Abstract
Avian plumage colours are model traits in understanding the evolution of sexually selected ornamental traits. Paradoxically, iridescent structural colours, probably the most dazzling of these traits, remain the most poorly understood. Though some data suggest that expression of bright iridescent plumage colours produced by highly ordered arrays of melanosomes and keratin is condition-dependent, almost nothing is known of their ontogeny and thus of any developmental mechanisms that may be susceptible to perturbation. Here, we use light and electron microscopy to compare the ontogeny of iridescent male and non-iridescent female feathers in blue-black grassquits. Feather barbules of males contain a single layer of melanosomes bounded by a thin layer of keratin-producing blue iridescent colour, while those of females contain disorganized melanosomes and no outer layer. We found that nanostructural organization of male barbules occurs late in development, following death of the barbule cell, and is thus unlikely to be under direct cellular control, contrary to previous suggestions. Rather, organization appears to be caused by entropically driven self-assembly through depletion attraction forces that pin melanosomes to the edge of barbule cells and to one another. These forces are probably stronger in developing barbules of males than of females because their melanosomes are (i) larger, (ii) more densely packed, and (iii) more homogeneously distributed owing to the more consistent shape of barbules during keratinization. These data provide the first proposed developmental pathway for iridescent plumage colours, and suggest that any condition dependence of iridescent barbules is likely driven by factors other than direct metabolic cost.
Keywords: Asakura–Oosawa depletion model, biophotonics, honest signalling, sexual selection, thin-films
1. Introduction
Since Darwin [1], numerous models have been proposed to explain the evolution of seemingly maladaptive traits through the role they play in intraspecific sexual contexts (reviewed in [2,3]). These models largely posit that mating preferences—and hence differential reproductive success—lead to an exaggeration of non-combat-related ornamental traits like long tails and colourful plumage. Honest signalling models have been most heavily studied (but see [4]) and posit that ornamental traits are costly to produce or maintain, and thus honestly convey information about their bearer.
Bird coloration is a model trait in the study of sexual selection [2,5]. Feather colours can be classified as either pigmentary, when resulting from selective absorption of light at certain wavelengths by pigments, or structural, when resulting from wavelength-dependent interference of light interacting with nanostructurally organized biophotonic tissues [6]. Understanding the mechanistic basis of colour production is essential to understanding the evolution of colourful plumage, because different mechanisms may vary in costliness of both production and maintenance [7–9]. While carotenoid-based sexual signals are now classic examples of condition-dependent sexual selection, the cost (if any) of structural colours is largely unknown. Iridescent structural colours in feather barbules are produced by interference effects from nanoscale arrangements of keratin, melanin (deposited in specialized organelles called melanosomes) and air [10]. When unorganized, these same materials (melanosomes and keratin) produce non-iridescent black, brown or grey colours [11–13]. Therefore, any differences in ontogenetic cost between structural and pigmentary-based feather colours should be caused by the processes driving nanoscale organization.
It is thus surprising that, despite numerous studies examining both the condition-dependence and sexual selection of structurally coloured traits in birds [7,14–18], their developmental mechanisms are still largely unknown. Only recently has a hypothesis for the organization of non-iridescent spongy layers been proposed [19,20]. No equivalent hypothesis has been proposed to explain the arrangement of melanin in iridescent feathers [21]. Because variations as small as a few nanometres can cause intraspecific variation in colour [22], it is reasonable to hypothesize that iridescent structural colours will be condition-dependent and signal developmental stability [7,23]. We can test this hypothesis by investigating iridescent colour ontogeny and identifying processes that may be physiologically costly or susceptible to perturbation.
We thus compared the development of iridescent and non-iridescent barbules in blue-black grassquit (Volatinia jacarina) feathers. These birds are ideal for this study because (i) they are sexually dichromatic, allowing us to directly compare the development of the two colours in the same species, (ii) the mechanism-producing iridescent colour in males has been previously described [24], and (iii) the iridescent nuptial plumage appears to be sexually selected [25,26], and condition-dependent [27,28]. By comparing male and female blue-black grassquit feather development, we hypothesize an ontogenetic pathway for the formation of the organized nanostructures responsible for iridescent colour production.
2. Material and methods
To induce feather moult, we plucked mantle feathers from 10 male and six female captive blue-black grassquits (for details on housing of captive birds, see [29]). Using forceps, we collected developing feathers erupting from the skin every other day for 10 days. Developing feathers were fixed overnight in 4 per cent glutaraldehyde in 0.1 M phosphate buffer solution, and then rinsed in phosphate buffer and post-fixed in 2 per cent OsO4 in buffer for 90 min. Samples were then rinsed three times in distilled water, dehydrated and embedded in Spurr resin following standard protocol (outlined in [24]).
