Abstract
Background & objectives:
HIV infection is characterized by a perturbation in T cell homeostasis, leading to alteration in T cell subsets. In addition to alteration in differentiation, HIV infection also leads to change in T cell survival and regenerative capacity, as suggested by differential expression of CD127 and CD57. We evaluated the expression patterns of CD127 and CD57 on CD4 and CD8 effector, memory and naïve T cell subsets in HIV-infected and uninfected individuals.
Methods:
We characterized T cell subsets based on expression of these markers, and compared their expression pattern in HIV infected subjects and uninfected controls. We further assessed therapy generated changes in these subsets and expression of CD127 and CD57 on them.
Results:
There was a generalized decrease in naïve CD4 and CD8 T cells in HIV infected subjects. These changes in T cell subset distribution were related to antigen load. CD127 expression was significantly reduced in T cells from HIV infected subject. In association to this, HIV infected subjects had higher percentage of T cell subsets expressing CD57. Increased CD57 and reduced CD127 expression correlated with plasma viraemia and CD8 T cell activation state. Incomplete restoration of T cell subset proportions was observed, despite suppression of viral replication and increase in CD4 T cell counts. Further, the improvement was more pronounced in CD127 expression.
Interpretation & conclusions:
HIV infected subjects have reduced T cell regenerative capacity along with increased senescence, highlighting decreased proliferation and effector activities.
Keywords: CD57, CD127, HIV-1 viral load, T cell activation, T cell subset
Naïve T cells, on productive interaction with their cognate antigen, undergo a complex maturation and differentiation process, leading to the formation of memory and effector type T cells1. Two cell surface markers, namely the lymphocyte antigen CD45RA and the chemokine receptor CCR7, have been used to delineate four chief subsets of human T cells. The naïve (CD45RA+CCR7+), effector memory (CD45RA-CCR7-, EM), central memory (CD45RA- CCR7+, CM), and effector cells re-expressing RA (TEMRA, CD45RA+CCR7-, EMRA) T cell subsets, have different functional capacities and the T cell homeostasis is tightly regulated2.
Chronic viral infection, as opposed to acute infections, are associated with an altered T cell response. Chronic infection leads to the formation of functionally exhausted T cells, which are in turn rapidly eliminated. Expression of several inhibitory receptors like PD-1, TRAIL, and LAG-3 and transcriptional factors like Blimp-3 have been implicated in T cell exhaustion3–5. Human immunodeficiency virus 1 (HIV-1) causes a gradual decline in circulating CD4 T cells, perturbation of the T-cell homeostasis due to direct or indirect effects of the virus and subsequently, activation of the immune system that results in alterations in the various T lymphocyte subpopulations6. HIV infection is associated with a reduction in the proportion of naïve T cells subsets, with a corresponding increase in activated memory/effector T cells7,8. Several chemokines play a vital role in maintenance of T cell subset homeostasis, of which interleukin-7 (IL-7) plays the pivotal role by stimulating thymopoiesis and controlling peripheral T cell subsets. Perturbation in IL-7 levels and expression pattern of IL-7Rα (CD127) expression are believed to be skewed in HIV infection, at least partly leading to the loss of T cell subset balance.
In addition to the depletion of CD4 T cell pool and breakdown of T cell homeostasis, HIV infection is also characterized by a generalized state of immune activation, which is now believed to play the predominant role in immunopathogenesis9,10. This uncontrolled state of immune activation and viral persistence affect the proliferative capacity of T cells11, and this loss of proliferative potential correlates with expression of the phenotypic marker of senescence: CD5712.
Thus, CD127 and CD57 are phenotypic markers identifying T cell subpopulations, proportions of which are distorted in HIV infection. This study focuses on the expression pattern of these cell surface markers on CD4 and CD8 effector, memory and naïve T cell subsets in HIV infected individuals, compared to their expression in HIV-uninfected T cell subsets. Also, the association of these marker expression patterns with markers of chronic T cell immune activation, CD38 and HLA-DR expression on CD8 T cells was studied.
