Abstract
Several studies on chondrocyte-seeded hydrogels in bioreactor culture report increased mechanical properties of mechanically loaded constructs compared with unloaded free swelling controls despite no significant differences in biochemical composition. One possible explanation is that changes in the collagen architecture of dynamically compressed constructs lead to improved mechanical properties. Collagen molecules are incorporated locally into the extracellular matrix with individual stress-free configurations and orientations. In this study, we computationally investigated possible influences of loading on the collagen architecture in chondrocyte-seeded hydrogels and their resulting mechanical properties. Both the collagen orientation and its stress-free configuration were hypothesized to depend on the local mechanical environment. Reorientation of the collagen network alone in response to dynamic compression leads to a prediction of constructs with lower compressive properties. In contrast, remodelling of the stress-free configuration of the collagen fibres was predicted to result in a more compacted tissue with higher swelling pressures and an altered pre-stressed state within the collagen network. Combining both mechanisms resulted in predictions of construct geometry and mechanical properties in agreement with experimental observations. This study provides support for the hypothesis that structural changes to the collagen network contribute to the enhanced mechanical properties of cartilaginous tissues engineered in bioreactors.
Keywords: mechanobiology, collagen remodelling, natural configuration, bioreactor, chondrocytes
1. Introduction
The aim of functional tissue engineering is to create viable substitutes to repair damaged tissues. Many tissue-engineering strategies rely on some form of scaffold or hydrogel that is seeded with and infiltrated by cells. The cells then synthesize phenotype-specific extracellular matrix (ECM), ideally generating a mechanically functional tissue. The specific biomolecules synthesized by the cells and incorporated into the ECM make up the tissue composition which in turn dictates the basic biomechanical properties of the tissue. The relationships between tissue composition and mechanical function have been the subject of many studies on articular cartilage [1–3].
Besides the biochemical composition another key determinant of biomechanical performance is the structural arrangement of the various constituents and their interactions. Connective tissues usually have a very distinct collagen architecture: articular cartilage exhibits a typical zonal variation in its collagen network ranging from parallel to the articular surface in the superficial zone to perpendicular in the deep zone; collagen fibres in the menisci are predominantly oriented circumferentially leading to a transversely isotropic material with a very high circumferential stiffness; other examples of soft tissues with a highly organized collagen structure include tendons, ligaments, periosteum and arteries.
Biological tissues adapt their structure to their mechanical environment [4]. A collagen architecture responsive to the mechanical environment has been observed and computational models have been used to study this phenomenon in many tissues, including cardiovascular [5–7], articular cartilage [8–11], tendon [12] and during skeletal tissue regeneration [13,14]. In many of these studies, the collagen network has been hypothesized to align with respect to the principal directions of local mechanical regulators. In addition to orientational remodelling, the notion of natural configurations has been documented. As proteins are incorporated into the ECM at different time points and different deformation states, they have individual stress-free configurations [15]. Owing to ongoing synthesis, degradation and remodelling, the tissue's stress-free configurations evolve. In the context of a collagen fibre, an implication of this is that the recruitment stretch, i.e. the stretch at which the fibre becomes uncrimped and begins to bear load, can evolve. Remodelling of this stress-free state plays a role in scaffold contraction [16] and tissue growth and remodelling [17–19].
Articular cartilage has been in the focus of numerous tissue-engineering studies [20–22]. Many of these have shown increases in metabolic or synthetic cell activities owing to dynamic loading in addition to enhanced mechanical properties [23–27]. Other studies on chondrocyte-seeded hydrogels in bioreactor culture have found increased mechanical properties of mechanically loaded constructs compared with unloaded free swelling controls despite no significant differences in biochemical composition [28–31]. Similar results have been observed for mesenchymal stem cells undergoing chondrogenesis while subjected to dynamic compression [32].
Enhanced structural organization has been suggested as one possible explanation for these latter results in the experimental literature [29,31,33,34]. However, this has not been directly tested experimentally and its influence—both in nature and in magnitude—on the mechanical properties remains largely unknown. Dynamic loading causes biochemical, biomechanical, compositional and nutritional alterations that are all potential contributors to the observed changes. Unravelling the relative contribution of the involved mechanisms is complex and challenging. Computational models offer the advantage of allowing systematic investigation of individual mechanisms without altering other aspects of the system, which is often not feasible experimentally. The hypothesis under investigation in this study is that changes in the local collagen orientation and/or stress-free configuration in response to loading can lead to enhanced bulk mechanical properties of tissue-engineered cartilaginous constructs in the absence of alterations to the biochemical composition, i.e. material parameters. To test this hypothesis, model predictions of changing construct geometry and mechanical properties owing to structural changes in the collagen network in response to dynamic compression will be compared with the results of bioreactor studies, where chondrocyte-seeded agarose constructs are subjected to dynamic compression [29,30]. Structural changes to the engineered tissues were studied using a previously developed remodelling framework [35]. This and similar frameworks have been successful in predicting changes in collagen fibre orientation and stress-free configuration in a large number of biological tissues and cell-seeded hydrogel systems which suggest that the underlying principles are of general significance in load-bearing tissues.
2. Material and methods
2.1. Material model
A large strain biphasic material model with osmotic swelling effects was used [36,37]. The total stress σ in the biphasic medium is given as:
| 2.1 |
Here, p is the hydraulic pore pressure, σE the solid extra Cauchy stress and Δπ the Donnan osmotic pressure inside the tissue given by
 |
2.2 |
where R = 8.3145 (N mm mmol K−1) is the universal gas constant, cext the external salt concentration, T the absolute temperature, cF0 the initial fixed charge density and J the determinant of the deformation gradient F. The initial porosity ϕF0 and solidity ϕS0 are linked via the saturation condition ϕF0 + ϕS0 = 1.
The solid extra stresses were derived from free Helmholtz energy density functions that were split into isotropic and anisotropic parts. For the isotropic part, we used a Neo-Hookean formulation
| 2.3 |
with the first and third principal invariant I1 and I3 of the right Cauchy-Green tensor, C = FTF.
To describe structural remodelling of the collagen network, an evolving remodelled configuration was introduced via a multiplicative decomposition of the deformation gradient
| 2.4 |
The part of the deformation denoted by Fr occurred stress-free in the collagen network, while the elastic deformation Fe contributed to the stress response. Conceptually, this can be visualized by the interplay between the free-rotating link element and the spring in the uniaxial model in figure 1. With the definition of elastic right Cauchy-Green tensors, Ĉe = FeTFe the energy potential for the anisotropic tissue response was modelled using a continuous angular fibre distribution following a formulation used in Ateshian et al. [38]
 |
2.5 |
Figure 1.
