Abstract
The ability of Lepidoptera, or butterflies and moths, to drink liquids from rotting fruit and wet soil, as well as nectar from floral tubes, raises the question of whether the conventional view of the proboscis as a drinking straw can account for the withdrawal of fluids from porous substrates or of films and droplets from floral tubes. We discovered that the proboscis promotes capillary pull of liquids from diverse sources owing to a hierarchical pore structure spanning nano- and microscales. X-ray phase-contrast imaging reveals that Plateau instability causes liquid bridges to form in the food canal, which are transported to the gut by the muscular sucking pump in the head. The dual functionality of the proboscis represents a key innovation for exploiting a vast range of nutritional sources. We suggest that future studies of the adaptive radiation of the Lepidoptera take into account the role played by the structural organization of the proboscis. A transformative two-step model of capillary intake and suctioning can be applied not only to butterflies and moths but also potentially to vast numbers of other insects such as bees and flies.
Keywords: Lepidoptera, capillarity, flexible microfluidics, permeability, plateau instability, porous materials
1. Introduction
The Lepidoptera, or butterflies and moths, constitute the second largest order of insects, with nearly 160 000 species [1], or about 16 per cent of all described insects. Using their proboscises, adults acquire nutrition, water and pheromonal precursors from diverse sources, such as soil, floral and extrafloral nectar, fruit, sap, plant surfaces, sugary exudates of plant-feeding insects, animal blood, carrion, dung, sweat, tears and urine [1–3]. The lepidopteran proboscis traditionally has been considered to function as a drinking straw, with the assumption that the insect depends only on the action of the sucking pump in its head to acquire liquids [4,5]. Given the remarkable diversity of feeding habits and the versatility of the proboscis, we asked (i) whether the conventional view of the proboscis as a drinking straw can explain the full range of functionality for fluid uptake, and (ii) how the mechanism of fluid uptake might have facilitated diversification of lepidopteran feeding habits and movement into adaptive zones such as fruit feeding.
The drinking-straw model becomes questionable when attempting to explain the ability of Lepidoptera to drink from limited nectar droplets and films in floral tubes or from porous substrates, such as rotting fruit and wet soil; fluid uptake would be hindered by the capillary pressure created by the liquid menisci. Yet, models of fluid feeding by butterflies and moths assume an exclusive role for the sucking pump [6]; if any significance is attributed to capillarity, it is merely a hypothesized role for the initial fluid intake [4]. Here, we provide the first experimental demonstration of the integrated role of the sucking pump and capillarity in insects, showing the essential role of capillary action in acquiring liquids from porous substrates.
2. Morphology of the lepidopteran proboscis
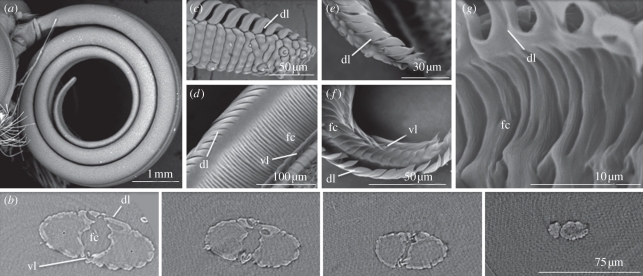
The lepidopteran proboscis (figure 1a), ranging in length from less than 1 mm to nearly 30 cm, consists of a pair of modified mouthparts, the maxillary galeae, joined by dorsal and ventral linkage devices termed legulae (figure 1b–g). The concave medial surfaces of the galeae form a food canal (figure 1b,d,f,g). The dorsal legulae consist of a series of partially overlapping plates with intervening spaces, whereas the ventral legulae are represented by tightly spaced, interlocking hooks [1,7]. The dorsal legulae of the apical 5–20% of the proboscis are more widely spaced than elsewhere (figure 1c,e), allowing liquid to enter what otherwise is putatively a fluid-tight tube sealed with glandular secretions [1,4,7]. The validity of a sealed tube, permeable only at the seam of the distal one-fifth or less of the dorsal legulae, however, has not been tested until now.
Figure 1.