Material & Methods
Study population: Thirty nine consecutive HIV infected individuals, 30 untreated (18 males and 12 females) and nine treated (6 males and 3 females) individuals (median duration of treatment of 10 months), at different stages of infection13 were recruited from AIDS clinic of the Department of Microbiology at All India Institute of Medical Sciences, New Delhi (from May 2010 to July 2010). The study protocol was approved by the Institute Ethics Committee and all patients gave written informed consent prior to inclusion in the study. Eight individuals (3 females and 5 males) negative for HIV-1/2 and for hepatitis B and C were included as healthy controls. The control samples used for the purpose of the study were laboratory members without any history of past infections and vaccination in the 6 months preceding the study period.
Five ml of blood was collected from all subjects by venipuncture in K3EDTA vacutainer tubes (Becton Dickinson, USA). Plasma samples were stored at -70°C.
Complete blood counts and CD4+ T cell enumeration: Complete blood counts including total and differential leucocyte counts were obtained from patients’ records. For CD4+ T cell enumeration, EDTA blood was processed according to manufacturer's instructions and the processed samples were analysed on FACSCount (Becton Dickinson).
Immunophenotyping: T cell subset phenotyping was performed as described previously14. The following monoclonal antibody panel was used for staining: allophycocynanin-H7 (APC-H7) labelled CD3, Pacific Blue labelled CD45RA, AmCyan labelled CD8, phycoerythrin (PE) labelled CCR7, peridinin chlorophyll protein (PerCP)-Cy5.5 labelled CD127, and fluorescein isothiocyanate (FITC)-labelled CD57 or CD38 and allophycocyanin (APC) labelled HLA-DR (Becton Dickinson, USA). The stained samples were further washed, lysed and resuspended in 250 μl paraformaldehyde before acquisition.
A total of 100,000 lymphocyte events were acquired for each tube on a LSR II (Becton Dickinson, USA) flow cytometer within 2 h of processing the blood samples. The acquisition gate was defined on lymphocytes using FSC and SSC. Further, CD3 staining was used to identify T cells. On this cell population, expression of CD4 and CD8 segregated the CD4+ and CD8+ T cells respectively15. All analysis for CD4+ and CD8+ T lymphocyte subsets was performed using Flowjo software (Treestar Inc. Oregon, USA). The dot plots were reviewed using a set of criteria for quality control. Background staining was assessed using appropriate fluorescence minus one (FMO) controls. Quadrant markers were established on these FMO controls and were carried on for all the samples.
T cell memory/naïve/effector subsets were identified based on expression of CD45RA and CCR7 (Fig. 1) (CD45RA+CCR7+ naïve T cells, CD45RA-CCR7- effector memory T cells, CD45RA+CCR7- TEMRA cells, and CD45RA-CCR7+ central memory T cells). The expression of IL-7Rα (CD127), senescence marker (CD57), were examined on the CD4 and CD8 T cell subsets and correlated with expression of activation markers (CD38, HLA-DR) on CD4 and CD8 T cells.
Fig. 1.
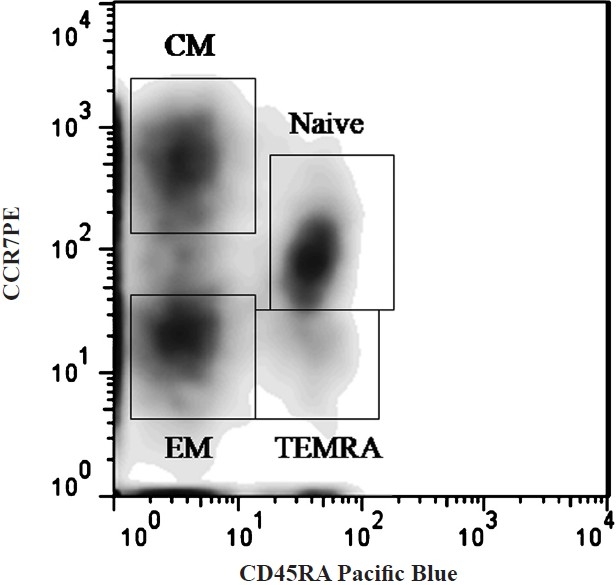
T cell subset differentiation based on expression of CD45RA and CCR7 on CD4 and CD8 T cells.
HIV-1 viral RNA quantification: HIV-1 viral RNA was extracted from plasma samples and quantified using COBAS TaqMan HIV-1 Test version 2.0 (Roche Diagnostics, Meylan, France) with a lower limit of detection of 40 copies/ml.