To visualize developmental patterns, we cut thick sections (approx. 0.7–1 µm) for light microscopy and thin sections (approx. 100–200 nm) for transmission electron microscopy at 1 mm intervals from the follicle base to its tip (i.e. from the least- to most-developed regions). Thick sections were placed on glass slides and stained with 1 per cent toluidine blue, and thin sections were collected using oval-slit carbon and formvar-coated copper grids in duplicate, and either stained with Reynold's lead citrate [30] or kept unstained.
We examined thick sections on an optical microscope (Leica DM 2500), and thin sections on a JEOL JEM-1230 transmission electron microscope operating at 120 kV. Initial observations revealed that samples collected eight days after moult induction showed complete sequences of feather development, so we focused on these for the remainder of the work.
3. Results
Results are organized by similarities and differences between males and females at each developmental stage.
3.1. 0 mm (ramogenic collar)
At this stage, feather development is similar in both sexes, with no evident distinctions. Barb ridges and barbule plates are barely evident. Keratinocytes are not fully differentiated and do not form clear ridges, are mostly unpigmented and are sometimes still mitotic (figure 1a,b). Melanocytes, and in some cases their dendrytic expansions, can be seen in the epidermal folds (figure 1a,c) and connected to keratinocytes (figure 1d). Several organelles, including endoplasmatic reticula and melanosomes in various stages of development can be seen in these expansions (figure 1c).
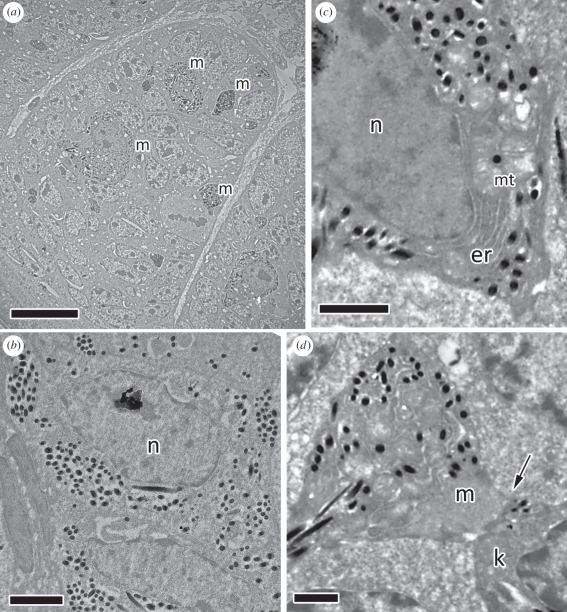
Figure 1.
Blue-black grassquit feathers early in development (0 mm, ramogenic collar). (a) Developing male barbules within the barb ridge (scale bar, 10 µm). Keratinocytes are undifferentiated and not organized in rows (barbule plates); melanocytes and melanocyte expansions (m) can be seen. (b) Female keratinocyte showing melanosomes (dark spots) and the large nucleus (n) (scale bar, 2 µm). (c) Female melanocyte with developing melanin granules; endoplasmatic reticulum (er) and mitochondria (mt) can be seen at the nucleus (n) edge (scale bar, 1 µm). (d) Female melanocyte expansion (m) with a fused membrane (arrow) to a barbule keratinocyte (k), for transfer of melanosomes (scale bar, 1 µm).
3.2. 1 mm
3.2.1. Similarities
Barbule ridges are differentiated, and barbules contain unorganized melanosomes (figure 2a,d). Nuclei are large and nucleoli are clearly identifiable, indicating active transcription and metabolism [31].
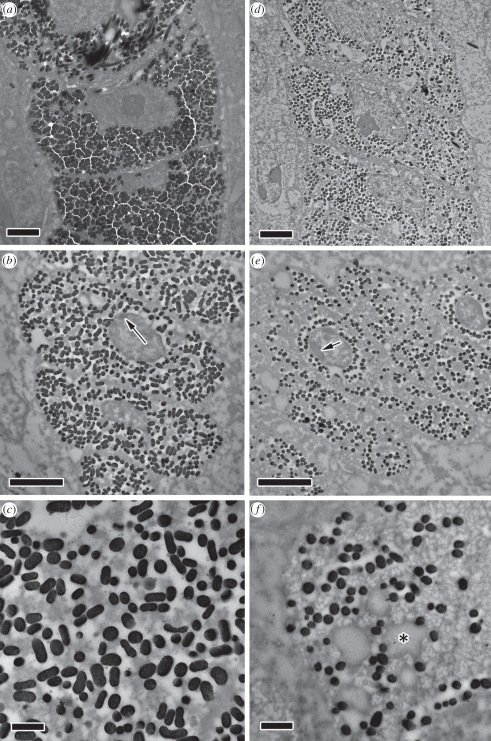
Figure 2.