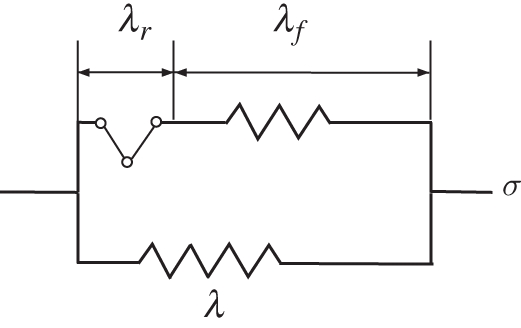
Conceptual visualization of the recruitment stretch. While the isotropic material (bottom spring; glycosaminoglycans, agarose) always carry load, the collagen network (top spring) only contributes to the stress response when stretched beyond the recruitment stretch, i.e. when the link element is straightened. The stretches λ, λr and λf are the one dimensional analogues to the three dimensional deformation gradients F, Fr and Fe, respectively.
with the anisotropic material parameters C4(a0) and β(a0) defining the (strain-dependent) stiffness in a fibre direction a0. The modified invariant I4e = tr(M̂Ĉe) = λf2 ensured that collagen fibres only contributed stresses once the fibre stretch reached a certain transition value. Here, M̂ is the unit structure tensor of a family of fibres in the remodelled configuration [35].
Collagen network anisotropy was described using an anisotropy tensor
 |
2.6 |
with the principal values ξi prescribing the degree of structural anisotropy. When the components of the direction vector a0 were expressed in terms of the basis {wi} as a0 = cosΘsinΦw1 + sinΘsinΦw2 + cosΦw3, the material parameter C4 in a fibre direction was derived via an ellipsoid representation [38] that could be scaled with a parameter m to allow for adjustment of the degree of anisotropy (increasing m leads to a higher degree of ellipticity in the material parameter distribution than given by Ξ)
 |
2.7 |
where C̄4 is a baseline material parameter and  the mean radius of the ellipsoid. The division by rm is performed such that in the case of a spherical tensor Ξ, one always gets C4(a0) = C̄4 irrespective of the magnitude of the scaled eigenvalues ξ1m = ξ2m = ξ3m. A numerical processing step ensured constant total fibre network stiffness independent of the degree of ellipticity (i.e. ‘constant’ amount of collagen).
the mean radius of the ellipsoid. The division by rm is performed such that in the case of a spherical tensor Ξ, one always gets C4(a0) = C̄4 irrespective of the magnitude of the scaled eigenvalues ξ1m = ξ2m = ξ3m. A numerical processing step ensured constant total fibre network stiffness independent of the degree of ellipticity (i.e. ‘constant’ amount of collagen).
2.2. Fibre reorientation owing to remodelling
The collagen network was assumed to remodel its orientation with respect to the local deformation such that the fibre network is reinforced in stretched directions and weakened in compressed directions. Using the spectral decomposition of the right Cauchy-Green tensor
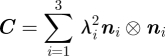 |
2.8 |
a rotation velocity vector ω could be derived (for details see Nagel & Kelly [35]) that describes the amount of rotation of the orthonormal basis {wi} of Ξ towards the orthonormal basis {ni} of C proportional to the angle θ between both systems and depending on a time constant τf:
| 2.9 |
where e is the rotational axis. With the skew-symmetric tensor  corresponding to the vector ω, the basis vectors of Ξ were then updated in a time increment Δt using an exponential map algorithm
corresponding to the vector ω, the basis vectors of Ξ were then updated in a time increment Δt using an exponential map algorithm
| 2.10 |
The eigenvalues of Ξ were updated based on the squared principal stretches using a linear rate equation:
| 2.11 |
2.3. Remodelling of the stress-free configuration
The stress-free configuration of the collagen network was modelled to evolve based on the assumption that collagen fibres remodel towards a homeostatic stretch value λh at which they reside in the matrix. Collagen fibres only contributed to load bearing once stretched above their recruitment stretch λr. Corresponding to the uniaxial analogy in figure 1, in three dimensions the corresponding geometric information was included into the definition of the remodelling part of the deformation gradient
 |
2.12 |
Once loading disturbs the homeostatic state of the network and fibres are no longer stretched at their homeostatic value λh the recruitment stretch has to change in order to restore tensional network homeostasis. This new desired recruitment stretch λ0 is related to the applied stretch λ via λ0 = λ /λh. The recruitment stretch evolution towards λ0 was governed by the rate equation
| 2.13 |
2.4. Extracellular matrix synthesis
The accumulation of ECM components such as proteoglycans (PG) and collagen (COL) in cell-seeded hydrogels leads to increases in their mechanical properties which changes the stimuli imposed by the bioreactor. As only the basic phenomenological aspects of biomolecule deposition were of interest, a bilinear model of constituent synthesis was assumed: initially, a constituent is secreted at a constant rate. Once it reaches a designated final concentration (m̄α) that level is maintained constant:
| 2.14 |
| 2.15 |
where mα is the current mass of constituent α, i.e. PG or COL. Final amounts of PG and COL were chosen to be 8% w/w and 16% w/w, respectively. We assumed that PG production levels off after 42 days and that collagen production is six times slower. This is in line with experimental observations showing that after 42 days in culture, COL content reaches only a fraction of native values whereas PG build up more quickly [29,30]. In accordance with the study objective and experimental observations [28–30], synthesis rates were modelled independent of mechanical stimuli. Therefore, differences in the predicted mechanical properties of dynamically compressed and free swelling constructs in these simulations are purely owing to changes in collagen orientation and configuration owing to loading.
It was assumed that the material properties of the isotropic ground phase C1, D2 remained at agarose values. The fixed charge density cF0 was directly related to the current proteoglycan content. The anisotropic material parameter C̄4 was related to collagen content. This is in general agreement with studies that relate tissue composition to mechanical properties [1–3,39,40]. For simplicity, we assumed a simple linear connection between the material parameters and the constituent levels:
| 2.16 |
| 2.17 |
2.5. Boundary conditions
In this study, boundary conditions were modelled according to the bioreactor culture protocols used in Lima et al. [29] and Bian et al. [30]. Cylindrical cell- seeded constructs were either left free swelling for the entire culture period (free swelling, FS group) or loaded for 3 h a day in cyclic unconfined compression and left to swell freely during the remaining 21 h (dynamically loaded, DL group).
