Abstract
Aims
Chronic kidney disease (CKD) and right ventricular (RV) dysfunction are important predictors of prognosis in heart failure (HF). We investigated the relationship between RV dysfunction and CKD in outpatients with chronic systolic HF, an association which remains poorly defined.
Methods and results
Outpatients (n = 373) with chronic HF and left ventricular ejection fraction (LVEF) ≤45% underwent clinical and echo-Doppler evaluations and were followed up for 31 ± 24 months. Tricuspid annular plane systolic excursion (TAPSE) assessed RV dysfunction. The estimated glomerular filtration rate (GFR) was measured by the simplified Modification of Diet in Renal Disease (MDRD) formula. Correlation analysis was used to characterize the association between TAPSE and estimated GFR. Odds ratios (ORs) for CKD and hazard ratios (HRs) for all-cause mortality were assessed using multivariable logistic or proportional hazards regression models. TAPSE and estimated GFR were significantly correlated (r = 0.38, P < 0.0001). TAPSE ≤14 mm was associated with elevated estimated right atrial pressure and N-terminal pro brain natriuretic peptide levels. TAPSE ≤14 mm increased the odds of estimated GFR <60 mL/min/1.73 m2, OR [95% confidence interval (CI)] = 2.51(1.44–4.39), P < 0.0001 and predicted all-cause mortality, HR (95% CI) = 1.80 (1.20–2.71) after multivariable adjustment.
Conclusions
Right ventricular dysfunction is cross-sectionally associated with CKD and prospectively predicts survival in outpatients with chronic systolic HF. These data suggest RV dysfunction to be one of the possible mechanistic links between HF and CKD.
Keywords: Congestion, Echocardiography, Glomerular filtration rate, Tricuspid annular plane systolic excursion
Introduction
Chronic kidney disease (CKD) is an important predictor of poor survival in patients with heart failure (HF).1–4 The pathophysiology of CKD in HF appears multifactorial. Traditional theories have pointed to a decrease in arterial perfusion pressure and renal blood flow (RBF) as the main cause of renal dysfunction in patients with impaired cardiac output.5,6 Contemporary reports have also associated venous congestion, as evidenced by high right atrial pressure (RAP), with renal dysfunction in acute7 and chronic HF,8 in pulmonary hypertension,9 and in an unselected population of patients undergoing right heart catheterization for various clinical indications.10 However, in the absence of cardiac imaging, these clinical and haemodynamic studies did not evaluate the association between specific cardiac abnormalities, such as right ventricular (RV) failure, and renal dysfunction.
Among the several echo-Doppler measures of RV function that have been proposed, tricuspid annular plane systolic excursion (TAPSE) is the one that has been more extensively studied.11−13 A TAPSE ≤14 mm has been associated with adverse outcomes in patients with chronic HF and reduced left ventricular (LV) function.11,12 A functional right ventricle is important for maintaining low venous pressure (significantly below the plasmatic oncotic pressure) as well as adequate cardiac output, either of which might mediate associations between RV dysfunction and CKD.14 However, despite this possible mechanistic link, the association between RV function and CKD in chronic systolic HF remains poorly defined. Thus, the primary aim of this study was to test the hypothesis that RV function, as assessed by TAPSE, is an independent predictor of CKD in outpatients with chronic systolic HF. The relationship between RV dysfunction and death in this patient population was also investigated.
Methods
Patient population
Outpatients referred for transthoracic echocardiography between January 2002 and June 2010 at the echocardiography labs of the Cardiac, Thoracic and Vascular Department of the University of Pisa were evaluated. The inclusion criteria were: history of chronic HF, left ventricular ejection fraction (LVEF) ≤45%, and aetiology due to ischaemic or hypertensive heart disease or idiopathic cardiomyopathy.15 Among n = 692 referred for transthoracic echocardiography, we included 373 consecutive patients in this study. The reasons for patient exclusions are as follows: chronic HF with isolated LV diastolic dysfunction (n = 62); asymptomatic LV systolic dysfunction (LVEF ≤45%) (n = 71); more than mild aortic or organic, mitral valve disease (n = 95); severe tricuspid regurgitation (n = 5); or chronic kidney replacement therapy (n = 6). Finally, n = 80 patients were excluded due to incomplete data for critical echo-Doppler or biochemical parameters such as LV measurements, creatinine, and N-terminal pro brain natriuretic peptide (NT-proBNP). All patients gave written informed consent.