Male (a–c) and (d–f) female blue-black grassquit feather barbules at intermediate stages of development. (a) Male barbules at 1 mm from the base of the developing feather. Melanosomes (dark spots) fill up most of the barbule volume that is not occupied by the nucleus. (b) Male barbule at 2 mm from the base of the developing feather. Barbule can be seen to elongate laterally, and some heterochromatin (arrow) can be seen at the nucleus edge. (c) Close-up: electron-dense keratin filaments can be seen being formed and phase-separating from the electron-pale cytoplasm. (d) Female barbule at 1 mm from the base of the developing feather, with similar pattern to male barbule, but with considerably fewer and smaller melanosomes. (e) Female barbule at 2 mm from the base of the developing feather, also with a heterochromatic nucleus (arrow). (f) Close-up: keratin filaments can also be seen, as well as large electron-pale vacuoles (exemplified by an asterisk), possibly of lipidic content. Scale bars (a,b,d,e), 2 µm; (c,f), 500 nm.
3.2.2. Differences
While melanosomes are scarce in female barbules, they occupy close to the entire barbule volume in males. Furthermore, the right-arm barbule plate of each barb ridge is more heavily pigmented than the left in females, but not in males (see figure 4a,b).
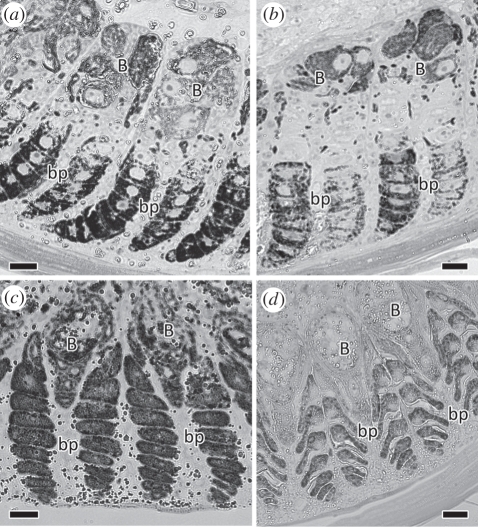
Figure 4.
Light microscopy of developing barb ridges of male (a,b) and female (c,d) blue-black grassquits during early (a,c; 1 mm from the base of the developing feather) and late (b,d; 3 mm from the base) development (B: barb; bp: barbule plates). During early development, there are contrasting patterns of melanization of barbules, with (a) male barbules being consistently heavily melanized, whereas (c) female barbules are more melanized in the outer barbule plate (bp; left) than the inner, and in both plates being less melanized than male barbules. During late development, the difference in shape of barbules is clearly visible, with (b) male barbules being elliptical in cross section, and (d) female barbules displaying an ‘arrowhead’ shape. Scale bars, 10 µm.
3.3. 2 mm
3.3.1. Similarities
Clumps of heterochromatin are seen on nuclei walls, indicating the reduction in nuclear activity that precedes cell necrosis (indicated by arrows in figure 2b,e). The cytoplasm of cells is very heterogeneous at this stage, as a consequence of small electron-dense keratin bundles forming and phase-separating from the cytoplasm, indicating initial stages of barbule keratinization (figure 2c,f). Electron-pale vesicules (evident in figure 2f), possibly lipidic bodies from the deterioration of cellular organelles [32,33] or cytoplasmatic ‘voids’ formed during initial keratinization [20], can be seen in both male and female barbules. Melanosomes remain unordered and are found in the unkeratinized cytoplasm.
3.3.2. Differences
Barbule shape is clearly dimorphic at this stage. In females, the internal side of barbules (relative to the barb ridge) is reduced in size, while male barbules maintain their elliptical shape (figure 2b,e). In females, the nucleus is shifted towards the outer edge of the barb ridge while in males, the nucleus is at approximately the centre of the barbule (figure 2b,e).