For remodelling studies, the current tissue deformation is of interest. Free swelling could be modelled as an equilibrium load step (p = 0). However, it is impractical owing to computational limitations to model 3 h of cyclic loading at 1 Hz (10 800 cycles) of a highly nonlinear material. Therefore, two simplifying model assumptions were compared:
— based on the behaviour of cyclically loaded biphasic materials relaxing to a mean deformation state (see electronic supplementary material, part A) and the assumption that characteristic time scales at which remodelling occurs are long compared with 1 s (‘biological inertia’, see electronic supplementary material, part B) cyclic loading was modelled as an equilibrium load step to the mean level of compression (10%);
— as remodelling could alternatively be directed towards the maximum deformation during a cycle the equilibrium step was followed by a quasi-incompressible compression by the dynamic amplitude (additional 5%). Incompressibility could be assumed owing to the high loading rate (see electronic supplementary material, part C).
2.6. Evaluated quantities
Construct properties were evaluated in terms of the nominal equilibrium modulus Enom and the apparent equilibrium Poisson's ratio νapp. Additionally, the FS geometry was evaluated in terms of volume and aspect ratio (defined as the ratio of lateral to axial strain in the FS state with respect to the reference geometry prior to swelling). The direction-dependent recruitment stretch λr and collagen fibre reinforcement ξ were plotted as well.
2.7. Performed simulations
The performed simulations are listed in table 1. Both FS and DL experiments were simulated. Unless otherwise stated in table 1, loading was applied for 3 h per day. Remodelling either took place with respect to the mean deformation (DL) or the maximum deformation (DL max) during compression.
Table 1.
Performed simulations.
| remodelled feature | parameters (%) | simulations |
|---|---|---|
| configuration | εh = 1.5 | FS, 3 h DL, 3 h DL (max) |
| εh = 2 | FS, 3 h DL, 3 h DL (max) | |
| εh = 3 | FS, 3 h DL, 3 h DL (max) | |
| orientation | m = 50 | FS, 3 h DL, 3 h DL (max) |
| combination | m = 50, | FS, 3 h DL, |
| εh = 2.0 | 6 h DL, 9 h DL | |
| m = 10, | FS 3 h DL | |
| εh = 2.0 |
In ‘configuration only’ simulations, only the recruitment stretch was remodelled and an isotropic fibre stiffness assumed (Ξ = I). The value of the homeostatic strain εh was subjected to a parameter variation.
In ‘orientation only’ simulations, the recruitment stretch was kept unchanged (Fr = I), while the fibre stiffness representing orientational effects was allowed to remodel. As varying m does not produce additional qualitative insight, only one representative simulation was performed.
The ‘combination’ simulations finally combined both effects. This group was used to study the effect of extending the duration of dynamic compression to 6 and 9 h d−1 with the m = 50 and εh = 2 per cent parameter set. To study the relative effects of m and εh, m was decreased to 10 in a second set of simulations. The parameter values used for all simulations are listed in table 2.
Table 2.
Material parameters.
| agarose | cartilage | |
|---|---|---|
| C1 (MPa) | 4.34 × 10−3 | 4.34 × 10−3 |
| ν (−) | 0.1 | 0.1 |
| ϕF0(−) | 0.98 | 0.8 |
| C̄4 (MPa) | 0 | 2.0 |
 |
2.5 | 2.5 |
| cF0(meq mm−3) | 0 | 0.0002 |
| k (m4 (N s)−1) | 6.61 × 10−13 | 7.5 × 10−15 |
| cext (mmol mm−3) | 0.00015 | 0.00015 |
| T (K) | 298 | 298 |
| τf (d) | 2 | 2 |
Unless otherwise stated, predicted values at day 56 are provided in §3. As both loading and geometry were axisymmetric, the material homogeneous and the model deterministic, constructs remained homogeneous throughout culture. Hence, presented results are representative of any point in the construct geometry.
3. Results
3.1. Fibre distribution
Remodelling of the fibre orientation was predicted to lead to anisotropy in loaded samples, whereas FS samples were predicted to remain isotropic (figure 2). Simulations where only fibre reorientation was considered (figure 2a) showed a slightly more anisotropic tissue when remodelling towards the maximum deformation during dynamic loading (DL max) than when remodelling to the mean configuration (DL). Maximum stiffness values were observed in the radial (and circumferential) direction (0° to horizontal) owing to fibre reorientation in this direction, whereas the lowest fibre stiffness was predicted in the axial direction (90° to horizontal).
Figure 2.
Direction-dependent relative structural anisotropy ξ(α)/ξm with ξm = (ξ1 + ξ2 + ξ3 )/3 at day 56. α is the angle to the horizontal (radial) direction. A value greater than one indicates higher than average fibre reinforcement, while a value smaller than one indicates a lower than average fibre reinforcement. (c) Simulation with combined effects (m = 50 and εh = 2.0%: black line, FS; grey line, DL 3). (a) Reorientation only simulation (m = 50: black line, FS; grey line, DL; dotted line, DL max). (b) Simulation with combined effects (m = 50 and εh = 2.0%: black line, FS; grey line, DL 3; black dotted line, DL 6; grey dotted line, DL 9).
This trend was maintained in the simulation where realignment was combined with reconfiguration (figure 2b,c). Increasing the time of dynamic loading from 3 to 6 and 9 h further increased the anisotropy of the tissue. When the scaling parameter m was decreased from 50 to 10, the collagen architecture itself was predicted to be more anisotropic (figure 2c).
3.2. Recruitment stretch distribution
The recruitment stretch distribution remained isotropic in the free swelling samples with anisotropy developing in the loaded samples (figure 3a–e). The transition stretch with respect to the reference configuration was predicted to increase in the horizontal (radial and circumferential) direction in loaded samples, while it was predicted to decrease in the axial direction. The anisotropy was slightly more pronounced when remodelling was driven by the maximum compressed deformation. The parameter variation of εh yielded lower recruitment stretch values for higher values of εh as well as a slightly decreasing degree of anisotropy (figure 3a–c). In the combined simulations (where both reorientation and reconfiguration occurred), the recruitment stretch distribution was less anisotropic with generally lower values for longer compression times (figure 3d).