Statistical analysis: The Mann-Whitney U test was used for comparisons between HIV-1-infected and uninfected control subjects. CD4+ T cell counts of the study population were used to form three groups, among which the CD127 and CD57 expression on T cell subsets were compared using Kruskal-Wallis test for equality of populations. Association of CD4+ T cell counts and HIV-1 viral load with T cell subset proportions and expression of CD127 and CD57, and with activation marker expression on CD4 and CD8 T cells was calculated using Spearman's ρ. Pair-wise comparisons were made to study the pattern of expression of these molecules on CD4 and CD8 T cells in the same subject using Wilcoxon signed ranks test. P<0.05 was considered significant. All the statistical analyses were performed using SPSS software version 17 (Chicago, IL).
Results
Study population: Thirty HIV-1 infected individuals, with a median age of 34 yr, nine HAART receiving subjects (median age, 37) and eight seronegative controls (median age, 29 yr) were included in the study. The median CD4+ T cell counts were 297/μl for HIV infected naïve subjects, 623/μl for HAART receiving subjects and 825/μl for control subjects (P<0.001). The median percentage of CD8 T cells expressing activation markers CD38 and HLA-DR in HIV infected untreated subjects was 51.2 per cent (range, 30.2; 88.1%) and 9.7% (range, 0.2; 49.2%) in HAART treated subjects. The treatment naïve subjects had a median viral load of 24,900 copies/ml, while the HAART receiving subjects had a median viral load of 71 copies/ml (P<0.001).
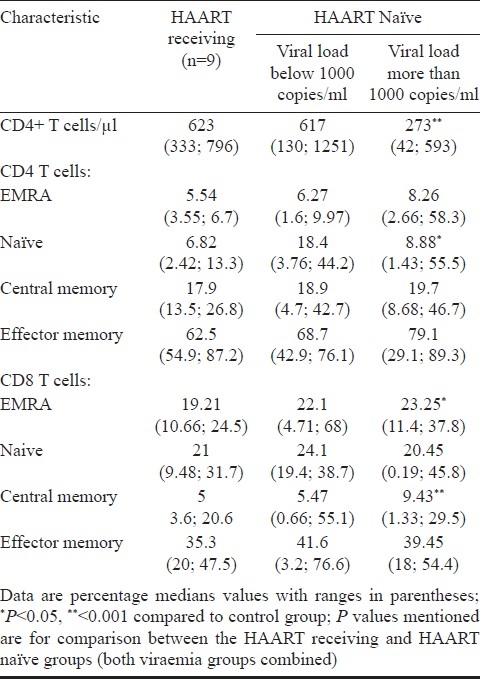
Altered T cell subsets in progressive HIV infection: HIV infected individuals had a significantly lower proportion of naïve CD8 T cells (median CD8 naïve T cells: control subjects, 48.1%; HIV-1-infected subjects, 19.6%; P<0.001), a similar decrease was also observed for naïve CD4 T cells, though the difference was not statistically significant. Further, there was an increase in CD8 EM cells (P<0.01). HIV infected subjects had an increased percentage of CD4 and CD8 EMRA cells, though the increase was not significant for either (Table I). To study the effect of viraemia on the T cell subsets, the subjects were divided into two categories based on their viral load: subjects with viral load less than 1000 copies/ml of HIV RNA, and with more than 1000 copies/ml of HIV RNA. CD8 EMRA T cells were lower in HAART naïve subjects with viral load below 1000 copies/ml when compared to therapy naïve subjects with viral load more than 1000 copies/ml (P<0.05). Similarly, central memory CD8 T cells were lower in subjects with viral loads lower than 1000 copies/ml when compared to subjects with viral load >1000 copies/ml, though the difference was not statistically significant. Similar trends were observed for CD4 T cells (Table II).
Table I.