3.4. 3 mm
3.4.1. Similarities
Large and electron-dense sheets of keratin are visible in barbules of both sexes as keratin increases in molecular weight and further phase separates from the cytoplasm. Melanosomes mostly occupy pockets of cytoplasm, which are now much smaller and scattered, except towards the edges and around nuclei (figure 3a,b,d,e). Nuclei at this stage are reduced in size and are highly heterochromatic (arrows in figure 3b,e).
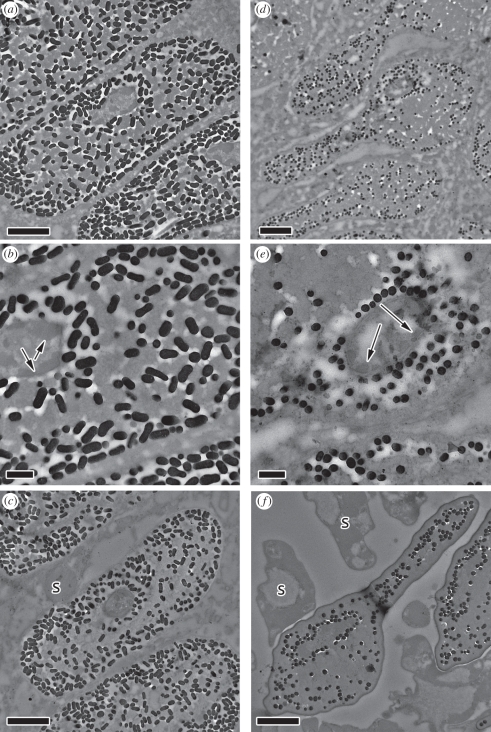
Figure 3.
Late-development (a–c) male and (d–f) female blue-black grassquit feather barbules. (a) Male barbule at 3 mm from the base of the developing feather. Large electron-dense bundles of keratin can be seen, and melanosomes (dark spots) are concentrated around the edges of the barbule. (b) Close-up: electron-pale areas can be seen in between melanosomes, as a result of their exclusion areas. Nucleus is small and heavily heterochromatic (arrows). (c) Male barbule at 4 mm from the base of the developing feather, mostly keratinized and with a small, electron-dense nucleus. Supportive cells (s) around barbules are also degenerated. (d) Female barbule at 3 mm from the base of the developing feather. As in males, large electron-dense bundles of keratin can be seen. However, the ‘arrowhead’ shape of the barbule and unorganized melanosomes contrast with the ovoid shape and organized melanosomes of the male. (e) Close-up of (d), illustrating as in males the electron lucent areas between melanosomes and the heterochromatin at the edge of the nucleus (arrows). (f) Female barbule at 4 mm from the base of the developing feather; fully keratinized, nucleus no longer visible. Scale bars (a,c,d,f), 2 µm; (b,e), 500 nm.
3.4.2. Differences
While female barbules are ‘arrowhead’-shaped (wider on the outer side relative to the inner side; [33]), male barbules retain their elliptical shape (figures 3a,d and 4b,d). In females, most melanosomes are haphazardly scattered and trapped between keratin sheets in the central portion of barbules, but in males, they are bundled together and pushed towards the edge of the barbule cells along with most of the unkeratinized cytoplasm.
3.5. 4 mm:
3.5.1. Similarities
Barbules are almost fully developed (figure 3c,f). Nuclei are either fully pyknoctic or fragmented. Shaft and supportive cells that surround the barb ridges also show late stages of degradation.
3.5.2. Differences
In females, keratin is mostly polymerized and homogeneous, while in males, areas of slightly lower density, coincident with high concentration of melanosomes, are visible near the edges of barbules (figure 3c,f). These form the layer bounding the thin keratin cortex around the barbule edge that is responsible for producing iridescent colour in males. Melanosomes remain unorganized in females, but are found in greater densities around the edge of the degrading nucleus.
4. Discussion
Comparisons of barbule development indicate dramatic differences between male and female blue-black grassquits that allowed us to develop, for the first time, a hypothesized ontogenetic pathway for iridescent feathers. We found no evidence of active cellular processes guiding placement of melanosomes. Indeed, most of the organizational steps we observed took place following the death of the barbule cell. This leads us to hypothesize that nanostructural arrangement occurs through self-assembly processes.