Figure 3.
Direction-dependent recruitment stretch distribution λr (α) at day 56. α is the angle to the horizontal (radial) direction. (a) εh = 1.5% (black line, FS; grey line, DL; dotted line, DL max). (b) εh = 2.0% (black line, FS; grey line, DL; dotted line, DL max). (c) εh = 3.0% (black line, FS; grey line, DL; dotted line, DL max). (d) m = 50 and εh = 2.0% (black line, FS; grey line, DL 3; black dotted line, DL 6; grey dotted line, DL 9). (e) m = 10 and εh = 2.0% (black line, FS; grey line, DL 3).
3.3. Sample geometry
A higher recruitment stretch in the horizontal direction will lead to preferred swelling into that direction, as fibres start to inhibit deformation at a later stage. A higher fibre stiffness in the horizontal direction however will lead to more swelling into the vertical direction. Hence, in the reconfiguration only simulations, samples with an aspect ratio of εr0/εz0 ≥ 1, i.e. lower and wider samples, were predicted (figure 4a–c). In the reorientation only simulations; however, the opposite trend, i.e. a higher and more slender sample, was predicted (figure 4d). For all simulation cases, the free swelling samples remained isotropic and hence maintained an aspect ratio of 1 (figure 4a–f).
Figure 4.
Sample volumes and aspect ratios in the free swelling state at day 56. The effect of extending the duration of dynamic compression per day has been studied in (e). (a) εh = 1.5% , (b) εh = 2.0% , (c) εh = 3.0% , (d) m = 50, (e) m = 50, εh = 2%, and (f) m = 10 εh = 2%. Shaded bars, aspect ratio; unfilled bars, FS volume.
With increasing homeostatic strain εh, the FS volume of the samples decreased significantly from approximately 66 mm3 for εh = 1.5 per cent to approximately 39 mm3 for εh = 3.0 per cent.
In the combined simulations, fibre reorientation led to a decreasing aspect ratio when the scaling parameter m was high (figure 4e) but increasing aspect ratios were predicted when a lower value of m = 10 was chosen (figure 4f). In both cases reconfiguration led to decreasing free swelling volumes in the loaded samples (figure 4e,f). When loading was simulated for 9 hour a day, the FS volume increased again compared with the sample loaded for 6 h. During the initial 14 days of culture, sample volumes and heights were predicted to decrease followed by an increase during the remaining culture period (figure 5b).
Figure 5.
Transient construct development for 0 and 3 h of loading at various time points in culture. (a) Young's moduli at 10% strain, collagen and GAG content (b) free swelling volumes and sample thicknesses for the m = 50, ɛh = 2% simulations. (a) Black bars, DL; grey bars, FS; dotted line, PG; solid line, COL. (b) Black bars (volume) and solid line (thickness), FS; grey bars (volume) and dotted line (thickness), DL 3.
3.4. Young's moduli
Strain-dependent Young's moduli predictions exhibited stress-softening effects (figure 6a–f). Reconfiguration lead to a stiffness increase in the loaded samples with little difference between remodelling to the maximum or mean deformation (figure 6a–c). The magnitude of homeostatic network strain εh furthermore has a pronounced effect on overall sample stiffness, covering a stiffness range at 10 per cent strain from approximately 0.4 to 1.4 MPa when increasing εh from 1.5 to 3 per cent. This was owing to the increased amount of pre-strain in the fibre network as well as the associated sample compaction (compare sample volumes in figure 4a with figure 4c). Reorientation, on the other hand, led to less fibres pre-strained in the loading direction and hence initially softer samples when the constructs were loaded (figure 6d).
Figure 6.
(a) Young's moduli at day 56. The effect of extending the duration of dynamic compression per day has been studied in the m = 50, ɛh = 2% group (e). (a) ɛh = 1.5% (black line, FS; grey line, DL; dotted line, DL max). (b) ɛh = 2% (black line, FS; grey line, DL; dotted line, DL max). (c) ɛh = 3.0% (black line, FS; grey line, DL; dotted line, DL max). (d) m = 50.0 (black line, FS; grey line, DL; dotted line, DL max). (e) m = 50, ɛh = 2% (black line, FS; grey line, DL 3; black dotted line, DL 6; grey dotted line, DL 9). (f) m = 10, ɛh = 2% (black line, FS; grey line, DL 3).
Combining realignment and reconfiguration caused combined effects. Loaded samples appeared softer than free swelling samples initially but were predicted to have higher stiffnesses with increasing deformation (figure 6e,f). This effect was more pronounced when loading for 6 h instead of 3 h. However, increasing the time of loading even further did not translate into increased mechanical properties but decreased construct stiffness again (figure 6e).
For the combined simulation with m = 50 and εh = 2 per cent, the transient construct development was evaluated at days 0, 12, 28 and 56 in terms of Young's moduli of the FS and DL3 samples (figure 5a). Loaded samples were predicted to be stiffer at all time points. The moduli increased monotonically until day 42. Despite an increasing collagen content after day 42, construct properties at day 56 were lower owing to the ongoing remodelling of the collagen network than at day 42 (figure 5a).
3.5. Poisson's ratios
Poisson's ratios were only marginally influenced by dynamic loading in the reconfiguration only simulations (figure 7a–c). Fibre realignment, however, led to significant decreases in the Poisson's ratios owing to dynamic loading (figure 7d). The orientational effect was even more pronounced in the combined simulations with an increasing time of loading leading to decreasing Poisson's ratios and less pronounced nonlinear behaviour (figure 7e). For a lower value of m, the decrease in Poisson's ratio was less pronounced but generally similar to the m = 50 simulation (figure 7f).
Figure 7.
Poisson's ratios at day 56. The effect of extending the duration of dynamic compression per day has been studied in the m = 50, ɛh = 2% group (e). (a) ɛh = 1.5% (black line, FS; grey line, DL; dotted line, DL max). (b) ɛh = 2% (black line, FS; grey line, DL; dotted line, DL max). (c) ɛh = 3.0% (black line, FS; grey line, DL; dotted line, DL max). (d) m = 50 (black line, FS; grey line, DL; dotted line, DL max). (e) m = 50, ɛh = 2% (black line, FS; grey line, DL 3; black dotted line, DL 6; grey dotted line, DL 9). (f) m = 10, ɛh = 2% (black line, FS; grey line, DL 3).