Photomicrographs of the proboscises of Danaus plexippus (monarch butterfly, Nymphalidae) and Dyseriocrania griseocapitella (Eriocraniidae), showing that physical features of the proboscis promoting capillarity were present in early (eriocraniid) and derived (monarch) lineages. (a) Coiled proboscis of monarch. (b) Series of X-ray tomography cross sections of monarch proboscis at distances (left to right) of 261.75, 139.50, 43.50 and 6.75 µm from the extreme apex, showing dorsal legulae (dl), ventral legulae (vl), food canal (fc) and absence of an apical straw-like opening. (c) Apex of single galea of monarch proboscis with enlarged dorsal legulae and inter-legular spaces. (d) Food canal of monarch proboscis showing annulations that probably aid capillarity. (e) Apex of single galea of the proboscis of D. griseocapitella, with enlarged dorsal legulae and inter-legular spaces. (f) Food canal of D. griseocapitella with plate-like grooves that might aid capillarity. (g) Close-up image of food canal of D. griseocapitella showing smaller striations, in addition to deeper annulations, that might facilitate capillarity.
3. Relation of proboscis morphology to feeding mechanism
3.1. Feeding from porous materials: critical pore size
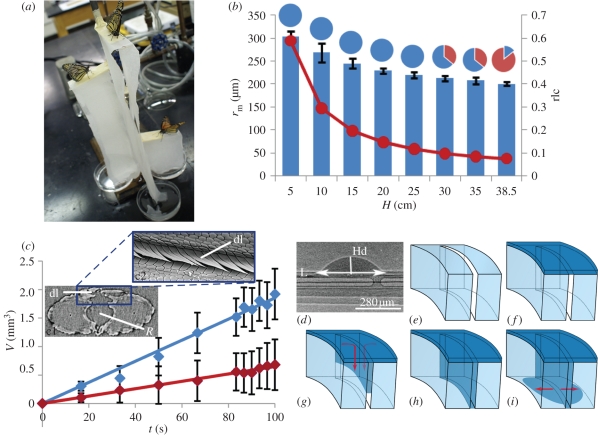
Although butterflies and moths can acquire fluids from a range of porous substrates, we hypothesized that they withdraw liquid from only a specific size range of pores because of fluid dynamics in relation to structure. We tested this hypothesis by placing monarch butterflies, Danaus plexippus, on a porous material (paper towels) saturated in 25 per cent sucrose solution (figure 2a). The saturated towels were suspended at different heights, with their ends submersed in the sucrose solution, until a hydrostatic equilibrium was reached. This equilibrium implied that the liquid was not uniformly distributed, and that the height and the size of the pores retaining the liquid were inversely correlated. Monarch butterflies then were placed on the towels to determine the heights at which they would feed (figure 2a), as determined by the extension and movements of the proboscis [8] (see §5). Butterflies generally would not drink from filled pores with a radius, rm, less than rm < 35 ± 5 µm (figure 2b, rlc)—about the average radius, R, of the food canal in the distal region of the proboscis.
Figure 2.
Determination of limiting pore size used by monarch butterflies. (a) Monarch butterflies were fed 25% aqueous sucrose solution from paper towels suspended at different heights to determine the limiting pore radius from which they could feed. (b) Minimum radius rm of open pores in the paper towel (filled circle solid curve) and towel-saturation level (grey bars) versus height H of the stage from which butterflies were fed. Pie charts represent the proportion of butterflies that drank (grey) or did not drink (dark grey) at each height. At heights of 5–25 cm, all butterflies fed. At 30 and 35 cm, five of 14 butterflies did not drink. At 38.5 cm, only two of 14 butterflies fed. The pore size at 38.5 cm is considered the critical minimum pore radius. The bar graph (with standard deviations) shows the liquid content at each height relative to the liquid content in the completely saturated towel (rlc) (see §5 for details). (c) Change of drop volume (V) as a function of time (t), demonstrating that the spaces between the overlapping dorsal legulae (dl) are permeable to water (with standard deviation bars). A drop was deposited 5 mm (dark grey) and 10 mm (grey) from the head. c1, cross-section of monarch proboscis at 261.75 µm from the apex, with radius of food canal (R) and effective porous surface boxed in grey. c2, dorsal view of effective porous surface of the proboscis showing slits between dorsal legulae. (d) Geometrical parameters used for calculation of absorption rate of the drop (Hd, height of drop; L, length of drop base; see §5). The liquid bridge belongs to a series of bridges moving towards the head (to the left). (e–i) Schematic of drop penetration through the slits between the dorsal legulae of the proboscs. Arrows indicate the direction of liquid flow. (Online version in colour.)