Echo-Doppler
Transthoracic two-dimensional and Doppler echocardiographic examination was performed with an Acuson Sequoia C256 ultrasound instrument (Mountain View, CA, USA) with second-harmonic imaging and a 3.5 MHz transducer. Recordings were made with the patient in left lateral decubitus during quiet respiration. Continuous single-lead electrocardiogram (ECG) monitoring was maintained during the study. Left ventricular volumes and EF were calculated from apical two- and four-chamber views using the modified Simpson's rule. Left ventricular volume indices and LV mass index were calculated.16 Right ventricular systolic function was evaluated by M-mode echocardiography using TAPSE.13 Measurement of TAPSE was not performed and patients were excluded if tricuspid regurgitation was deemed to be severe, because assessment of RV systolic function by TAPSE would not be accurate in the presence of severe tricuspid regurgitation.17 Pulsed-wave Doppler mitral flow was analysed for peak E and peak A velocities, E/A ratio, and E wave deceleration time. Mitral regurgitation severity was graded according to the vena contracta method.18 Patients were considered to have moderate to severe mitral regurgitation if they had a vena contracta width ≥0.5 cm in the parasternal long axis view. The estimated pulmonary artery systolic pressure was obtained by the sum of the Doppler-derived transtricuspid gradient and the estimated right atrial pressure, as assessed by the inspiratory collapse of the inferior vena cava.19
Clinical assessment and measurement of biological variables
Medical history, physical examination, laboratory results, and medical management of HF were registered at the time of the index echocardiogram. The HF score was calculated according to a scoring system based on Framingham criteria by an experienced cardiologist at the time of the index echocardiogram.20 The estimated glomerular filtration rate (GFR) was calculated on the basis of the Modification of Diet in Renal Disease (MDRD) formula.21 Patients were divided according to the estimated GFR into those with normal or relatively preserved renal function (estimated GFR ≥60 mL/min/1.73 m2) vs. those with renal dysfunction (estimated GFR <60 mL/min/1.73 m2). NT-proBNP plasma levels were measured by an Elecsys 2010 analyser (measuring range, 5–35 000 pg/mL) using a chemiluminescent immunoassay kit (Roche Diagnostics, Grenach-Wyhlen, Germany).
Follow-up data
The study endpoint was all-cause mortality. Survival data were obtained through follow-up visits of patients and, in the case of a missed visit, vital status was verified through telephone contacts, local authority registry and hospital records. In the absence of confirmed mortality, survival times were based on last contact (clinical visit or phone call).
Statistical analyses
SAS version 9.2 was used for analyses. Analysis of variance and Mann–Whitney tests (continuous variables) or χ2 tests (categorical variables) were used to test for statistically significant differences across levels of either CKD or TAPSE in descriptive analyses. Stepwise logistic regression models were used to identify predictors of prevalent CKD (cross-sectional analyses). Independent variables were required to have a P-value of <0.10 to enter the stepwise selection and a P-value of <0.05 to remain in the final model. Receiver operator characteristic (ROC) curves considered multiple dichotomies of TAPSE to verify that our a priori cut-off point of ≤14 mm11,12 was appropriate for both renal and mortality outcomes; specifically, dichotomies based on thresholds of 11, 12, 13, 14, 15, and 16 mm were evaluated. A non-parameteric approach was used to compare the areas under the curve (AUCs) for these multiple definitions and to determine the best TAPSE threshold for the prediction of prevalent CKD.22 To construct a concise mortality prediction model (longitudinal analyses), stepwise Cox proportional hazards regression models were used as described above.
Results
Baseline characteristics
Relevant demographic, clinical, echo-Doppler, and biochemical characteristics of the patient population are summarized in Table 1. The study patients had a mean age of 68 years, 80% were male, and 23% had LVEF <25%. CKD as defined by estimated GFR <60 mL/min/1.73 m2 was present in 53% of participants. Patients with estimated GFR <60 mL/min/1.73 m2 were older, had a higher prevalence of hypertension, diabetes, atrial fibrillation, and ischaemic aetiology of their cardiomyopathy, and were more often treated with diuretics and less often with angiotensin-converting enzyme (ACE) inhibitors and beta-blockers. In addition, CKD patients exhibited more advanced HF score and New York Heart Association (NYHA) class, had lower diastolic blood pressure and LVEF, and higher left atrial size, NT-proBNP levels, and estimated RAP. In patients with estimated GFR >60 mL/min/1.73 m2, TAPSE was significantly higher than in patients with CKD (P < 0.0001).
Table 1.