Summary of comparison between uninfected control subjects and HIV-1 infected study subjects
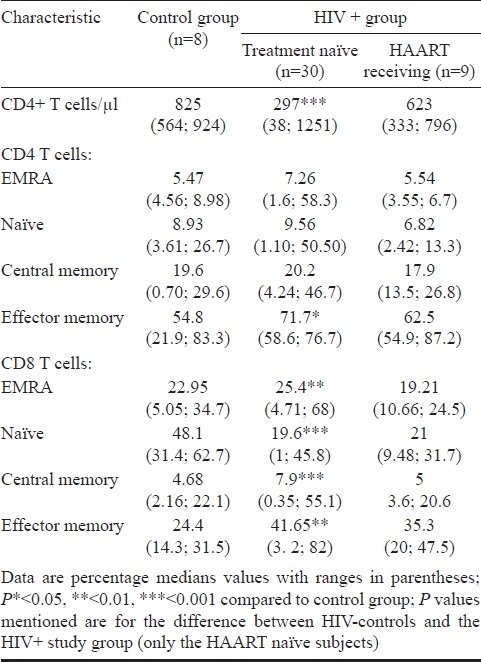
Table II.
T cell subsets in study groups based on plasma viraemia
CD4 and CD8 naïve T cell proportion correlated positively with CD4 T cell counts (CD4, ρ = 0.688, P< 0.001; CD8, ρ = 0.384, P= 0.04). The CD4 and CD8 EM cells correlated negatively with CD4 T cell counts, (CD4 EM, ρ = -0.505, P= 0.005; CD8 EM, ρ = -0.396, P= 0.033). Plasma HIV-1 viraemia correlated negatively with CD8 naïve T cells (ρ = -0.364, P=0.037).
Decreased CD127 expression on CD4 and CD8 T cell subsets: HIV infected subjects had a significantly reduced proportion of CD127 expressing CD4 T cells (median percentage: HIV infected subjects, 62.65%; uninfected controls, 75.75%; P=0.005) and CD8 T cells (median percentage: HIV infected subjects, 17.15%; uninfected controls, 53.45%; P<0.001). Further, a statistically significant decrease was seen in CD127 expressing T cells in all the T cell compartments, and for both CD4 and CD8 T cells (Fig. 2A). Loss of CD127 was more pronounced in CD8 T cell compartment (Fig. 2B).
Fig. 2.
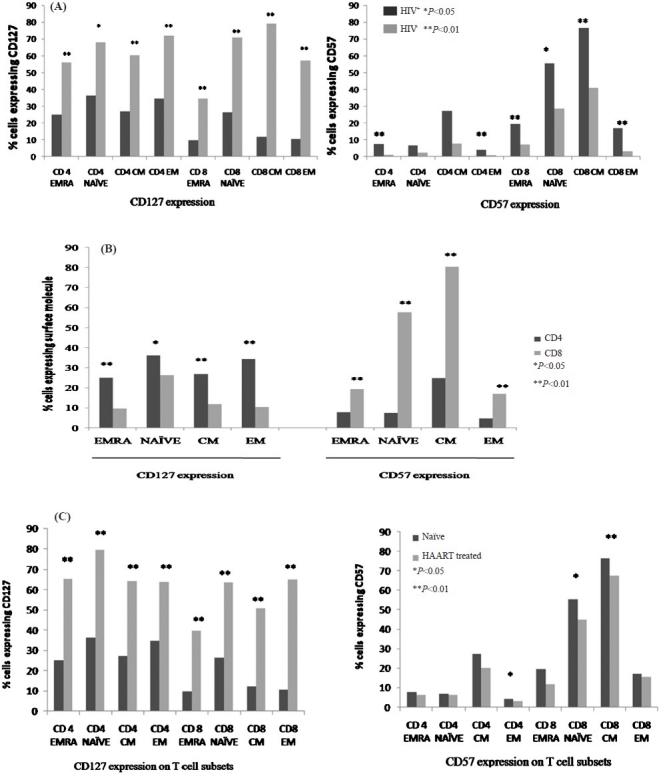
CD127 and CD57 expression pattern on T cell subsets. (A) CD127 and CD57 expression pattern in HIV infected subjects, therapy naïve subjects (n=30) and HIV uninfected controls (n=8). (B) Pattern of expression of CD127 and CD57 on CD4 and CD8 T cells (n = 30, HAART naïve study subjects). (C) Expression of CD127 and CD57 in HAART treated (n=9) and HAART naïve study subjects (n=30). EMRA, CD45 RA expressing terminally differentiated effector memory; CM, central memory; EM, effector memory.