The approximate equality between rm and R suggests that the food canal would initiate fluid uptake by functioning like a capillary tube, relying on capillary rise. However, our scanning electron microscopy and X-ray tomography (figure 1a,b) demonstrate that the food canal tapers distally and lacks a distinct terminal opening. Other lepidopteran species are claimed to have no terminal opening [1,9]. The lack of a terminal opening would prevent capillary rise, drawing into question the similarity of the proboscis to a drinking straw. The test butterflies also did not probe the porous substrates with the end of their proboscises, but instead pressed the dorsal surface of the distal region to the substrate, leaving the extreme apex unengaged; similar behaviour is characteristic of butterflies and moths feeding from rotting fruits and other wet substrates [2,10–12].
This feeding behaviour is explained by the specific structure of the proboscis. The dorsal legulae, comprising the roof of the food canal, have spaces between them. In the distal, so-called drinking region, the spaces averaged 2.6 ± 0.12 µm (n = 3 females) (figure 1c), which are smaller than the radius of the substrate pores (figure 2b), suggesting that menisci form between the legulae as a means of initial fluid intake from the substrate pores. This method of fluid intake, however, does not explain the approximate equality between rm and R; the spaces between the dorsal legulae are at least 15 times smaller than the average radius of the food canal, raising the question of why rm is comparable with R but not with the inter-legular spaces.
3.2. X-ray phase-contrast imaging of feeding from pores and small droplets
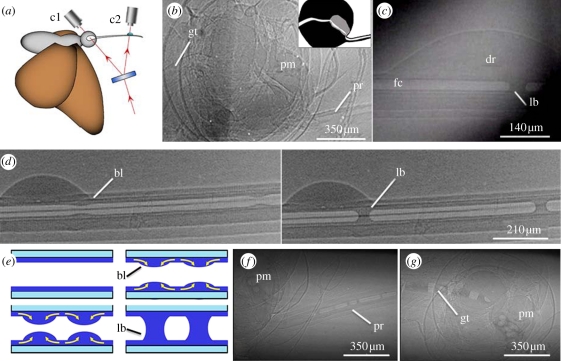
To further explore the mechanism of liquid uptake and the relationship between rm and R, we employed X-ray phase-contrast imaging [13] in a series of experiments with monarch butterflies offered water only or a 15 per cent aqueous sucrose solution with 40 per cent iodine (Isovue 370, Bracco Diagnostics, USA). X-ray phase-contrast imaging could distinguish fluids (saliva, water and sucrose solutions) and air in the food canal from the surrounding cuticle (figure 3b–d,f,g). When butterflies fed from a pool of the sucrose and iodine solution, a continuous liquid column formed in the food canal. However, when butterflies fed from a porous material (Kimwipes) saturated with sucrose and iodine solution, they withdrew the fluid from the substrate pores as a series of liquid bridges in the food canal separated by air bubbles (figure 3d,f,g). Although adult Lepidoptera can produce saliva to dilute viscous fluids and solubilize dried substances such as sugars [14], the butterflies in this experiment did not introduce saliva into their food canal before feeding. In other words, before contact with a saturated substrate or drop, no menisci were in the food canal and no visible liquid film was on the wall. If any liquid (e.g. glandular secretion) was in the proboscis before feeding, the amount was not detectable as a liquid bridge.
Figure 3.
X-ray phase-contrast imaging of the monarch butterfly proboscis and formation of liquid bridges. (a) Schematic displaying the split-beam (arrows) X-ray phase-contrast imaging used to simultaneously view liquid-bridge formation in the proboscis and action of the sucking pump, using cameras c1 and c2. (b,c) Raw split-screen images acquired from c1 and c2, respectively. (b) Camera 1 displays an empty food canal in the proboscis (pr) that leads to the sucking pump (pm; empty here). Fluid travels through the pump into the gut (gt); inset indicates the path that fluid travels (white) and musculature of the sucking pump (grey). (c) Camera 2 shows a liquid drop (dr) on the dorsal legulae of the proboscis, with a liquid bridge (lb) in the food canal (fc). (d) Series of two X-ray phase-contrast images showing a liquid drop on the proboscis and the formation of two bulges (bl) that have collapsed into liquid bridges. (e) Schematic of meniscus formation. Fluid moves into the food canal, forming a film that enlarges into a bulge. The liquid bulge enlarges until it collapses into a liquid bridge due to Plateau instability. Arrows show fluid motion. (f,g) Series of liquid bridges separated by air pockets in the proboscis, (f) pump and (g) gut of the butterfly that travel through the uptake system. (b,c) Acquired using a split-beam set-up; remaining X-ray phase-contrast images were acquired with a single beam. Brightness of the food canal in (c,d,f,g) were digitally enhanced. (Online version in colour.)