In regenerating feathers, keratinization is a complex process involving at least two chronologically separated phases: the synthesis phase, where keratin molecules are synthesized by ribosomes from free amino acids, and the keratogenous (or transitional) phase, where other cellular constituents are broken down and large-scale polymerization follows a reduction in metabolism associated with cellular necrosis [34–36]. The developmental steps that form the outer melanin layer and the thin keratin cortex responsible for iridescent colour production are only observed at these very last stages of cellular differentiation (as also observed by [37]). It is unlikely that direct metabolic processes are active at these stages [33,38], and organization should thus instead proceed through entropic, free-energy-minimizing interactions, of which we suggest depletion attraction [39] plays a fundamental role.
Though the emergence of organized systems through increasing entropy may seem paradoxical, the Asakura–Oosawa model of attraction forces resulting from osmotic depletion have been extensively documented in colloidal mixtures [40], and have been shown or suggested to play critical roles in many biological processes [41–43]. In mixtures of hard (‘non-interacting’) particles and polymers (in this case, melanosomes and keratin), the centre of mass of the polymer cannot occupy an area of the particle's surface equal to its own radius, creating an area of excluded volume surrounding the particle. As the large particles approach one another, these areas of excluded volume overlap, resulting in a larger volume that can be occupied by the polymer and therefore a loss of free energy [44]. This overlap leads to the depletion of the polymer in the gaps between particles, creating an osmotic gradient that attracts particles to one another (figure 5a).
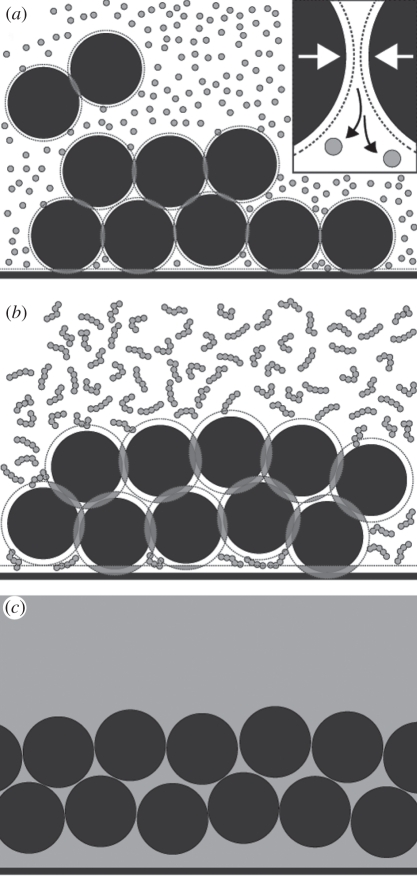
Figure 5.
Schematic of depletion attraction forces during keratinization of iridescent feather barbules. (a) keratin units (grey circles) cannot occupy the excluded volume (dashed lines) around melanosomes (black circles). When they are close to one another or to the barbule edge, these exclusion areas overlap (grey areas), resulting in a greater total volume that can be occupied by keratin, and therefore in less free energy in the system. Because difference in size between melanosomes and keratin units is very large, keratin can occupy gaps between melanosomes and the barbule edge. Inset: since keratin cannot occupy the area between melanocytes, the difference in concentration of the area between melanosomes and the rest of the system results in osmotic pressure that draws solute from that area and back into the system, resulting in an attractive force between particles. (b) As keratin polymerizes and cross-links during keratinization, the range and the strength of these forces increase, resulting in even greater free energy loss from the packing of melanosomes. Keratin units that were ‘trapped’ between melanosomes and the barbule cell membrane also increase in molecular weight, causing these regions to connect and form the keratin thin layer, which will produce iridescence. (c) The product of these interactions results in the formation of a thin film of keratin overlaying a layer of melanosomes when the feather is fully keratinized (as represented by the homogeneous keratin grey area), characteristic of iridescent feather barbules.
The range of these interactions is proportional to the size of the polymer and their magnitude is proportional to its concentration; therefore, as polymerization proceeds, the effect of depletion interactions on particle arrangement constantly increases (figure 5b; [39,45–47]). Furthermore, at vesicle walls, the overlap of particle and wall-excluded volumes result in a free energy loss approximately twice that between two particles [40,48,49]. As a consequence, the concentration of dilute polymers necessary for attraction depletion forces is much lower, potentially leading to the coexistence of an organized, particle-rich phase near the walls and a polymer-rich phase in the bulk (figure 5c; [44,50,51]).