On comparing between CD4 T cell subsets, highest expression of CD127 was observed on naïve CD4 T cells (median 36.25%, range, 23.35; 48.2%), followed by EM CD4 T cells (median: 34.5%, range: 24.75; 43.2%), with least expression on EMRA CD4 T cells (median: 25%, range: 20; 34.3%). These differences in levels of CD127 expressing CD4 T cell subsets were not statistically significant. In the CD8 T cell subsets, the same trend was observed with least proportion of CD127 expressing CD8 T cells in the EMRA compartment (9.74%), and highest in naïve CD8 T cell compartment (26.4%), with these differences being statistically significant (P<0.001).
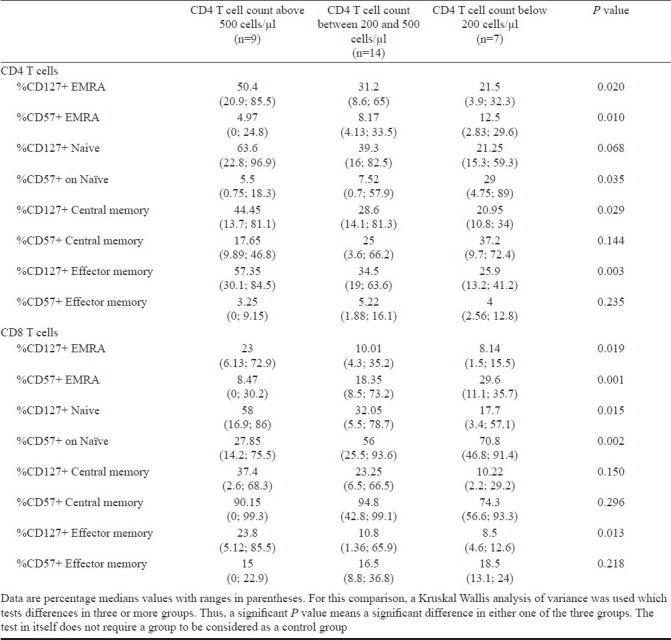
When compared between subjects at various stages of HIV infection; segregated on basis of their CD4 T cell counts; we observed least expression of this molecule in subjects with CD4 T cell counts below 200 cells/μl. This decrease in CD127 expression was statistically significant for CD4 TEMRA, and CD4 EM cells, and all subsets of CD8 T cells (Table III).
Table III.
Expression of CD127 and CD57 on T cell subsets in study groups based on CD4 T cell counts
Expansion of CD57 expressing CD4 and CD8 T cells: We observed a higher percentage of CD57 expressing total CD4 T cells in infected subjects compared to study controls (median percentage of CD4 T cells expressing CD57: HIV infected subjects, 7.14%; uninfected controls, 4.64%; P<0.05). CD57 expressing total CD8 T cells were also expanded in HIV infected subjects compared to controls, though this increase was not statistically significant.
A higher percentage of CD57 expressing CD4 T cell subsets was observed in HIV infected subjects compared to uninfected controls, with this difference being significant for CD4 EMRA cells (P<0.01), and CD4 EM cells (P<0.01). Similarly, CD8 T cell subsets expressing CD57 were also expanded, with this increase statistically significant for all CD8 T cell subsets .
As for CD127 expression, the expansion of CD57 expressing T cells was more for CD8 T cells compared to CD4 T cells (Fig. 2B). Proportion of CD4 T cells expressing immunosenescence marker CD57 were highest in the central memory compartment (median, 24.9%), and least in the effector memory compartment (median, 4.75%). These differences were statistically significant (P<0.001). CD57 expression on T cell subsets increased with severity of HIV disease, as signified by highest expression of CD57 on T cell subsets from subjects with CD4 T cell counts below 200 cells/μl (Table III).
Altered expression associates with HIV disease progression: We investigated the presence of association between alteration in the CD127 and CD57 expression on T cell subsets and biological markers of HIV disease progression, i.e. CD4 T cell counts, CD4 T cell percentage, activation status (CD8 T cells expressing CD38 and HLA-DR), and HIV-1 plasma viraemia. CD127 expression on T cell subsets correlated positively with CD4 T cell counts and CD4 T cell percentages. Also, the loss of CD127 expressing CD4 and CD8 T cells correlated negatively with activated CD8 T cells expressing CD38 and HLA-DR. CD127 expression on most of the CD4 and CD8 T cell subsets correlated negatively with plasma viraemia, with this correlation being statistically significant for CD127 expression on most CD4 and CD8 EM cells and also CD8 EMRA cells (Table IV).