4. Discussion
In this study, we used a computational model based on previous work [35] to investigate changes in the mechanical properties of cell-seeded agarose constructs in bioreactor culture from a structural perspective. To the best of our knowledge, this study represents the first theoretical investigation of mechanically induced changes in the orientation and stress-free configuration of the collagen network in a swelling hydrogel during bioreactor culture, and furthermore demonstrates for the first time how alterations to this network can lead to improvements in the mechanical functionality of engineered cartilage tissue. We hypothesized that remodelling of the collagen architecture of tissue-engineered constructs in response to dynamic compression can lead to enhanced bulk mechanical properties in the absence of alterations to the biochemical composition, as reported experimentally [28–32]. Traditionally, collagen fibre orientation is the main architectural feature considered. Fibre reorientation in response to loading, however, caused decreases in bulk construct compressive stiffness owing to the charged nature of the material [37]. The present model additionally considered the local natural configuration of the collagen network. The model predicted that collagen network reconfiguration leads to increased equilibrium moduli while reorientation leads to lowered Poisson's ratios. The model further predicted that the FS geometry of loaded and unloaded samples differs depending on the dominant remodelling mechanism involved. Only when both conformational and orientational changes were considered, could the trends in Young's modulus, Poisson's ratio and sample geometry be predicted simultaneously.
Changes to the collagen's natural configuration were predicted to impact tissue properties by altering the volumetric compaction of the developing tissue and the state of pre-strain in the collagen network. During loading, the constructs occupy less volume than in their FS state owing to the exudation of fluid. Collagen fibres are laid down and/or remodelled in this configuration. Therefore, for a given homeostatic strain value, the DL samples are compacted to a smaller volume in their unloaded states and hence appeared stiffer owing to higher swelling pressures [37]. A lower volume in DL constructs (52.9 mm3) compared with FS samples (53.1 mm3) at day 42 has been observed experimentally [34]. In addition to altering the volumetric compaction, remodelling the natural configuration also caused changes in the stress-softening phenomena associated with the tension-compression nonlinearity [37,38,41], with higher collagen network pre-strains predicted to increase the apparent mechanical properties of the construct at equilibrium. Additionally, altering the stress-free state of the collagen network owing to loading was predicted to lead to increased aspect ratios, i.e. increased sample radii and decreased heights. Corresponding results have also been reported experimentally [34] where flattening and widening of the samples were observed with a height-to-radius ratio of 1.02 and 0.91 for FS and DL samples, respectively. Dynamically loaded samples that were up to 20 per cent thinner in the loading direction than free swelling controls have also been reported [28].
That collagen is incorporated into cartilage ECM under pre-stretch with respect to the free swelling configuration can be demonstrated by digesting the collagen in a cartilage plug and observing its subsequent re-swelling [42,43]. The actual value of εh is speculative and the natural configurations can only be approximated using inverse simulations. The parameter variation of εh showed that increasing its value will have a significant effect on construct stiffness.
Experimental evidence is also available for reorientation of the collagen network within engineered cartilaginous constructs in response to extrinsic mechanical signals. For example, no preferred collagen angle has been found using polarized light microscopy in free-swelling tissue-engineered cartilage while a maximum intensity perpendicular to the loading axis indicates horizontal fibre alignment in dynamically compressed samples [34]. The model also predicted an isotropic fibre architecture in free swelling samples while the maximum value of the anisotropy tensor Ξ was predicted in the horizontal direction within the loaded samples. The apparent Poisson's ratio of engineered cartilaginous constructs has been shown to be lower for loaded (≈0.17) than for free swelling (≈ 0.23) samples [34]. Reorientation of the collagen network was predicted to result in changes similar to the Poisson's ratio of dynamically compressed constructs. Horizontal fibres are more efficient at resisting lateral expansion, which explains the decreases in the Poisson's ratio of the loaded samples. In contrast, the experimentally observed increases in the equilibrium moduli could not be explained by fibre reorientation. Fibre reorientation alone was predicted to lead to higher constructs with a smaller diameter, not consistent with experimental observations [28,34]. Therefore, while fibre reorientation can potentially explain certain experimentally observed phenomena during bioreactor culture such as decreasing Poisson's ratios, it alone cannot explain the effect of dynamic compression on the structural development of engineered cartilaginous constructs.
When combining collagen network realignment and recruitment stretch reconfiguration, a combination of the individual results was predicted. While lower construct properties in the loaded samples were predicted in the initial small strain range, loading led to increased equilibrium properties at higher strains (≈5% strain and higher). A lower Poisson's ratio was also predicted. Thus, combining reorientation and reconfiguration allowed the simultaneous prediction of increased Young's moduli and decreased Poisson's ratios as well as geometrical changes. The interaction of the various constituents was predicted to lead to initial decreases in construct thickness and volume during the initial 14 days of culture followed by an increase thereafter. Initial decreases in sample thickness with subsequent thickening have also been reported experimentally [28]. Our model also predicted a slight increase in equilibrium modulus for 6 h of dynamic compression compared with 3 h (at 10% applied strain). However, for 9 h of loading, the modulus was predicted to decrease again to the level reached after 3 h of loading. This should be seen as a qualitative result. The exact duration of dynamic compression that will produce the stiffest constructs will depend on the parameter values chosen, which are yet to be identified, and other biological effects. Experimentally, no increase in the Young's modulus of engineered constructs has been observed for increasing the daily duration of dynamic compression from 3 to 6 h [44].
In addition to the daily duration of applied dynamic compression, the total duration of culture will also determine construct mechanical properties. For example, a levelling off of glycosaminoglycan accumulation has been reported for the last two weeks of an eight week study, while the collagen content continued to increase [28]. Despite that, stiffness values plateaued or even decreased [28]. Our model similarly predicted that after day 42, construct mechanical properties decreased despite increasing collagen content. This was owing to ongoing remodelling, namely reconfiguration of the collagen fibres slowly releasing any excess tension in the collagen network previously built up owing to increasing swelling pressures. This result emphasizes the importance of tissue structure and can partly explain the difficulties in obtaining composition–function relationships. The latter are usually obtained relating bulk biochemical content to biomechanical properties and, as the simulations show, can only be an estimate if structural aspects are neglected.