We hypothesize that ontogeny of the nanostructural organization of male blue-black grassquit barbules proceeds through self-assembly via depletion attraction forces. Specifically, these forces pin melanosomes to the cell wall and to each other, forming a stable homogeneous layer around the outer edge of male barbule cells. Two critical differences between developing male and female barbules should lead to greater and more consistent depletion attraction forces, and hence self-assembly, in males. First, the larger size and density of male melanosomes increase their excluded volume overlap during keratinization and, assuming all other properties are constant, the scale of depletion attraction [52]. The small and sparsely distributed female melanosomes should only form local and randomly dispersed aggregates, which we indeed observe (figure 3). Second, the uniform elliptical shape of male barbules should lead to a more homogeneous range of interactions between melanosomes and the cell wall than in female barbules, resulting in a more discrete and consistent layer formation. A rough, heterogeneously shaped barbule surface would lead to local differences in interaction forces. For example, a particle in a concave portion of the surface would have a greater exclusion area overlap, as the curvature will provide a greater contact area between the surface and the particle [48]; thus, melanosomes would aggregate more densely and closer to the surface in these portions, whereas the opposite would occur in convex portions and invaginations. Indeed, melanosomes in female barbules aggregate around the degraded nucleus and also form larger aggregates in the thinner portion of the barbule (where they are locally more concentrated), suggesting weaker and heterogeneously distributed depletion attraction forces.
The thin keratin film overlying the melanosome layer in males may also be produced by these depletion interactions. Given that melanosome diameter is orders of magnitude larger than keratin units at initial stages, keratin can occupy gaps between melanosomes and the cell boundary [49]. As keratin polymerizes and increases in molecular weight and fibre unit size, melanosomes are attracted to the cell boundary, but can only reach the edge where keratin is polymerized or is forming longer bundles (figure 5b). The thickness of this edge, and hence outer keratin layer (which largely determines the colour of the feather), may thus be determined by and negatively associated with both the speed of keratinization and melanosome size.
This hypothesis makes clear, testable predictions that can be explored in the future. For example, both a higher concentration of keratin and faster keratinization would facilitate depletion attraction of melanosomes, thereby affecting nanostructural organization and feather colour. Indeed, indirect evidence suggests that the speed of nuptial plumage acquisition affects plumage colour in male blue-black grassquits [13]. In addition, because keratin is the main cytoskeleton protein in keratinocytes [53,54], its concentration may also affect their elliptical cross-sectional shape. We further predict that barbules with thin-film iridescence should have a greater volume fraction of melanosomes than non-iridescent barbules, and this is supported by evidence from a comparison of iridescent and non-iridescent barbules from jungle crows (Corvus machrorhynchos; [55]) and population-level variation in the chestnut-bellied monarch (Monarcha castaneiventris; [56]).
Finally, the interactions between depletion attraction and other short-range forces (such as Coulombic forces) may contribute to the diversity of iridescent structures. The known morphological diversity in melanosome morphology [57,58] should affect the strength of such interactions. Furthermore, it is reasonable to expect equivalent variation in surface charge (resulting from the protein content on the membranes of these organelles), which may affect the relative intensity of attraction depletion and short-range repulsion forces. This in turn will influence transitions between organized and unorganized patterns [43], as well as transitions between the laminar aggregation of melanosomes found here and the hexagonal packing found in other species [10]. Finally, it will be interesting to evaluate how these forces may integrate to produce multi-layer arrays of melanosomes found in birds-of-paradise (Paradiseidae; [59]), among other avian families [60]. Together, these conditions provide the first testable set of predictions for the development and evolution of thin-film iridescence in feathers.
4.1. Sexual selection and the evolution of iridescent plumage
Previous authors have suggested that the process of precise organization needed for iridescent structural colours was physiologically costly and thus condition-dependent [7,10,28]. Our data show that direct cellular control of this process is unlikely and hence that it is not energetically demanding, as has also been suggested for non-iridescent structural colours in feathers [19,20]. However, the conditions needed for phase separation kinetics may be strict and controlled [20,51,61]. Thus, though the nanostructural organization per se emerges through entropic interactions, the properties of the system in which it occurs may indeed be subject to developmental perturbations [23]. In other words, though the direct organization of colour-producing nanostructure under this scenario is not physiologically demanding, other costs may be involved in ‘setting the stage’ for self-assembly to emerge properly, rendering iridescent-coloured feathers adaptive signals of individual quality. Whether this is the case or not remains to be investigated, but our results provide clear predictions as to where such costs are to be found. For example, whether melanin production is costly or not is still hotly debated [62,63], but, if so, the large amount of melanin needed for self-assembly, or pleiotropic effects of melanin expression on other metabolic pathways [64–66], may indeed create an energetic cost. Also, keratinization is likely associated with the speed at which the feather grows [34,38], potentially explaining previously reported associations between colour and growth bar width [22,28]. The rate of feather keratinization is strongly influenced by the initial amount and concentration of keratin monomers [67], which in turn is mostly limited by keratin gene transcription and mRNA abundance [68]. Given that keratin gene expression is regulated by various transcription factors that are highly responsive to extracellular condition and hormones [69], and keratinization itself is sensitive to the pH of the cellular environment [67], this crucial step in nanostructural organization is likely to be sensitive to, and therefore indicative of, developmental stressors and individual condition [70–72].