Table IV.
Summary of association between expression of CD127 and CD57 on T cell subsets and disease progression markers
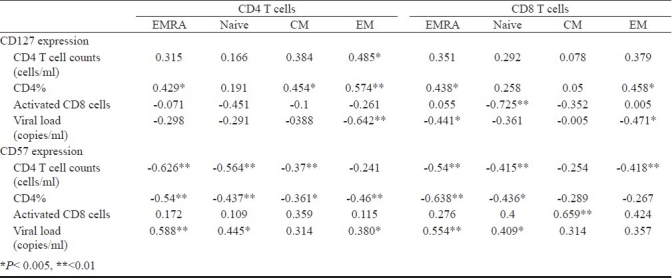
The expansion of CD57 expressing T cells correlated with CD4 T cell loss (CD4 T cell counts and CD4 T cell percentage), with this negative correlation being statistically significant for most subsets (Table IV). Further, CD57 expressing CD4 and CD8 T cell subsets also correlated negatively with CD4 T cell percentages. CD57 expression on CD4 and CD8 T cells correlated positively with plasma viraemia, with this association being significant for most subsets (Table IV).
Effect of highly active antiretroviral therapy (HAART) on T cell phenotype: The percentage of EMRA CD8 T cells was significantly reduced in HAART treated HIV infected study subjects (median, 19.21%) in comparison to treatment naïve subjects (median, 25.4%, P<0.001). Similar decrease was observed in EMRA CD4 T cell proportion in HAART treated subjects when compared to treatment naïve study subjects (median: HAART treated subjects, 5.54%; HAART naïve subjects, 7.26%; P<0.05). CD8 CM cell proportion was significantly lowered in HAART receiving subjects in comparison to HAART naïve subjects (median: HAART treated subjects, 5%; HAART naïve subjects, 7.9%; P<0.001).
Antiretroviral therapy resulted in an increase in expression of IL-7 receptor CD127 on all CD4 and CD8 T cell subsets, with these alterations being significant (Fig. 2C). Therapy experienced subjects had lowered CD57 expressing CD4 and CD8 T cell subsets, though this decrease in CD57 expression on HAART was significant only for CD4 effector cells (P<0.05), CD8 naïve cells (P<0.05), and CD8 central memory T cells (P<0.001) (Fig. 2C).
Discussion
A massive and continuous depletion of CD4 T cells occurs at all stages of HIV infection, and is the disease hallmark6,9. However, in addition to the overall CD4 T cell depletion, a perturbation in T cell homeostasis, leading to a differential loss of certain T cell subsets has been observed16,17. Untreated HIV infected individuals in general show a progressive loss of resting T cell subsets, preferentially naïve T cells. We observed a significant reduction in naïve CD8 T cell subsets in our HIV infected study subjects, in comparison to the uninfected controls. An increase was observed in the memory CD8 T cell subsets, especially the effector memory CD8 T cell subset (CD8+CD45RA-CCR7-), as has been reported in previous studies18,19. The expanded effector population correlated negatively with CD4 T cell counts highlighting the negative impact of this perturbation on host immune status, and also could be a result of compensative increase in place of the depleting naïve T cell population. In addition, this negative correlation with CD4 T cell counts could also highlight the increased cytotoxic activity of these effector cells resulting in killing of the CD4 T cells. An expansion of effector T cells in HIV infection is associated with a loss of effector functions like cytokine production, degranulation, and proliferation20,21. Thus, even with an expanded effector T cell population, the immune system is unable to control viral replication.