Baseline characteristics of patients classified according to renal function
| Variable | All | eGFR ≥60 mlmL/m/1.73 m2 (n = 177) | eGFR <60 mL/min/1.73m2 (n = 196) | P-value |
|---|---|---|---|---|
| Age (years) | 68 ± 12 | 64 ± 13 | 73 ± 10 | <0.0001 |
| Male (%) | 80 | 88 | 74 | 0.001 |
| Heart rate (b.p.m.) | 75 ± 15 | 74 ± 16 | 76 ± 14 | 0.24 |
| BMI | 26.4 ± 4.3 | 26.5 ± 4.0 | 26.2 ± 4.5 | 0.50 |
| Ischaemic CM (%) | 47 | 37 | 57 | 0.006 |
| Hypertensive CM (%) | 15 | 14 | 16 | 0.37 |
| Idiopathic CM (%) | 37 | 49 | 27 | 0.0001 |
| DM (%) | 21 | 13 | 28 | <0.0001 |
| History of hypertension | 40 | 38 | 42 | 0.05 |
| Atrial fibrillation (%) | 19 | 14 | 23 | 0.03 |
| Loop diuretics (%) | 89 | 83 | 94 | <0.0001 |
| ACE inhibitors (%) | 74 | 84 | 64 | <0.0001 |
| Beta-blockers (%) | 69 | 78 | 60 | 0.0002 |
| Aldosterone antagonists (%) | 58 | 56 | 60 | 0.64 |
| Digoxin (%) | 19 | 18 | 19 | 0.64 |
| NYHA class >II (%) | 45 | 29 | 60 | <0.0001 |
| Framingham HF score | 1.0 (0.5–2.0) | 1.0 (0.5–1.5) | 1.5 (0.5–2.5) | <0.0001 |
| Systolic BP (mmHg) | 124 ± 16 | 125 ± 10 | 122 ± 10 | 0.09 |
| Diastolic BP (mmHg) | 73 ± 10 | 75 ± 9 | 72 ± 10 | 0.002 |
| LV EDVi (ml/m2) | 113 ± 34 | 110 ± 32 | 116 ± 35 | 0.09 |
| LV ESVi (mL/m2) | 80 ± 31 | 76 ± 28 | 83 ± 33 | 0.04 |
| LVEF (%) | 31 ± 9 | 32 ± 8 | 30 ± 9 | 0.03 |
| Left atrial size (mm) | 49 ± 7 | 48 ± 7 | 50 ± 6 | 0.02 |
| Mitral regurgitation (%) | 30 | 23 | 37 | 0.003 |
| E/A ratio | 1.8 ± 1.5 | 1.7 ± 1.4 | 1.8 ± 1.6 | 0.71 |
| EDT (ms) | 156 ± 53 | 163 ± 54 | 150 ± 51 | 0.03 |
| TAPSE (mm) | 18 ± 5 | 19 ± 5 | 17 ± 5 | <0.0001 |
| PASP (mmHg) | 40 ± 12 | 39 ± 12 | 42 ± 11 | 0.006 |
| eRAP (mmHg) | 6 ± 3 | 6 ± 3 | 7 ± 4 | 0.11 |
| NT-proBNP (pg/mL)a | 1687 (749–3888) | 1285 (507–2596) | 2644 (1090–5835) | <0.0001 |
| Creatinine (mg/dL) | 1.4 ± 0.7 | 1.8 ± 0.8 | 1.0 ± 0.2 | <0.0001 |
ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; CM, cardiomyopathy; DM, diabetes mellitus; EDT, E wave deceleration time; EDVi, end-diastolic volume index; ESVi, end-systolic volume index; eGFR, estimated glomerular filtration rate; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic blood pressure; eRAP, estimated right atrial pressure; TAPSE, tricuspid annular plane systolic excursion.
aMedian and interquartile range.
Validation of TAPSE cut-off points for CKD and mortality prediction
An initial ROC analysis confirmed the <14 mm cut-off11,12 as an appropriate threshold for creating a dichotomous TAPSE variable. For the prediction of both CKD and mortality, TAPSE ≤14 mm and TAPSE ≤15 mm were not meaningfully different. AUCs for TAPSE ≤14 mm and TAPSE ≤15 mm were 60% and 63%, respectively (P = 0.02), for the prediction of estimated GFR <60 mL/min/1.73 m2, whereas for the prediction of mortality the respective AUCs were 61% and 60% (P=non-significant), respectively.
Correlates of impaired renal function
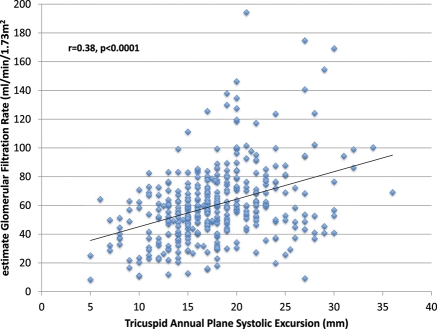
TAPSE and estimated GFR demonstrated a highly statistically significant linear relationship, with a correlation of 0.38 (P < 0.0001, Figure 1). Among 373 patients included in the study, 84 had TAPSE ≤14 mm (25%). TAPSE averaged (25th percentile, 75th percentile) 12 (10,14) and 20 (17,22) mm in participants with low (≤14 mm) vs. high (>14 mm) TAPSE, respectively. Mean (± SEM) values of estimated RAP and NT-proBNP between patients with low vs. high TAPSE were 8 ± 0.3 vs. 6 ± 0.2 mmHg (P < 0.0001) and 6837 ± 873 vs. 2709 ± 244 pg/mL (P < 0.001), respectively. Systolic and diastolic blood pressures were reduced by 7 (P < 0.001) and 3 (P < 0.05) mmHg, respectively, among patients with low TAPSE vs. high TAPSE.
Figure 1.
Association between tricuspid annular plane systolic excursion (TAPSE) and estimated glomerular filtration rate (GFR); r = 0.38, P < 0.0001.