Our observations demonstrate that liquid-bridge formation is facilitated by the sponge-like capillary activity of the spaces between the dorsal legulae. When the dorsal part of the proboscis is brought in contact with a liquid, the liquid is pulled from the source to the spaces between the legulae owing to capillary action. The dorsal linkage structure can be considered an effective porous medium with multiple spaces; that is, it acts like a sponge. When the proboscis, as a sponge, is completely filled with the liquid, the capillary forces do not counteract release of the liquid into the food canal. When the meniscus invades a curved slit-like space of the dorsal linkage and then approaches its inner edge, it wets the slit edges. Even if the final liquid surface is parallel to the walls of the food canal, the negative curvature of the food canal reduces the pressure in the invading liquid, pushing the liquid to overflow the space (see electronic supplementary material, movie S1). Supported by the wetting forces acting over the contact line, the liquid continues to overflow the linkage, forming a wetting film inside the food canal (figure 2e–i). This process should apply to all Lepidoptera with a functional proboscis.
X-ray phase-contrast imaging revealed that liquid uptake occurs not only between the distal dorsal legulae of the proboscis, typically referred to as ‘drinking slits’ [1] (figure 1c,e), but also between the dorsal legulae proximal to this region (figures 1d and 2c). Drops of water applied to the dorsal linkage of the straightened proboscis at 5 and 10 mm from the head (n = 3 butterflies per region; mean proboscis length = 17 ± 2 mm, n = 8) produced a series of liquid bridges in the food canal that were transported to the head, demonstrating that the proximal region of the proboscis is not sealed to all liquids. The drop disappears faster at 10 mm, suggesting that the slit-like spaces increase in size distally. The structure of the dorsal linkage thus consists of multiple capillary channels forming a porous structure with a pore-size gradient. From the rate of drop absorption, with experimental controls accounting for evaporation, we estimated the size of these slit-like spaces between the dorsal legulae to be 96 ± 27 nm, and 162 ± 18 nm at 5 and 10 mm, respectively, from the head (see §5). These differences suggest that the dorsal legulae in the distal region transport the liquid more easily. The larger slits in this region should decrease viscous resistance to flow.
When a thin cylindrical film forms in the food canal, it becomes subject to Plateau instability [15–18]; the liquid body seeks a new configuration to decrease its surface energy. Plateau instability [15–18] is manifested as liquid bulges on the walls of the food canal (figure 3d,e). Plateau instability assumes that any thermal or mechanical perturbation of the film surface will amplify only if the perturbation spans a length greater than the circumference of the food canal, or more correctly, greater than 2 × 21/2 πR [16]. The average basal length of the liquid bulge calculated from the images is 188 ± 9 µm and the average circumference of the proboscis at the onset of Plateau instability is 111 ± 24 µm (n = 5); the incoming fluid enlarges the bulge until it collapses into a liquid bridge, confirming that the process is driven by Plateau instability (figure 3d). Multiple bulges form and grow simultaneously along the food canal (figure 3d), collapsing into liquid bridges that are transported into the gut by negative pressure generated by the sucking pump (figure 3b,f,g; see electronic supplementary material, movie S2). The pump action does not affect the liquid film, which remains on the surface of the food canal during the entire uptake process. The liquid film acts as a continuous junction between the external liquid and the liquid bridges in the food canal. Annulations of the food canal (figure 1d,g) might enhance spreading of the film on the inner walls of the proboscis and lead to the formation of multiple bridges along the channel to improve the efficiency of the liquid uptake. We also suspect that hydrophilicity of the proboscis is an important component of liquid uptake, and we are currently investigating the hydrophilic properties.
Simultaneous recordings of bridge formation and pump activity, using split-beam X-ray phase-contrast imaging, reveal that the bridges form in 0.15 s, at least three times faster than the observed pump cycle of 0.45–0.7 s. We discovered that liquid bridges form even when the pump is not activated, implying that they are a product of the architecture of the proboscis and independent of the sucking pump. Even when inter-legular spaces fill, fluid uptake is possible only if rm is larger than R because stable liquid bridges cannot form when opposed by counter-capillary forces exerted by the substrate (i.e. when rm < R).