The perturbations were more pronounced for CD8 T cell subsets in comparison to the CD4 T cell subsets, and are because of the compensatory increase in memory and effector CD8 T cells during HIV infection in order to maintain T cell homeostasis. There was also a marked increase in the proportion of terminally differentiated effector CD4 and CD8 T cells (CD45RA+CCR7-) compared to uninfected controls, though the differences were statistically significant only for CD8 EMRA T cells. In comparison to previous studies, we observed no correlation pattern between EMRA T cell proportion and CD4 T cell counts or viral load19,22,23. The perturbation in T cell homeostasis, leading to an over-representation of memory phenotypes has been attributed to several factors. A major player among these factors is altered cytokine milieu, resulting in aberrant differentiation and maturation of T cells in HIV infection. IL-7 is one such crucial cytokine involved in the generation, activation, and homeostasis of the T cell compartment of the immune system24,25. The alpha chain of IL-7 receptor is expressed exclusively on T cells, and the IL-7R signaling is required for homeostatic expansion of naïve and memory T cells26,27. As reported previously27–29, we observed a decreased expression of CD127 on CD4 and CD8 T cells in HIV infected subjects, with this depletion more pronounced in subjects in advanced disease stage. To further examine the relation between CD127 expression on T cells and HIV disease progression, the association of CD127 expression on T cells with disease progression markers CD4 T cell counts, CD4 T cell percentage, and also activated CD8 T cell levels was studied. We found that CD127 expression on both CD4 and CD8 T cells correlated positively with CD4 T cell counts and percentages, and negatively with activation status and plasma viraemia, thus underscoring the importance of preservation of this receptor on T cell survival in chronic HIV infection.
A significant reduction in naïve, effector, and memory T cell subsets expressing CD127 was observed, with the loss more pronounced for the CD8 T cell subsets. However, the loss of CD127 expressing cells was more marked for the central and effector memory cell subsets, as has been reported28. As signaling through CD127-IL-7 receptor complex is fundamental to T cell survival, a depletion of CD127 from these memory cells signify a functional aberration of these cells that can result in loss of these cells eventually in the course of infection. Further, in comparison to EMRA and effector memory T cells, central memory T cells are generally endowed with capacity for self-renewal and better proliferation30. A loss of CD127 from the surface of these cells therefore, dramatically decreases their proliferation and life span, thereby further debilitating the virus-specific response.
Although its precise function on T cells remains unknown, CD57 expression defines a senescent T cell subset which has been shown to be elevated in HIV infection31. CD57 expression occurs in association with the ability to produce cytolytic enzyme perforin, and thus marks a highly cytotoxic T cell population. Further, these cells have shorter telomeres, and a higher replicative history, thus also denoting a terminally differentiated population of T cells. HIV infection is associated with chronic immune activation, and massive clonal expansion of specific T cell populations. We studied the expression of CD57 on CD4 and CD8 T cells in HIV infected individuals. As expected, we observed significant increase in CD4 and CD8 T cells expressing CD57 in HIV infected individuals. Further this increase in senescence marker was highest for subjects with CD4 T cell count below 200 cells/μl, and correlated positively with activated CD8 T cell levels and plasma viral load, and negatively with CD4 T cell counts, thus signifying the effect of HIV on T cells. Thus, progressive HIV infection is characterized by an activated immune state which is reflected in the increase in CD57 expression on T cells.
We characterized the pattern of expression of CD57 on the naïve, memory and effector T cells subsets to see which cell compartment was most affected by HIV induced immune activation. It was observed that in HIV infected subjects, central memory CD4 and CD8 T cells expressed most CD57, with this increase significantly more distinct for CD8 T cells. Thus the central memory T cells in settings of HIV infection appear to be worse affected with a significant decrease in CD127 and a pronounced increase in CD57, marking a cell population with limited regenerative capacity and survival capability, while at the same time being senescent32.
Persistent HIV replication in chronic HIV infection leads to tremendous alteration in T cell subsets. Highly active antiretroviral therapy (HAART) restores the immune status by blocking the viral replication. We, therefore, studied the effect of HAART on the T cells subsets and expression of markers CD127 and CD57. HAART resulted in partial restoration of some of the T cell subsets, especially a reduction in EMRA CD4 and CD8 T cells. Thus, by removing persistent antigenaemia, some amount of immune restoration is brought about, though a return to normal levels is almost never reported. HAART also resulted in increased CD127 expression on all CD4 and CD8 T cell subsets. CD57 expression was reduced with therapy, though the decrease was not as pronounced as increase in CD127 expression. Thus, even in presence of highly active antiretroviral therapy, the T cell subsets remain largely immunosenescent and thus dysfunctional. This can be attributed to the myriad of inhibitory molecules that are upregulated on T cells of HIV infected individuals, and recently it has been shown that HIV pulsed dendritic cells prime and expand a T cell population rich in these inhibitory molecules (PD-1, LAG-3, CTLA-4, Blimp-3, TIM-3) with decreased cytokine secretion profile4,5,33. A longitudinal analysis of CD57 expression on T cell subsets along with functional assays of T cell immune response will help in understanding whether this defect resolves with increased duration of HAART or remains despite therapy induced betterment of immune status. An in-depth analysis of expression of inhibitory molecule expression on T cells and their role in T cell exhaustion would also add to our knowledge of HIV-1 subtype C immunopathogenesis.