A number of assumptions had to be made in developing this model. To capture the evolution of the material parameters used in the constitutive model, they were related to the main ECM constituents: PG and COL. This evolution was described by a simple bilinear relationship capturing the basic trends observed in bioreactor culture: faster PG and slower COL production as well as the resulting increase of construct properties over time. If the synthesis (and degradation) of the constituents itself becomes the focus of study, then more sophisticated models will be required. The increases in PGs led to increasing construct volumes owing to swelling. Because the offset strain associated with the dynamic compression regime is applied with respect to day 0 geometry, this caused the applied tare strain in the model to change over time in culture. In the isotropic case, an increase from 10 to over 20 per cent (see electronic supplementary material, part C), paralleling experimental observations [29], was predicted.
Both agarose and cartilage are porous media in which the pore liquid contributes significantly to the material behaviour. Flow-dependent viscoelastic behaviours can be captured with biphasic models [45]. The bioreactor loading regimen used in this study could be conveniently split up into an equilibrium part, where hydraulic fluid pressurization was negligible, and a short-term quasi-instantaneous part, where fluid flow is negligible. This enabled us to use single phasic constitutive models and compare remodelling towards the mean and the maximum deformation. Based on the equilibration behaviour of cyclically loaded biphasic tissues and the assumption that biological remodelling takes place on a time scale significantly larger than 1 s, we were able to simulate a complete day of loading rather than merely one representative loading cycle. Owing to the small time-step size, it would be computationally infeasible to simulate the complete loading protocol of a day in detail for 56 days with a full biphasic model.
Our phenomenological model makes no mechanistic distinction between cell-mediated and non-cell-mediated remodelling. Collagen network remodelling has been observed both in the presence and absence of cells [16]. In tissues with a low cellularity, such as articular cartilage, non-cell-mediated mechanisms might play an important role. Strain-dependent collagen–collagenase interactions have been reported [46] such that fibres perpendicular to the direction of tensile loading become resorbed, which ultimately causes alignment. As long as the mechanism of remodelling in cartilaginous constructs has not been resolved experimentally, simulation can potentially provide insight into the consequences of remodelling.
Using a computational approach, we have been able to provide support for the hypothesis that a mechanoregulated collagen architecture can lead to enhanced bulk mechanical properties of tissue engineered constructs owing to mechanical loading with the same biochemical composition as free swelling controls. We further showed that reorientation alone, the traditionally considered architectural feature, is insufficient to capture the experimental observations. This does not invalidate or exclude other hypotheses related to the collagen network that could explain the observed phenomena. Alternative mechanisms likely to be involved include collagen cross-linking and the synthesis of other ECM proteins, such as other collagen types [34,47,48]. Studies on heart valve tissue engineering [49] have observed that dynamic loading did not enhance or even decreased bulk collagen content but did lead to increased cross-linking and mechanical properties of the tissue-engineered constructs. Yan et al. [48] found low levels of collagen IX and mature collagen cross-linking to be a major contributing factor to poor mechanical properties of in vitro engineered cartilage. This study also demonstrated that physical stimulation, via centrifugal forces, enhances the mechanical properties of tissue-engineered cartilage, implicating enhanced levels of collagen IX and collagen cross-linking for the improvements in construct functionality. These studies suggest an important role for cross-linking and its promotion via dynamic loading in engineering living tissue substitutes. In phenomenological constitutive models, both increases in cross-linking and bulk collagen content could be captured via elevated material parameters. This will clearly lead to increases in the apparent mechanical properties in the simulations but does not elucidate whether such phenomena are responsible for the increased mechanical properties reported. While increased properties owing to increases in material parameters is an intuitively obvious result, changes in configurational parameters such as fibre orientation and stress-free configuration might be less transparent especially in charged swelling materials and require appropriate models to investigate their possible contribution to the observed changes. Similarly, a number of experimental studies have also shown that dynamic loading can lead to changes in both biochemical composition and mechanical properties [23–27]. Models such as that presented here might in the future be able to help decouple the relative roles played by compositional and structural changes in determining the mechanical properties of engineered tissues. Another possible mechanism is the altered diffusion of ECM proteins within the samples owing to DL. Despite equivalence of the bulk biochemical content, DL could lead to different distributions of the ECM proteins and hence affect mechanical properties [47]. However, finite-element studies [50] on the local distribution of ECM in tissue-engineered cartilage concluded that the global aggregate modulus and permeability were largely insensitive to the microscopic matrix distribution.
While biochemical assays can help determine composition–function relationships, tissue organization is a determinant of biomechanical functionality in its own right. The effect of organizational alterations is difficult to investigate experimentally, as tissue structure is not easily altered and certain structural features aside from orientation such as natural configurations are difficult to quantify. Uncoupling the relative roles of tissue composition, distribution and organization at various hierarchical levels is a task amenable to simulation methods. In future, this modelling framework will be extended to other cell types, particularly MSCs, combined with tissue differentiation algorithms and applied to the study of in vivo healing scenarios [14]. For example, a native-like zonal architecture is crucial for successful chondral and osteochondral defect repair. It is for this reason that mechanoregulation algorithms need to include both tissue differentiation and architecture simultaneously in order to understand how environmental factors regulate tissue form and function during skeletal regeneration.
Acknowledgements
We acknowledge funding by IRCSET (G30345) and a Science Foundation Ireland PIYRA award (08/YI5/B1336).