4. Discussion
4.1. Relation between proboscis morphology and feeding mechanism
Our work suggests that the lepidopteran proboscis is a complex fluidic system with at least two levels of channel hierarchy. Spaces between the dorsal legulae constitute the first nanolevel of channel hierarchy; these spaces enhance capillary action for liquid uptake in a manner similar to a sponge. The food canal, in conjunction with the sucking pump, represents a second microlevel by supporting the flow of liquid bridges to the mouth, similar to a drinking straw. The butterfly proboscis thus represents a sponge and a drinking straw in a single embodiment.
The hierarchical organization of the proboscis provides a model for artificial probes capable of acquiring diverse liquids from different environments (e.g. pooled fluids, damp materials); at least two levels of pores are needed: nanopores to provide strong capillarity and micropores to facilitate fluid transport. This channel hierarchy supports the uptake of liquids from porous substrates and small droplets in a two-step process. Liquid bridges are formed by capillary action and then transported to the gut by the sucking pump. The lepidopteran fluidic system offers a unique model for the integration of nano- and microchannels, shifting the existing microfluidic paradigm from stationary channel-like structures towards fibre-based microfluidic devices providing distributed actuation, sensing and manipulation of minute amounts of fluids.
4.2. Role of the proboscis in diversification of lepidopteran feeding habits
Structural similarities in the proboscis of ancient (e.g. family Eriocraniidae) and derived (e.g. butterfly) lineages of Lepidoptera (figure 1c–f) suggest that the mechanism of liquid uptake through the dorsal legulae has been in place since the evolution of the earliest Lepidoptera with a proboscis (100–146 Mya [19,20]). Capillarity, thus, must have had an early selective advantage, perhaps in exploiting limited availability of moisture or sugary exudates. Scanning electron micrographs of the proboscis representing a wide range of additional lepidopteran groups [1,9,10] confirm the structural organization that promotes capillarity, particularly the spaces between the dorsal legulae. The pleated food canal and loosely spaced ventral legulae of the proboscis of numerous Lepidoptera (figure 1f; [9]) probably augment the capillarity of the dorsal legulae, as would the annulations and finer cuticular striations (figure 1g).
The structural organization of the proboscis would have overcome the physical challenge of withdrawing liquids from porous substrates and from films and droplets trapped in crevices of vegetation. Thus, with capillarity enabling feeding from sources with limited liquids, early Lepidoptera could have exploited not only nectar in floral tubes but also the increased availability of rotting fruit resulting from diversification of the flowering plants. The dual functionality of the proboscis also would have enabled opportunistic and specialized feeding from damp soil, animal products and other sources.
Most explanatory models of lepidopteran biodiversification focus on the larva–foodplant interface [21]. The possible role of the proboscis in facilitating the adaptive radiation of butterflies and moths, such as the exclusively fruit-feeding butterflies [12], has received scant attention. The vast majority of Lepidoptera have a proboscis that is believed to have evolved once [1]. We suggest that future studies explore the possible role that the proboscis has played as a key innovation fostering the enormous biodiversification of the Lepidoptera, perhaps facilitated by slight structural modifications [13] in the associated cuticle. The widespread porous and fibrillar structures of mouthparts used for fluid uptake in additional insect groups, such as flies and bees [22], suggest that capillary action also has played a significant, yet unexplored, role in the diversification of their feeding strategies.
5. Experimental materials and procedures
5.1. Species investigated
We selected the monarch butterfly, D. plexippus L., as a model species capable of drinking from both floral tubes and porous substrates, and supplemented our structural analyses with anatomical observations of the proboscis of one of the earliest lineages of the Lepidoptera with a proboscis, the Eriocraniidae, represented by Dyseriocrania griseocapitella (Walsingham). Monarchs were wild caught in Clemson, SC, USA or obtained from the Shady Oak Butterfly Farm (Brooker, FL, USA) and Flutterby Gardens (Bradenton, FL, USA). Eriocraniids were obtained from the collection of the McGuire Centre for Lepidoptera and Biodiversity; the specimens originally were collected in the southeastern United States.
5.2. Scanning electron microscopy and tomography
Each proboscis, attached to the head, was moved through a series of ethanol washes (80–100%, 48 h each), followed by chemical drying with hexamethyldisilazane. The dried heads were mounted on carbon-graphite tape affixed to aluminium stubs. Samples were imaged without sputter-coating at variable pressure in a Hitachi S3400 scanning electron microscope (20 kv) (figure 1a,d), or with gold sputter-coating for 2–3 min at full vacuum in a Hitachi TM3000 (15 kv) (figures 1c,e–g and 2c, c2). The auto-contrast function in Adobe Photoshop was applied to images in figure 1.