HIV infection is characterized by a loss of less differentiated cells, and a progressive accumulation of activated, highly differentiated and senescent cells. Thus the course of HIV infection is marked by a decrease in regenerative potential, as evident by the loss of CD127 expressing cells leading to decrease in survival power of the cells. These cells are, in turn, replaced by terminally differentiated late-stage cells, evident by CD57 expression. Thus, CD127 and CD57 are important markers for study of T cell differentiation and maturation in HIV immunopathogenesis, and a study of cell populations based on these markers will help in further elucidating the HIV induced maturation defects in T cells.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Marsden VS, Kappler JW, Marrack PC. Homeostasis of the memory T cell pool. Int Arch Allergy Immunol. 2006;139:63–74. doi: 10.1159/000090000. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Che KF, Sabado RL, Shankar EM, Tjomsland V, Messmer D, Bhardwaj N, et al. HIV-1 impairs in vitro priming of naive T cells and gives rise to contact-dependent suppressor T cells. Eur J Immunol. 2010;40:2248–58. doi: 10.1002/eji.201040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–9. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 7.Schaerli P, Moser B. Chemokines: control of primary and memory T-cell traffic. Immunol Res. 2005;31:57–74. doi: 10.1385/IR:31:1:57. [DOI] [PubMed] [Google Scholar]
- 8.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003;112:821–4. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 12.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 13.Interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec. 1990;65:221–4. [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 15.Vithayasai V, Sirisanthana T, Sakonwasun C, Suvanpiyasiri C. Flow cytometric analysis of T-lymphocytes subsets in adult Thais. Asian Pac J Allergy Immunol. 1997;15:141–6. [PubMed] [Google Scholar]
- 16.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 17.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–84. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 19.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–18. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aandahl EM, Quigley MF, Moretto WJ, Moll M, Gonzalez VD, Sonnerborg A, et al. Expansion of CD7(low) and CD7(negative) CD8 T-cell effector subsets in HIV-1 infection: correlation with antigenic load and reversion by antiretroviral treatment. Blood. 2004;104:3672–8. doi: 10.1182/blood-2004-07-2540. [DOI] [PubMed] [Google Scholar]
- 21.Bandera A, Trabattoni D, Pacei M, Fasano F, Suardi E, Cesari M, et al. Fully immunocompetent CD8+ T lymphocytes are present in autologous haematopoietic stem cell transplantation recipients despite an ineffectual T-helper response. PLoS One. 2008;3:e3616. doi: 10.1371/journal.pone.0003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northfield JW, Loo CP, Barbour JD, Spotts G, Hecht FM, Klenerman P, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J Virol. 2007;81:5759–65. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, Brenchley J, et al. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol. 2009;182:4751–61. doi: 10.4049/jimmunol.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 26.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SY, Zhang Z, Fu JL, Kang FB, Xu XS, Nie WM, et al. Progressive CD127 down-regulation correlates with increased apoptosis of CD8 T cells during chronic HIV-1 infection. Eur J Immunol. 2009;39:1425–34. doi: 10.1002/eji.200839059. [DOI] [PubMed] [Google Scholar]
- 28.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Delfraissy JF, et al. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients-reversal by highly active anti-retroviral therapy (HAART) Clin Exp Immunol. 2006;143:398–403. doi: 10.1111/j.1365-2249.2006.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Jacod S, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients - effects of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:277–85. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 31.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 32.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–7. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol Med. 2011;17:229–40. doi: 10.2119/molmed.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]