In univariable analysis, we found several variables to be related to estimated GFR <60 mL/min/1.73 m2 (Tables 1 and 2). In stepwise logistic regressions, older age, male gender, diabetes, NYHA class >II, and TAPSE ≤14 mm were all associated with estimated GFR <60 mL/min/1.73 m2 (Table 2). Participants with TAPSE ≤14 mm vs. >14 mm had a substantially increased odds of estimated GFR <60 mL/min/1.73 m2: odds ratio (OR) = 2.51 [95% confidence interval (CI) 1.44–4.39], P < 0.0001. Of note, this association was even stronger when considering patients with more severe CKD. In multivariable models, increased NT-proBNP levels, age, body mass index, and decreased TAPSE were all associated with increased odds of estimated GFR <30 mL/min/1.73 m2. The OR for estimated GFR <30 mL/min/1.73 m2 when comparing TAPSE ≤14 mm vs. >14 mm was 4.06 (95% CI 1.80–9.17), P < 0.0001.
Table 2.
Univariable and multivariable predictors of impaired renal function (estimated GFR <60 mL/min/1.73 m2) among 373 patients with systolic heart failure
| Variable | Univariate |
Multivariate |
P-value | ||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | Selection order (score χ2) | ||
| Age (per 5 year increase) | 1.41 (1.27–1.56) | <0.0001 | 1.55 (1.35–1.77) | First (48) | <0.0001 |
| Male | 0.40 (0.23–0.70) | 0.001 | 2.24 (1.15–4.36) | Fifth (6) | 0.02 |
| BMI (per 1 m/kg2 increase) | 0.98 (0.94–1.03) | 0.50 | |||
| Heart rate (per 10 b.p.m. increase)a | 1.09 (0.94–1.27) | 0.24 | |||
| Idiopathic vs. ischaemic CM | 0.35 (0.22–0.56) | <0.0001 | |||
| Hypertensive vs. ischaemic CM | 0.79 (0.43–1.45) | 0.44 | |||
| DM | 2.61 (1.53–4.47) | 0.0005 | 2.01 (1.17–3.45) | Fourth (6) | 0.01 |
| History of hypertension | 1.18 (0.78–1.79) | 0.43 | |||
| Atrial fibrillation | 1.90 (1.10–3.27) | 0.02 | |||
| NYHA class >II vs. ≤II | 3.56 (2.31–5.48) | <0.0001 | 2.39 (1.44–3.95) | Third (11) | 0.0009 |
| Framingham HF score (per 1 unit increase) | 1.04 (1.02–1.05) | <0.0001 | |||
| Systolic BP per 10 mmHg increase | 0.90 (0.79–1.02) | 0.09 | |||
| Diastolic BP per 10 mmHg increase | 0.71 (0.57–0.88) | 0.002 | |||
| LV EDVi (per 35 mL/m2 increase) | 1.20 (0.97–1.48 | 0.09 | |||
| LV ESVi (per 31 mL/m2 increase) | 1.24 (1.01–1.53) | 0.05 | |||
| LVEF (per 5% increase) | 0.88 (0.78–0.99) | 0.04 | |||
| Mitral regurgitation | 1.99 (1.26–3.14) | 0.003 | |||
| EDT (per 52 ms increase)a | 0.78 (0.62–0.98) | 0.03 | |||
| TAPSE ≤14 mm vs. >14 mm | 3.09 (1.85–5.14) | <0.0001 | 2.51 (1.44–4.39) | Second (19) | <0.0001 |
| PASP >35 mmHg vs. ≤35 mmHga | 2.46 (1.57–3.86) | <0.0001 | |||
| eRAP >6 mmHg vs. ≤6 mmHg | 1.71 (0.94–3.08) | 0.08 | |||
| NT-pro-BNP (per 3000 pg/mL increase) | 1.85 (1.44–2.36) | <0.0001 | |||
BP, blood pressure; CI, confidence interval; CM, cardiomyopathy; DM, diabetes mellitus; EDT, E wave deceleration time; EDVi, end-diastolic volume index; eGFR, estimated glomerular filtration rate; eRAP, estimated right atrial pressure; ESVi, end-systolic volume index; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio; PASP, pulmonary artery systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
an = 343 participants included in heart rate univariable analysis; n = 364 available for PASP univariable analysis; n = 334 patients included in EDT univariable analysis; heart rate, PASP, and EDT were not eligible for inclusion in stepwise regressions due to missing data. Adding these variables to manually constructed multivariable models did not result in meaningful changes to the findings summarized in the table.
TAPSE and other predictors of mortality
During a mean follow-up time of 31 months (range 1.0–90 months), 121 patients died; overall mortality rate = 32%. Several parameters were associated with increased risk of death in univariable models (Table 3).
Table 3.