In summary, based on the timing and steps of the organization of colour-producing nanostructures, our evidence strongly opposes the suggestion that nanostructural organization results from active cellular processes, instead pointing to the role of energy-minimizing entropic interactions for achieving such patterns. The differences between the development of male and female barbules, outlined above, thoroughly fit with the expectations under self-assembly through depletion attraction interactions, and we therefore hypothesize this as a mechanism for the development of iridescent feathers. Our results are not only the first report on how melanin granules organize in feather barbules to produce thin-film iridescence, but also pioneer clear predictions as to the mechanisms that might influence both the evolution and diversification of iridescent colours, bringing a new understanding to the role that such colours might play in sexual selection and animal communication.
Acknowledgements
We thank Liliana D'Alba, Chad Eliason, Don W. Ott, Jutta Luettmer-Strathmann and four anonymous reviewers for comments on previous versions of the manuscript and discussion of our findings, Luiza Brasileiro, Roberto Lacava and João Victor Caetano for help with bird housing and sample preparation. This work was supported by University of Akron startup funds and AFOSR grant FA9550-09-1-0159 (M.D.S.) and National Geographical Society and CAPES/CNPq grants (R.H.M.).
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 2.Andersson M. 1994. Sexual selection. New Jersey, NJ: Princeton University Press [Google Scholar]
- 3.Searcy W. A., Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. New Jersey, NJ: Princeton University Press [Google Scholar]
- 4.Prum R. O. 2010. The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for the meaning, honesty, and design in intersexual signals. Evolution 64, 3085–3100 10.1111/j.1558-5646.2010.01054.x (doi:10.1111/j.1558-5646.2010.01054.x) [DOI] [PubMed] [Google Scholar]
- 5.Bennett A. T. D., Cuthill I. C., Partridge J. C., Lunau K. 1997. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA 94, 8618–8621 10.1073/pnas.94.16.8618 (doi:10.1073/pnas.94.16.8618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shawkey M. D., Morehouse N. I., Vukusic P. 2009. A protean palette: colour materials and mixing in birds and butterflies. J. R. Soc. Interface 6, S221–S231 10.1098/rsif.2008.0459.focus (doi:10.1098/rsif.2008.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGraw K. J., Mackillop E., Dale J., Hauber M. E. 2002. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 205, 3747–3755 [DOI] [PubMed] [Google Scholar]
- 8.Peters A., Delhey K., Johnsen A., Kempenaers B. 2007. The condition-dependent development of carotenoid-based and structural plumage in nestling blue tits: males and females differ. Am. Nat. 169, S122–S136 10.1086/510139 (doi:10.1086/510139) [DOI] [PubMed] [Google Scholar]
- 9.Eliason C. M., Shawkey M. D. 2011. Decreased hydrophobicity of iridescent feathers: a potential cost of shiny plumage. J. Exp. Biol. 214, 2157–2163 10.1242/jeb.055822 (doi:10.1242/jeb.055822) [DOI] [PubMed] [Google Scholar]
- 10.Prum R. 2006. Anatomy, physics and evolution of structural colors. In Bird coloration, vol 1. Mechanisms and measurements (eds Hill G., McGraw K.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- 11.Shawkey M. D., Hauber M. E., Estep L. K., Hill G. E. 2006. Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae). J. R. Soc. Interface 3, 777–786 10.1098/rsif.2006.0131 (doi:10.1098/rsif.2006.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E., Aoyama M., Sugita S. 2009. Microstructure of the feather in Japanese Jungle Crows (Corvus macrorhynchos) with distinguishing gender differences. Anat. Sci. Int. 84, 141–147 10.1007/s12565-009-0022-5 (doi:10.1007/s12565-009-0022-5) [DOI] [PubMed] [Google Scholar]