References
- 1.Williamson A. K., Chen A. C., Sah R. L. 2001. Compressive properties and function–composition relationships of developing bovine articular cartilage. J. Orthop. Res. 19, 1113–1121 10.1016/S0736-0266(01)00052-3 (doi:10.1016/S0736-0266(01)00052-3) [DOI] [PubMed] [Google Scholar]
- 2.Wilson W., Huyghe J. M., van Donkelaar C. C. 2007. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomech. Model. Mechanobiol. 6, 43–53 10.1007/s10237-006-0044-z (doi:10.1007/s10237-006-0044-z) [DOI] [PubMed] [Google Scholar]
- 3.Julkunen P., Jurvelin J., Isaksson H. 2009. Contribution of tissue composition and structure to mechanical response of articular cartilage under different loading geometries and strain rates. Biomech. Model. Mechanobiol. 9, 237–245 10.1007/s10237-009-0169-y (doi:10.1007/s10237-009-0169-y) [DOI] [PubMed] [Google Scholar]
- 4.Taber L. A. 1995. Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487–545 10.1115/1.3005109 (doi:10.1115/1.3005109) [DOI] [Google Scholar]
- 5.Gleason R. L., Humphrey J. D. 2004. A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J. Vasc. Res. 41, 352–363 10.1159/000080699 (doi:10.1159/000080699) [DOI] [PubMed] [Google Scholar]
- 6.Driessen N. J. B., Bouten C. V. C., Baaijens F. P. T. 2005. Improved prediction of the collagen fiber architecture in the aortic heart valve. J. Biomech. Eng. 127, 329–336 10.1115/1.1865187 (doi:10.1115/1.1865187) [DOI] [PubMed] [Google Scholar]
- 7.Kuhl E., Holzapfel G. 2007. A continuum model for remodeling in living structures. J. Mater. Sci. 42, 8811–8823 10.1007/s10853-007-1917-y (doi:10.1007/s10853-007-1917-y) [DOI] [Google Scholar]
- 8.Grodzinsky A. J., Levenston M. E., Jin M., Frank E. H. 2000. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2, 691–713 10.1146/annurev.bioeng.2.1.691 (doi:10.1146/annurev.bioeng.2.1.691) [DOI] [PubMed] [Google Scholar]
- 9.Wilson W., Driessen N. J. B., van Donkelaar C. C., Ito K. 2006. Prediction of collagen orientation in articular cartilage by a collagen remodeling algorithm. Osteoarthritis Cartilage 14, 1196–1202 10.1016/j.joca.2006.05.006 (doi:10.1016/j.joca.2006.05.006) [DOI] [PubMed] [Google Scholar]
- 10.Klisch S. M., Asanbaeva A., Oungoulian S. R., Masuda K., Thonar E. J. M., Davol A., Sah R. L. 2008. A cartilage growth mixture model with collagen remodeling: validation protocols. J. Biomech. Eng. 130, 031006. 10.1115/1.2907754 (doi:10.1115/1.2907754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Turnhout M., Kranenbarg S., van Leeuwen J. 2010. Contribution of postnatal collagen reorientation to depth-dependent mechanical properties of articular cartilage. Biomech. Model. Mechanobiol. 10, 269–279 10.1007/s10237-010-0233-7 (doi:10.1007/s10237-010-0233-7) [DOI] [PubMed] [Google Scholar]
- 12.Giori N. J., Beaupré G. S., Carter D. R. 1993. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J. Orthop. Res. 11, 581–591 10.1002/jor.1100110413 (doi:10.1002/jor.1100110413) [DOI] [PubMed] [Google Scholar]
- 13.Cullinane D. M., Fredrick A., Eisenberg S. R., Pacicca D., Elman M. V., Lee C., Salisbury K., Gerstenfeld L. C., Einhom T. A. 2002. Induction of a neoarthrosis by precisely controlled motion in an experimental mid-femoral defect. J. Orthop. Res. 20, 579–586 10.1016/S0736-0266(01)00131-0 (doi:10.1016/S0736-0266(01)00131-0) [DOI] [PubMed] [Google Scholar]
- 14.Nagel T., Kelly D. J. 2010. Mechano-regulation of mesenchymal stem cell differentiation and collagen organisation during skeletal tissue repair. Biomech. Model. Mechanobiol. 9, 359–372 10.1007/s10237-009-0182-1 (doi:10.1007/s10237-009-0182-1) [DOI] [PubMed] [Google Scholar]
- 15.Humphrey J. D. 1999. Remodeling of a collagenous tissue at fixed lengths. J. Biomech. Eng. 121, 591–597 (doi:org/10.1115/1.2800858) [DOI] [PubMed] [Google Scholar]
- 16.Thomopoulos S., Fomovsky G. M., Holmes J. W. 2005. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J. Biomech. Eng. 127, 742–750 10.1115/1.1992525 (doi:10.1115/1.1992525) [DOI] [PubMed] [Google Scholar]
- 17.Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 10.1038/nrm809 (doi:10.1038/nrm809) [DOI] [PubMed] [Google Scholar]
- 18.Foolen J., van Donkelaar C. C., Soekhradj-Soechit S., Ito K. 2010. European Society of Biomechanics S. M. Perren Award 2010: an adaptation mechanism for fibrous tissue to sustained shortening. J. Biomech. 43, 3168–3176 10.1016/j.jbiomech.2010.07.040 (doi:10.1016/j.jbiomech.2010.07.040) [DOI] [PubMed] [Google Scholar]
- 19.Donkelaar C. C. v., Wilson W. 2006. Chondrocyte hypertrophy requires matrix turnover. In Proc. 2006 Summer Bioengineering Conf., June 21–25, Amelia Island, FL: Amelia Island Plantation [Google Scholar]
- 20.Temenoff J. S., Mikos A. G. 2000. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 21, 431–440 10.1016/S0142-9612(99)00213-6 (doi:10.1016/S0142-9612(99)00213-6) [DOI] [PubMed] [Google Scholar]
- 21.Hunziker E. B. 2002. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10, 432–463 10.1053/joca.2002.0801 (doi:10.1053/joca.2002.0801) [DOI] [PubMed] [Google Scholar]
- 22.Koga H., Engebretsen L., Brinchmann J., Muneta T., Sekiya I. 2009. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg. Sports Traumatol. Arthrosc. 17, 1289–1297 10.1007/s00167-009-0782-4 (doi:10.1007/s00167-009-0782-4) [DOI] [PubMed] [Google Scholar]
- 23.Mauck R. L., Soltz M. A., Wang C. C., Wong D. D., Chao P. H., Valhmu W. B., Hung C. T., Ateshian G. A. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122, 252–260 10.1115/1.429656 (doi:10.1115/1.429656) [DOI] [PubMed] [Google Scholar]
- 24.Buschmann M. D., Gluzband Y. A., Grodzinsky A. J., Hunziker E. B. 1995. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 108, 1497–1508 [DOI] [PubMed] [Google Scholar]
- 25.Davisson T., Kunig S., Chen A., Sah R., Ratcliffe A. 2002. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J. Orthop. Res. 20, 842–848 10.1016/S0736-0266(01)00160-7 (doi:10.1016/S0736-0266(01)00160-7) [DOI] [PubMed] [Google Scholar]
- 26.Tsuang Y. H., Lin Y. S., Chen L. T., Cheng C. K., Sun J. S. 2008. Effect of dynamic compression on in vitro chondrocyte metabolism. Int. J. Artif. Organs 31, 439–449 [DOI] [PubMed] [Google Scholar]