5.3. Preparation of porous samples with different liquid content
Paper towels were chosen as a model porous substrate because they have a broad range of pore sizes, from tens to several hundred of micrometres. A 0.03 cm thick paper towel (Mardi-Gras White, 709-2 Ply sheets, 22.3 × 27.9 cm, Georgia-Pacific Consumer Products) was cut into strips of different lengths, and wetted in 25 per cent aqueous sucrose solution (surface tension of solution: σ ≈ 70 mN m–1; Kruss DSA). Individual strips were removed, shaken to remove excess fluid and wrapped in polyethylene film to restrict evaporation. The wrapped strips were hung over flat 2.5 × 7.5 cm2 stages at heights (H) of 5, 10, 15, 20, 25, 30, 35 and 38.5 cm, with their exposed ends submerged in a dish of the sucrose solution.
To estimate the capillary pressure maintaining the menisci inside the pores of paper towels at different heights, we modelled the pores as an assembly of capillary tubes of different radii r. According to the Jurin law of capillarity [23], liquid rises to the equilibrium height H, defined by the equation H = 2σ/ρrg, where σ is the surface tension of the sucrose solution, ρ its density and g the acceleration due to gravity. Therefore, at height H, pores larger than r > rH = 2σ/ρHg were empty, while pores smaller than r < rH were filled. The capillary pressure holding liquid in the pores at height H is estimated through the Laplace equation Patmospheric − Pliquid = Pc = 2σ/rH. This method allowed us to prepare substrates with different sizes of filled pores.
After 16, 18 and 42 h, the films were removed and samples were cut (4.0 × 2.5 cm2) and weighed to determine saturation level and time to reach equilibrium. After 16 h, equilibrium was reached, which was based on the stabilization of the paper towel weight; subsequent tests used 16 h strips. Weighing the partially wet, completely saturated and dry samples, we calculated the resulting saturation of the towel at each H as S(H) = (M(H) − Mdry)/(Msaturated − Mdry), where M was the sample weight. The liquid fraction gradually decreased as the stage height increased. To confirm that the measured liquid content was independent of size of the sample piece, we cut 2.0 × 2.5 cm pieces and observed that the liquid fraction was approximately the same (±5%). The change in saturation levels supports the hypothesis of uneven effective pore saturation at different heights.
5.4. Determination of minimum pore size used by butterflies
Samples of paper towels were prepared as above. Each monarch butterfly (n = 14) was placed on the paper towel at each stage height, with the highest stage tested first, followed by each lower stage. A butterfly was considered to feed if it uncoiled its proboscis and laid it on the paper towel for at least 30 s. If the butterfly did not initially uncoil its proboscis, we uncoiled it with an insect pin and pressed it against the saturated substrate. If the butterfly continued to recoil the proboscis after probing the substrate two or three times, we placed the butterfly on the next lowest stage and repeated the procedure. The butterfly was categorized as non-drinking at a particular height if it began drinking at the next lower height.
5.5. X-ray phase-contrast imaging experiments
To mimic feeding from porous substrates, we used a glass slide wrapped in a double layer of solution-saturated Kimwipes (KIMTECH, Kimberly Clark). A butterfly was mounted on a stage with its wings secured horizontally and its proboscis coiled around a metal needle attached to a linear positioning stage (VT-21, MICOS USA). The position of the proboscis relative to the X-ray beam and saturated Kimwipe was adjusted remotely by controlling the position of the needle. Feeding was recorded at 30 fps (Sony digital camera) with a beam intensity of 33.2 keV.
To test fluid intake along the proboscis, the butterfly was secured with wax paper and pins on a piece of Styrofoam. The proboscis was uncoiled and laid across two blocks of rubber (5 × 2 × 4 mm) glued to the Styrofoam, creating a space between the proboscis and the Styrofoam. Movement of the proboscis was restricted by crossing insect pins near the head of the butterfly and near the tip of the proboscis. A drop of water was placed on a metal wire above the proboscis, which was attached to the positioning stage and used to apply the drop to the dorsal side of the proboscis at 5 and 10 mm from the head.