Univariable and multivariable predictors of all cause mortality among 373 patients with systolic heart failure
| Variable | Univariate |
Multivariate |
P-value | ||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | Selection order (score χ2) | ||
| Age (per 5 year increase) | 1.18 (1.08–1.30) | 0.0005 | 1.15 (1.04–1.27) | Seventh (6) | 0.02 |
| Male | 1.32 (0.82–2.11) | 0.25 | 1.98 (1,19–3.30) | Sixth (6) | 0.01 |
| BMI (per 1 m/kg2 increase) | 0.94 (0.90–0.99) | 0.02 | |||
| Heart rate (per 10 b.p.m. increase)a | 1.14 (1.01–1.28) | 0.04 | |||
| Idiopathic vs. ischaemic CM | 0.78 (0.51–1.18) | 0.24 | |||
| Hypertensive vs. ischaemic CM | 1.59 (0.96–2.62) | 0.07 | |||
| DM | 1.57 (1.07–2.32) | 0.02 | |||
| Hypertension | 1.19 (0.83–1.71) | 0.93 | |||
| Atrial fibrillation | 1.40 (0.89–2.19) | 0.14 | |||
| NYHA class >II vs. ≤II | 2.31 (1.60–3.34) | <0.0001 | |||
| Framingham HF score (per 1 unit increase) | 1.04 (1.03–1.05) | <0.0001 | 1.02 (1.01–1.03) | Second (17) | <0.0001 |
| Systolic BP (per 10 mmHg increase) | 0.84 (0.75–0.94) | 0.004 | |||
| Diastolic BP (per 10 mmHg increase) | 0.72 (0.58–0.87) | 0.001 | |||
| LV EDVi (per 35 mL/m2 increase) | 1.54 (1.31–1.80) | <0.0001 | 1.46 (1.24–1.72) | Fourth (11) | 0.0008 |
| LV ESVi (per 31 mL/m2 increase) | 1.59 (1.36–1.86) | <0.0001 | |||
| LVEF <25% vs. ≥25% | 1.94 (1.32–2.87) | 0.0008 | |||
| Mitral regurgitation | 1.97 (1.37–2.82) | 0.0002 | |||
| EDT per 52 ms increasea | 0.68 (0.54–0.85) | 0.0007 | |||
| TAPSE ≤14 mm vs. >14 mm | 2.72 (1.88–3.93) | <0.0001 | 1.80 (1.20–2.71) | Fifth (8) | <0.01 |
| PASP >35 mmHg vs. ≤35 mmHga | 1.96 (1.28–.00) | 0.002 | |||
| eRAP >6 mmHg vs. ≤6 mmHg | 3.15 (2.11–4.70) | <0.0001 | 1.79 (1.08–2.97) | Eighth (3) | 0.02 |
| NT-proBNP (per 3000 pg/mL increase) | 1.29 (1.21–1.37) | <0.0001 | First (76)b | <0.0001 | |
| eGFR <60 mL/min/1.73 m2 | 2.75 (1.83–4.14) | <0.0001 | 1.86 (1.19–2.90) | Third (11) | 0.0008 |
BP, blood pressure; CI, confidence interval; CM, cardiomyopathy; DM, diabetes mellitus; EDT, E wave deceleration time; EDVi, end-diastolic volume index; eGFR, estimated glomerular filtration rate; eRAP, estimated right atrial pressure; ESVi, end-systolic volume index; HF, heart failure; HR, hazard ratio; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
an = 343 participants included in heart rate univariable analysis; n = 364 available for PASP univariable analysis; n = 334 patients included in EDT univariable analysis; heart rate, PASP, and EDT were not eligible for inclusion in stepwise regressions due to missing data. Adding these variables to manually constructed multivariable models did not result in meaningful changes to the findings summarized in the table.
bNT-proBNP entered into the model first but was removed in the final multivariable model. The HR for a 3000 pg/mL increase in NT-proBNP was 1.07 (0.99–1.16).
In crude analyses, the hazard ratio (HR) for all-cause mortality among patients with TAPSE ≤14 mm vs. those with TAPSE >14 mm was 2.72 (95% CI 1.88–3.93), P < 0.0001. The crude HR for estimated GFR <60 mL/min/1.73 m2 predicting mortality was 2.75 (95% CI 1.83–4.14); P < 0.05. TAPSE, age, male gender, Framingham HF score, LV end-diastolic volume index, estimated RAP >6 mmHg, and estimated GFR <60 mL/min/1.73 m2 were all selected as predictors of mortality in the final multivariable model (Table 3). NT-proBNP predicted mortality in univariable models (Table 3) and was the first parameter selected in stepwise regression. However, after the full multivariable model was constructed, NT-proBNP was not statistically significantly associated with mortality: HR 1.07 (95% CI0.99–1.16); P = 0.09.
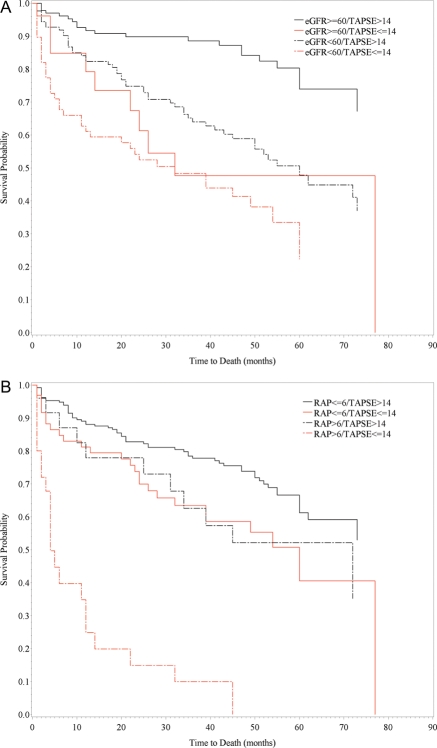
The combination of TAPSE with either CKD or estimated RAP was also evaluated to determine the ability of minimal prognostic models to predict mortality (Figure 2). Mortality was lowest in patients with TAPSE >14 mm and estimated GFR >60 mL/min/1.73 m2 (Figure 2A). Patients with CKD, presumably from aetiologies other than RV dysfunction (their TAPSE being >14 mm) had an intermediate prognosis. Of note, prognosis was also intermediate in those patients who were able to maintain normal kidney function despite low TAPSE (≤14 mm). The combination of severe RV and renal dysfunction was associated with poor prognosis in our patient cohort: 50% survival at 2 years. There was marginal statistical evidence for an interaction between CKD and TAPSE (P = 0.06).
