen D, Henderson SA, Johnson A, Sterjovski J, Moore K, La J, et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS One. 2010;5:e12309. doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Loughlin J, Millwood IY, McDonald HM, Price CF, Kaldor JM, Paull JR. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): a dose ranging, phase I study. Sex Transm Dis. 2010;37:100–4. doi: 10.1097/OLQ.0b013e3181bc0aac. [DOI] [PubMed] [Google Scholar]
- 91.Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl) adenine. Science. 1995;270:1197–9. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 92.Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother. 2009;53:1797–807. doi: 10.1128/AAC.01096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.NC USA: Research Triangle Park; 2010. Jul, FHI and the Centre for the AIDS Programme of Research in South Africa: Factsheet: Results of the CAPRISA 004 trial on the effectiveness of tenofovir gel for HIV prevention. [Google Scholar]
- 95.MTN, Microbicide trials Network, USA. (NAID-NIH funded) [accessed on October 24, 2011]. Available from: http://www.mtnstopshiv.org/node/70 .
- 96.Balzarini J, Pelemans H, Aquaro S, Perno CF, Witvrouw M, Schols D, et al. A highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol Pharmacol. 1996;50:394–401. [PubMed] [Google Scholar]
- 97.van Herrewege Y, Michiels J, Van Roey J, Fransen K, Kestens L, Balzarini J, et al. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother. 2004;48:337–9. doi: 10.1128/AAC.48.1.337-339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Fabio S, Van Roey J, Giannini G, van den Mooter G, Spada M, Binelli A, et al. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS. 2003;17:1597–604. doi: 10.1097/00002030-200307250-00003. [DOI] [PubMed] [Google Scholar]
- 99.Yang H, Parniak MA, Isaacs CE, Hillier SL, Rohan LC. Characterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J. 2008;10:606–13. doi: 10.1208/s12248-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Talwar GP, Raghuvanshi P, Mishra R, Banerjee U, Rattan A, Whaley KJ, et al. Polyherbal formulations with wide spectrum antimicrobial activity against reproductive tract infections and sexually transmitted pathogens. Am J Reprod Immunol. 2000;43:144–51. doi: 10.1111/j.8755-8920.2000.430303.x. [DOI] [PubMed] [Google Scholar]
- 101.Joshi SN, Katti U, Godbole S, Bharucha K, B KK, Kulkarni S, et al. Phase I safety study of Praneem polyherbal vaginal tablet use among HIV-uninfected women in Pune, India. Trans R Soc Trop Med Hyg. 2005;99:769–74. doi: 10.1016/j.trstmh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Joglekar NS, Joshi SN, Navlakha SN, Katti UR, Mehendale SM. Acceptability of Praneem polyherbal vaginal tablet among HIV uninfected women & their male partners in Pune, India - Phase I study. Indian J Med Res. 2006;123:547–52. [PubMed] [Google Scholar]
- 103.Joshi SN, Dutta S, Kumar BK, Katti U, Kulkarni S, Risbud A, et al. Expanded safety study of Praneem polyherbal vaginal tablet among HIV-uninfected women in Pune, India: a phase II clinical trial report. Sex Transm Infect. 2008;84:343–7. doi: 10.1136/sti.2007.029207. [DOI] [PubMed] [Google Scholar]
- 104.Talwar GP, Dar SA, Rai MK, Reddy KV, Mitra D, Kulkarni SV, et al. A novel polyherbal microbicide with inhibitory effect on bacterial, fungal and viral genital pathogens. Int J Antimicrob Agents. 2008;32:180–5. doi: 10.1016/j.ijantimicag.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 105.AIDS Vaccine Advocacy Coalition. Ongoing and planned clinical trials of topical microbicide candidates (June 2010) 2010. [accessed on June 10, 2010]. Available from: http://www.avac.org/ht/a/GetDocumentAction/i/3109 .
- 106.Fichorova RN. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr. 2004;37(Suppl 3):S184–93. [PMC free article] [PubMed] [Google Scholar]
- 107.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Public Report, Microbicide Donors Committee Quick Working Group, Meeting #9, Population Council New York, NY, USA. 2009. Feb, [accessed on September 11, 2010]. Available from: http://www.microbicide.org/uploads/3/1/2/1/3121935/qwg_meeting_9_final_public_report .