- 27.Nicodemus G. D., Bryant S. J. 2010. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage 18, 126–137 10.1016/j.joca.2009.08.005 (doi:10.1016/j.joca.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 28.Mauck R. L., Wang C. C. B., Oswald E. S., Ateshian G. A., Hung C. T. 2003. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage 11, 879–890 10.1016/j.joca.2003.08.006 (doi:10.1016/j.joca.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 29.Lima E. G., Bian L., Ng K. W., Mauck R. L., Byers B. A., Tuan R. S., Ateshian G. A., Hung C. T. 2007. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthritis Cartilage 15, 1025–1033 10.1016/j.joca.2007.03.008 (doi:10.1016/j.joca.2007.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian L., Fong J. V., Lima E. G., Stoker A. M., Ateshian G. A., Cook J. L., Hung C. T. 2010. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng. Part A 16, 1781–1790 10.1089/ten.TEA.2009.0482 (doi:10.1089/ten.TEA.2009.0482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoenig E., Winkler T., Mielke G., Paetzold H., Schuettler D., Goepfert C., Machens H. G., Morlock M. M., Schilling A. F. 2011. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng. Part A 17, 1401–1411 (doi:10.1089/ten.tea.2010.0395) [DOI] [PubMed] [Google Scholar]
- 32.Huang A., Farrell M., Kim M., Mauck R. 2010. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur. Cell Mat. 19, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauck R. L., Wang C. C. B., Oswald E. S., Ateshian G. A., Hung C. T. 2003. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage 11, 879–890 10.1016/j.joca.2003.08.006 (doi:10.1016/j.joca.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 34.Kelly T. A. N., Ng K. W., Wang C. C. B., Ateshian G. A., Hung C. T. 2006. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J. Biomech. 39, 1489–1497 10.1016/j.jbiomech.2005.03.031 (doi:10.1016/j.jbiomech.2005.03.031) [DOI] [PubMed] [Google Scholar]
- 35.Nagel T., Kelly D. 2011. Remodelling of collagen fibre transition stretch and angular distribution in soft biological tissues and cell-seeded hydrogels. Biomech. Model. Mechanobiol. (doi:10.1007/s10237-011-0313-3) [DOI] [PubMed] [Google Scholar]
- 36.Görke U. J., Günther H., Nagel T., Wimmer M. A. 2010. A large strain material model for soft tissues with functionally graded properties. J. Biomech. Eng. 132, 074502. 10.1115/1.4001312 (doi:10.1115/1.4001312) [DOI] [PubMed] [Google Scholar]
- 37.Nagel T., Kelly D. J. 2010. The influence of fibre orientation on the equilibrium properties of neutral and charged biphasic tissues. J. Biomech. Eng. 132, 114506. 10.1115/1.4002589 (doi:10.1115/1.4002589) [DOI] [PubMed] [Google Scholar]
- 38.Ateshian G. A., Rajan V., Chahine N. O., Canal C. E., Hung C. T. 2009. Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J. Biomech. Eng. 131, 061003. 10.1115/1.3118773 (doi:10.1115/1.3118773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korhonen R. K., Laasanen M. S., Töyräs J., Lappalainen R., Helminen H. J., Jurvelin J. S. 2003. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J. Biomech. 36, 1373–1379 10.1016/S0021-9290(03)00069-1 (doi:10.1016/S0021-9290(03)00069-1) [DOI] [PubMed] [Google Scholar]
- 40.Ficklin T. et al. 2007. Articular cartilage mechanical and biochemical property relations before and after in vitro growth. J. Biomech. 40, 3607–3614 10.1016/j.jbiomech.2007.06.005 (doi:10.1016/j.jbiomech.2007.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chahine N. O., Wang C. C. B., Hung C. T., Ateshian G. A. 2004. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J. Biomech. 37, 1251–1261 10.1016/j.jbiomech.2003.12.008 (doi:10.1016/j.jbiomech.2003.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maroudas A. I. 1976. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 260, 808–809 10.1038/260808a0 (doi:10.1038/260808a0) [DOI] [PubMed] [Google Scholar]
- 43.Bank R. A., Soudry M., Maroudas A., Mizrahi J., TeKoppele J. M. 2000. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 43, 2202–2210 (doi:10.1002/1529-0131(200010)43:10<2202::AID-ANR7>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 44.Ng K. W., Mauck R. L., Wang C. C. B., Kelly T. A. N., Ho M. M. Y., Chen F. H., Ateshian G. A., Hung C. T. 2009. Duty cycle of deformational loading influences the growth of engineered articular cartilage. Cell Mol. Bioeng. 2, 386–394 10.1007/s12195-009-0070-x (doi:10.1007/s12195-009-0070-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mow V. C., Kuei S. C., Lai W. M., Armstrong C. G. 1980. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J. Biomech. Eng. 102, 73–84 10.1115/1.3138202 (doi:10.1115/1.3138202) [DOI] [PubMed] [Google Scholar]
- 46.Huang C., Yannas I. V. 1977. Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J. Biomed. Mater. Res. 11, 137–154 10.1002/jbm.820110113 (doi:10.1002/jbm.820110113) [DOI] [PubMed] [Google Scholar]
- 47.Kelly T. A. N., Wang C. C. B., Mauck R. L., Ateshian G. A., Hung C. T. 2004. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology 41, 223–237 See http://iospress.metapress.com/content/FDYYBTYEWHR0VCGH [PubMed] [Google Scholar]
- 48.Yan D., et al. 2009. The impact of low levels of collagen IX and pyridinoline on the mechanical properties of in vitro engineered cartilage. Biomaterials 30, 814–821 10.1016/j.biomaterials.2008.10.042 (doi:10.1016/j.biomaterials.2008.10.042) [DOI] [PubMed] [Google Scholar]
- 49.Balguid A., Rubbens M. P., Mol A., Bank R. A., Bogers A. J. J. C., van Kats J. P., de Mol B. A., Baaijens F. P., Bouten C. V. 2007. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets—relevance for tissue engineering. Tissue Eng. 13, 1501–1511 10.1089/ten.2006.0279 (doi:10.1089/ten.2006.0279) [DOI] [PubMed] [Google Scholar]
- 50.Sengers B., van Donkelaar C., Oomens C., Baaijens F. 2004. The local matrix distribution and the functional development of tissue engineered cartilage, a finite element study. Ann. Biomed. Eng. 32, 1718–1727 10.1007/s10439-004-7824-3 (doi:10.1007/s10439-004-7824-3) [DOI] [PubMed] [Google Scholar]