Figure 2.
Kaplan–Meier plots showing survival (A) in patients with systolic chronic heart failure categorized according to tricuspid annular plane systolic excursion (TAPSE) ≤14 mm or >14 mm and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or ≥60 mL/min/1.73 m2; and (B) in patients categorized according to TAPSE ≤14 mm or >14 mm and right atrial pressure (RAP) ≤6 mmHg or >6 mmHg. P-values for (A) and (B) < 0.0001.
When evaluating the combination of TAPSE and estimated RAP, patients with preserved RV function and normal right side filling pressures had the lowest mortality. Patients with TAPSE ≤14 mm and estimated RAP <6 mmHg as well as those with increased estimated RAP and TAPSE >14 mm had an intermediate prognosis. Low TAPSE and high estimated RAP identified a unique population of patients with particularly poor survival (Figure 2B). Although the survival pattern observed suggests an interaction between TAPSE and estimated RAP, there was no statistical evidence for interaction (P = 0.45).
Discussion
The present results indicate that after multivariable adjustment TAPSE ≤14 mm as a measure of RV dysfunction was strongly associated with prevalent CKD as well as risk for death in outpatients with chronic systolic HF. Patients with RV dysfunction experienced an ∼2.5-fold increase in the odds for estimated GFR <60 mL/min/1.73 m2, and an 80% increase in the risk for death during up to 8 years of longitudinal follow-up.
Relationship of right ventricular dysfunction with chronic kidney disease
The pathogenic mechanisms responsible for worsening renal function in acute and chronic HF are multifactorial and not fully characterized. Historically, several studies have demonstrated that reduced cardiac output and RBF are strong determinants of GFR in patients with chronic HF, so-called forward failure.5,6 However, while decreased resting cardiac output may contribute to decreased RBF and decreased GFR in advanced HF, elevated renal venous pressure from backward failure due to RV dysfunction may also play an important and possibly earlier role in the pathophysiology of impaired renal function in chronic HF.23,24 Under this framework, elevated renal venous pressure will decrease GFR by: (i) increasing interstitial and tubular hydrostatic pressures within the kidneys (which are encapsulated organs); and (ii) decreasing renal perfusion pressure and RBF. Hypoxia as well as local and systemic neurohormonal activation from elevated venous pressure may further compromise kidney function in these patients.25−27
Recent reports have associated increased RAP with worsening renal function in acute7 and chronic HF8 in pulmonary hypertension,9 and in an unselected population of patients who had various clinical indications for right heart catheterization.10 However, little is known as to whether specific cardiac abnormalities such as RV dysfunction contributed to renal impairment. This is a key issue as renal dysfunction, by causing fluid retention, may also raise RAP regardless of concomitant cardiac dysfunction. Testani et al. studied unstable patients who were hospitalized for acute decompensated HF and were acutely treated with a high dose of intravenous diuretics.28 The authors found that the presence of RV dysfunction and venous congestion predicts improvement and not worsening of renal function during the index hospitalization. They further suggest that relief of congestion in a setting of aggressive diuresis probably mediated these findings. In the present study we investigated the relationship between a comprehensive set of clinical, echo-Doppler, and biochemical parameters, and CKD in stable outpatients with chronic systolic HF. Our results showed that RV dysfunction as measured by TAPSE ≤14 mm was an important determinant of CKD in this patient population. Of note, LVEF and blood pressure were not associated with decreased estimated GFR after multivariate adjustment. Although our results are somewhat inconsistent with earlier reports,8,29 we believe it is possible that previous studies reporting LVEF and blood pressure to be significantly related to CKD in multivariable models might do so because they have not adequately accounted for parameters related to RV dysfunction (such as TAPSE). From a mechanistic standpoint, we also show that reduced TAPSE is associated with elevated RAP and NT-proBNP levels as well as lower blood pressure, suggesting venous congestion and reduced renal perfusion pressure (the latter eventually resulting from both high RAP and low cardiac output) to be possible mechanistic links between RV dysfunction and CKD. Importantly, from this perspective, coupling between LV and RV function may also impair cardiac output and, thereby, arterial perfusion pressure and RBF. The observed association between reduced TAPSE and lower blood pressure may suggest limited forward flow to be an important contributor to the relationship between RV dysfunction and CKD.