5.6. Estimation of spacing between dorsal legulae
We tested fluid intake along the proboscis by measuring the permeability of the proboscis in two regions: 5 and 10 mm from the head. Each butterfly was set up as in the water application experiment in the beam-room, with constant temperature of 24.7°C and relative humidity of 23 per cent. A drop of water was applied to the dorsal side of the straightened proboscis, and liquid uptake was video-recorded. Each experiment was repeated three times. A control water drop was placed on a wire behind the proboscis to compare fluid uptake kinetics with evaporation kinetics. Drop shape was approximated by a spherical cap of height Hd and base L. We concluded that the decrease in drop volume was a linear function of time (figure 2c). Thus, Darcy's Law [24] could be applied to describe the permeability between the dorsal legulae of the proboscis: dV/dt = (kA/η) · ΔP/h, where dV is an incremental change of the drop volume in time interval dt, A is the total area of the linkage under the drop, η = 0.001 Pa s is water viscosity, ΔP is the pressure drop causing water wicking, h = 30 µm is the distance from the external portion of the dorsal legulae to the food canal, and κ is the permeability. The pressure drop ΔP is equal to capillary pressure ΔP = 2σ/Rc, where σ = 0.072 N m–1 is water surface tension and Rc=35 µm is the radius of the food canal in the regions tested. Although the drop height changed significantly, the base length L ≈ 2 mm remained nearly the same until the drop disappeared. The area A = L·w was calculated as a product of the drop base L and the width of the linkage w ≈ 31 µm. Using these parameters and the measured wicking rate, we estimated the permeability k of the first region (5 mm from the head) as k ≈ 0.76 × 10−15 ± 0.51 × 10−15 m2 and the permeability of the second region (10 mm from the head) as k ≈ 2.2 × 10−15 ± 0.51 × 10−15 m2. Assuming that pores between dorsal legulae have a slit-like shape, we estimate the spacing between adjacent legulae as g = (12·k)1/2.
Acknowledgements
We thank Andrew Warren (McGuire Centre for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida) for the eriocraniid specimens, staff at the Electron Microscope Facility (Clemson University), and Thomas C. Emmel (University of Florida) and J. Mark Scriber (Michigan State University) for reviewing a draft of the manuscript. We are grateful to Vijoya Sa and Chen-Chih Tsai for helping us with experiments at different stages of the research and to high school students Kara Edmond, Caleb Klipowics, Campbell Yore, Steven Rea and Zak Bartholomew who worked in our laboratory during the summers of 2007–2010. This work was supported by National Science Foundation grant no. EFRI 0937985. We also acknowledge Sigma Xi Grants-in-Aid of Research G20100315153485 and G20100315153500. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357.
References
- 1.Krenn H. W. 2010. Feeding mechanisms of adult Lepidoptera: structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 55, 307–327 10.1146/annurev-ento-112408-085338 (doi:10.1146/annurev-ento-112408-085338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler P. H. 1982. Soil- and puddle-visiting habits of moths. J. Lepid. Soc. 36, 161–173 [Google Scholar]
- 3.Smedley S. R., Eisner T. 1995. Sodium uptake by puddling in a moth. Science 270, 1816–1818 10.1126/science.270.5243.1816 (doi:10.1126/science.270.5243.1816) [DOI] [PubMed] [Google Scholar]
- 4.Kingsolver J. G., Daniel T. L. 1995. Mechanics of food handling by fluid-feeding insects. In Regulatory mechanisms in insect feeding (eds Chapman R. F., de Boer G.), pp. 32–74 New York, NY: Springer [Google Scholar]
- 5.Chapman R. F. 1998. The insects: structure and function, 4th edn. Cambridge, MA: Cambridge University Press [Google Scholar]