The casual relationship between RV function and venous congestion has been extensively characterized by Guyton in his seminal work on cardiovascular equilibrium.30−34 Guyton superimposed venous return curves on cardiac output curves to reveal their intersection at a specific level of RAP and blood flow, which is influenced by the contractile properties of the right ventricle. According to this concept, when the right ventricle fails RAP is driven up to a new equilibrium that makes cardiac output and venous return equal. This basic concept provides a causal foundation to our findings since advanced RV dysfunction (as reflected in our study by a severely reduced TAPSE) may precede and directly contribute to increased RAP that, in turn, by promoting systemic congestion, may mechanistically account for the association between impaired RV function and reduced GFR.
Prognostic implications
Right ventricular dysfunction and CKD are associated with a poor outcome in chronic systolic HF. Ghio et al.11 as well as Kjaergaard et al.12 found that based on multivariable comparison the same cut-off for TAPSE ≤14 mm adds significant prognostic information in patients with chronic HF. A recent meta-analysis of >18 000 patients showed that an increase in serum creatinine ≥0.2 mg/dL or a corresponding decrease in estimated GFR ≥5 mL/min/1.73 m2 was associated with a 50% higher risk for mortality and 30% higher incidence of hospitalization.35 Our current results confirmed that after accounting for conventional predictors of mortality in HF such as NT-proBNP, LV function, and CKD, TAPSE ≤14 mm (and estimated RAP) was also a strong predictor of mortality in patients with chronic systolic HF.
We also analysed the ability of minimal prognostic models to predict mortality. With respect to the combination of TAPSE with CKD, we found that mortality was lowest in patients with TAPSE >14 mm and estimated GFR >60 mL/min/1.73 m2, and intermediate in those with mild to moderate CKD and normal TAPSE as well as in those who were able to maintain normal kidney function despite reduced TAPSE ≤14 mm. This latter finding suggests that RV dysfunction may affect mortality independently from its renal effects, by limiting, for example, cardiac output in advanced HF (ventricular coupling). Not surprisingly, the combination of severe RV and renal dysfunction was associated with the worst prognosis.
We also explored the prognostic value of combining RV function and estimated RAP, and found that that TAPSE ≤14 mm and normal filling pressures were associated with increased mortality, again suggesting that RV dysfunction may negatively impact survival from mechanisms other than venous congestion (see above). Of note, mortality was similarly increased in those patients who had increased estimated RAP despite TAPSE >14 mm, implying that systemic venous congestion from aetiologies other than RV dysfunction, for example from CKD, may lower survival in patients with chronic systolic HF. Prognosis in patients with severe RV dysfunction and elevated RAP was dismal, with only a 20% survival at 2 years.
Limitations
The present study has several limitations. It comprises a selected population of outpatients with chronic systolic HF who had a clinical indication for transthoracic echocardiography. Our report is also limited by the lack of invasive haemodynamic data and RBF in particular. We also did not directly measure RAP. However, the latter was assessed non-invasively in all patients using a reliable method for estimation of mean RAP from the inspiratory collapse of the inferior vena cava.36 In addition, grading of tricuspid regurgitation is not available in our echocardiographic database. This limitation is important as tricuspid regurgitation increases RAP. Tricuspid regurgitation also reduces RV afterload, thereby attenuating the decrease in TAPSE. However, in the present study, patients were excluded if tricuspid regurgitation was severe (as detailed in the Methods section) which probably minimized the impact of this confounder on our final results. Future studies are needed to examine whether the addition of tricuspid regurgitation to the statistical model removes or diminishes the observed strong associations between TAPSE, CKD, and mortality in patients with chronic systolic HF. Finally, our reported associations between TAPSE and estimated GFR are cross-sectional, precluding any temporal assessment and substantially weakening causal inference on whether the relationship between RV function and estimated GFR is from heart to kidney, bidirectional, or actually the reverse. Nevertheless, from an epidemiological perspective, cross-sectional data are quite valuable in combination with other reports7,28 supporting a link between venous congestion and RV and renal function as they demonstrate consistency of results exploring this hypothesis.
Conclusion
Right ventricular dysfunction, as assessed by TAPSE ≤14 mm, is strongly associated with CKD and poor prognosis in outpatients with chronic systolic HF. These data further support the concept that venous congestion from backward cardiac failure might be as important as forward failure in the pathophysiology of renal impairment in HF. As previously reported,10 this concept challenges the intuitive notion that volume overload is beneficial from the point of view of preservation of renal function. These findings set the stage for follow-up studies that can address whether serial measurements of TAPSE, possibly resulting in early and aggressive treatment of RV dysfunction, for example through reduction of pulmonary pressures, may favourably impact the progression of CKD and the overall survival in patients with chronic systolic HF.
Funding
National Institutes of Health (grant R01 HL092144 to P.C.C. grant R00 DE018739 to R.T.D.).
Conflict of interest: none declared.