- 6.Borrell B. J., Krenn H. W. 2006. Nectar feeding in long-proboscid insects. In Ecology and biomechanics: a mechanical approach to the ecology of animals and plants (eds Herrel A., Speck T., Rowe N. P.), pp. 85–212 Boca Raton, FL: CRC Press [Google Scholar]
- 7.Eastham L. E., Eassa Y. E. E. 1955. The feeding mechanism of the butterfly Pieris brassicae. L. Phil. Trans. R. Soc. Lond. B 239, 1–43 10.1098/rstb.1955.0005 (doi:10.1098/rstb.1955.0005) [DOI] [Google Scholar]
- 8.Krenn H. W. 1990. Functional morphology and movements of the proboscis of Lepidoptera (Insecta). Zoomorphology 110, 105–114 10.1007/BF01632816 (doi:10.1007/BF01632816) [DOI] [Google Scholar]
- 9.Krenn H. W., Kristensen N. P. 2000. Early evolution of the proboscis of Lepidoptera (Insecta): external morphology of the galea in basal glossatan moths lineages, with remarks on the origin of the pilifers. Zool. Anz. 239, 179–196 [Google Scholar]
- 10.Krenn H. W., Zulka K. P., Gatschnegg T. 2001. Proboscis morphology and food preferences in nymphalid butterflies (Lepidoptera: Nymphalidae). J. Zool. 254, 17–26 10.1017/S0952836901000528 (doi:10.1017/S0952836901000528) [DOI] [Google Scholar]
- 11.Büttiker W., Krenn H. W., Putterill J. 1996. The proboscis of eye-frequenting and piercing Lepidoptera (Insecta). Zoomorphology 116, 77–83 10.1007/BF02526872 (doi:10.1007/BF02526872) [DOI] [Google Scholar]
- 12.Knopp M. C. N., Krenn H. W. 2003. Efficiency of fruit juice feeding in Morpho peleides (Nymphalidae, Lepidoptera). J. Insect Behav. 16, 67–77 10.1023/A:1022849312195 (doi:10.1023/A:1022849312195) [DOI] [Google Scholar]
- 13.Westneat M. W., Socha J. J., Lee W. K. 2008. Advances in biological structure, function, and physiology using synchrotron x-ray imaging. Annu. Rev. Physiol. 70, 119–142 10.1146/annurev.physiol.70.113006.100434 (doi:10.1146/annurev.physiol.70.113006.100434) [DOI] [PubMed] [Google Scholar]
- 14.Eberhard S. H., Krenn H. W. 2003. Salivary glands and salivary pumps in adult Nymphalidae (Lepidoptera). Zoomorphology 122, 161–167 10.1007/s00435-003-0081-4 (doi:10.1007/s00435-003-0081-4) [DOI] [Google Scholar]
- 15.Plateau J. A. F. 1863. Experimental and theoretical researches on the figures of equilibrium of liquid mass withdrawn from the action of gravity. (Transl.). Annual Report of the Board Regents Smithsonian Institution, pp. 207–285 Washington, DC: Government Printing Office.
- 16.Goren S. L. 1962. The instability of an annular thread of fluid. J. Fluid Mech. 12, 309–319 10.1017/S002211206200021X (doi:10.1017/S002211206200021X) [DOI] [Google Scholar]
- 17.Lautrup B. 2011. Physics of continuous matter. Boca Raton, FL: CRC Press [Google Scholar]
- 18.Kornev K. G., Neimark A. V., Rozhkov A. N. 1999. Foam in porous media: thermodynamic and hydrodynamic peculiarities. Adv. Colloid Interface Sci. 82, 127–187 10.1016/S0001-8686(99)00013-5 (doi:10.1016/S0001-8686(99)00013-5) [DOI] [Google Scholar]
- 19.Kristensen N. P. 1984. Studies on the morphology and systematics of primitive Lepidoptera (Insecta). Steenstrupia 10, 141–191 [Google Scholar]
- 20.Grimaldi D., Engel M. S. 2005. Evolution of the insects. New York, NY: Cambridge University Press [Google Scholar]
- 21.Scriber J. M. 2010. Integrating ancient patterns and current dynamics of insect–plant interactions: taxonomic and geographic variation in herbivore specialization. Insect Sci. 17, 471–507 10.1111/j.1744-7917.2010.01357.x (doi:10.1111/j.1744-7917.2010.01357.x) [DOI] [Google Scholar]
- 22.Krenn H. W., Plant J., Szucsich N. U. 2005. Mouthparts of flower-visiting insects. Arthropod Struct. Develop. 34, 1–40 10.1016/j.asd.2004.10.002 (doi:10.1016/j.asd.2004.10.002) [DOI] [Google Scholar]
- 23.Jurin J. 1717. An account of some experiments shown before the Royal Society; with an enquiry into the cause of the ascent and suspension of water in capillary tubes. Phil. Trans. 30, 739–747 10.1098/rstl.1717.0026 (doi:10.1098/rstl.1717.0026) [DOI] [Google Scholar]
- 24.Scheidegger A. E. 1974. The physics of flow through porous media. Toronto, ON: The University of Toronto Press [Google Scholar]