References
- 1.Waldum B, Westheim AS, Sandvik L, Flonaes B, Grundtvig M, Gullestad L, Hole T, Os I. Renal function in outpatients with chronic heart failure. J Card Fail. 2010;16:374–380. doi: 10.1016/j.cardfail.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Martins D, Ani C, Pan D, Ogunyemi O, Norris K. Renal dysfunction, metabolic syndrome and cardiovascular disease mortality. J Nutr Metab. 2010;2010:167162. doi: 10.1155/2010/167162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurth T, de Jong PE, Cook NR, Buring JE, Ridker PM. Kidney function and risk of cardiovascular disease and mortality in women: a prospective cohort study. BMJ. 2009;338:b2392. doi: 10.1136/bmj.b2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DD, Hubbard RB, Bulpitt CJ, Fletcher AE. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis. 2009;53:950–960. doi: 10.1053/j.ajkd.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Cody RJ, Ljungman S, Covit AB, Kubo SH, Sealey JE, Pondolfino K, Clark M, James G, Laragh JH. Regulation of glomerular filtration rate in chronic congestive heart failure patients. Kidney Int. 1988;34:361–367. doi: 10.1038/ki.1988.189. [DOI] [PubMed] [Google Scholar]
- 6.Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and ‘modifiable’ risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009;98:121–129. doi: 10.1007/s00392-008-0732-z. [DOI] [PubMed] [Google Scholar]
- 7.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damman K, Voors AA, Hillege HL, Navis G, Lechat P, van Veldhuisen DJ, Dargie HJ. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. doi: 10.1093/eurjhf/hfq118. [DOI] [PubMed] [Google Scholar]
- 9.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 11.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 12.Kjaergaard J, Akkan D, Iversen KK, Kober L, Torp-Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 14.Furey SA, 3rd, Zieske HA, Levy MN. The essential function of the right ventricle. Am Heart J. 1984;107:404–410. doi: 10.1016/0002-8703(84)90402-2. [DOI] [PubMed] [Google Scholar]
- 15.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Bennett SJ, Burke G, Eagle KA, Krumholz HM, Linderbaum J, Masoudi FA, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association task force on performance measures (writing committee to develop heart failure clinical performance measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol. 2005;46:1144–1178. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao SH, Lin SK, Wang WC, Yang SH, Gin PL, Liu CP. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr. 2006;19:902–910. doi: 10.1016/j.echo.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Hall SA, Brickner ME, Willett DL, Irani WN, Afridi I, Grayburn PA. Assessment of mitral regurgitation severity by doppler color flow mapping of the vena contracta. Circulation. 1997;95:636–642. doi: 10.1161/01.cir.95.3.636. [DOI] [PubMed] [Google Scholar]
- 19.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, McGregor K, Morais J, Oto A, Smiseth OA, Barbera JA, Gibbs S, Hoeper M, Humbert M, Naeije R, Pepke-Zaba J. Guidelines on diagnosis and treatment of pulmonary arterial hypertension The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 21.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 23.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1:1033–1035. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 24.Tang WH, Mullens W. Cardiorenal syndrome in decompensated heart failure. Heart. 2010;96:255–260. doi: 10.1136/hrt.2009.166256. [DOI] [PubMed] [Google Scholar]
- 25.Ganda A, Onat D, Demmer RT, Wan E, Vittorio TJ, Sabbah HN, Colombo PC. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr Heart Fail Rep. 2010;7:66–74. doi: 10.1007/s11897-010-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damman K, Kalra PR, Hillege H. Pathophysiological mechanisms contributing to renal dysfunction in chronic heart failure. J Ren Care. 2010;36(Suppl 1):18–26. doi: 10.1111/j.1755-6686.2010.00172.x. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 28.Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, Kirkpatrick JN. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight EL, Glynn RJ, McIntyre KM, Mogun H, Avorn J. Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: results from the studies of left ventricular dysfunction (SOLVD) Am Heart J. 1999;138:849–855. doi: 10.1016/s0002-8703(99)70009-8. [DOI] [PubMed] [Google Scholar]
- 30.Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35:123–129. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- 31.Guyton AC. Circulartory Physiology: Cardiac Output and its Regulation. Philadelphia, PA: Saunders; 1963. [Google Scholar]
- 32.Guyton AC, Jones CE, Coleman TG. Effect of Right Atrial Pressure on Venous Return—The Normal Venous Return Curve. Philadelphia, PA: Saunders; 1973. [Google Scholar]
- 33.Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol. 1957;189:609–615. doi: 10.1152/ajplegacy.1957.189.3.609. [DOI] [PubMed] [Google Scholar]
- 34.Guyton AC, Lindsey AW, Kaufmann BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Physiol. 1955;180:463–468. doi: 10.1152/ajplegacy.1955.180.3.463. [DOI] [PubMed] [Google Scholar]
- 35.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Pepi M, Tamborini G, Galli C, Barbier P, Doria E, Berti M, Guazzi M, Fiorentini C. A new formula for echo-doppler estimation of right ventricular systolic pressure. J Am Soc Echocardiogr. 1994;7:20–26. doi: 10.1016/s0894-7317(14)80414-8. [DOI] [PubMed] [Google Scholar]