- 13.Maia R., Macedo R. H. 2011. Achieving luster: prenuptial molt pattern predicts iridescent structural coloration in blue-black grassquits. J. Ornithol. 152, 243–252 10.1007/s10336-010-0576-y (doi:10.1007/s10336-010-0576-y) [DOI] [Google Scholar]
- 14.Bitton P.-P., Dawson R. D., Ochs C. L. 2008. Plumage characteristics, reproductive investment and assortative mating in tree swallows Tachycineta bicolor. Behav. Ecol. Sociobiol. 62, 1543–1550 10.1007/s00265-008-0583-7 (doi:10.1007/s00265-008-0583-7) [DOI] [Google Scholar]
- 15.Hill G. E., Doucet S. M., Buchholz R. 2005. The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 69, 387–394 10.1016/j.anbehav.2004.03.013 (doi:10.1016/j.anbehav.2004.03.013) [DOI] [Google Scholar]
- 16.Loyau A., Gomez D., Moureau B., Thery M., Hart N. S., Saint Jalme M., Bennett A. T. D., Sorci G. 2007. Iridescent structurally based coloration of eyespots correlates with mating success in the peacock. Behav. Ecol. 18, 1123–1131 10.1093/beheco/arm088 (doi:10.1093/beheco/arm088) [DOI] [Google Scholar]
- 17.Keyser A. J., Hill G. E. 1999. Condition-dependent variation in the blue-ultraviolet coloration of a structurally based plumage ornament. Proc. R. Soc. Lond. B 266, 771–777 10.1098/rspb.1999.0704 (doi:10.1098/rspb.1999.0704) [DOI] [Google Scholar]
- 18.Siefferman L. M., Hill G. E. 2005. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim. Behav. 69, 67–72 10.1016/j.anbehav.2003.12.026 (doi:10.1016/j.anbehav.2003.12.026) [DOI] [Google Scholar]
- 19.Dufresne E. R., Noh H., Saranathan V., Mochrie S. G. J., Cao H., Prum R. O. 2009. Self-assembly of amorphous biophotonic nanostructures by phase separation. Soft Matter 5, 1792–1795 10.1039/b902775k (doi:10.1039/b902775k) [DOI] [Google Scholar]
- 20.Prum R. O., Dufresne E. R., Quinn T., Waters K. 2009. Development of colour-producing beta-keratin nanostructures in avian feather barbs. J. R. Soc. Interface 6, S253–S265 10.1098/rsif.2008.0466.focus (doi:10.1098/rsif.2008.0466.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiradella H. T., Butler M. W. 2009. Many variations on a few themes: a broader look at development of iridescent scales (and feathers). J. R. Soc. Interface 6, S243–S251 10.1098/rsif.2008.0372.focus (doi:10.1098/rsif.2008.0372.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doucet S. M., Shawkey M. D., Hill G. E., Montgomerie R. 2006. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 209, 380–390 10.1242/jeb.01988 (doi:10.1242/jeb.01988) [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick S. 1998. Colour schemes for birds: structural coloration and signals of quality in feathers. Ann. Zool. Fenn 35, 67–77 [Google Scholar]
- 24.Maia R., Caetano J. V. O., Bao S. N., Macedo R. H. 2009. Iridescent structural colour production in male blue-black grassquit feather barbules: the role of keratin and melanin. J. R. Soc. Interface 6, S203–S211 10.1098/rsif.2008.0460.focus (doi:10.1098/rsif.2008.0460.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida J., Macedo R. H. 2001. Lek-like mating system of the monogamous blue-black grassquit. Auk 118, 404–411 10.1642/0004-8038(2001)118[0404:LLMSOT]2.0.CO;2 (doi:10.1642/0004-8038(2001)118[0404:LLMSOT]2.0.CO;2) [DOI] [Google Scholar]
- 26.Carvalho C. B. V., Macedo R. H., Graves J. A. 2006. Breeding strategies of a socially monogamous neotropical passerine: extra-pair fertilizations, behavior and morphology. Condor 108, 579–590 10.1650/0010-5422(2006)108[579:BSOASM]2.0.CO;2 (doi:10.1650/0010-5422(2006)108[579:BSOASM]2.0.CO;2) [DOI] [Google Scholar]
- 27.Costa F., Macedo R. H. 2005. Coccidian oocyst parasitism in the blue-black grassquit: influence on secondary sex ornaments and body condition. Anim. Behav. 70, 1401–1409 10.1016/j.anbehav.2005.03.024 (doi:10.1016/j.anbehav.2005.03.024) [DOI] [Google Scholar]
- 28.Doucet S. M. 2002. Structural plumage coloration, male body size, and condition in the blue-black grassquit. Condor 104, 30–38 10.1650/0010-5422(2002)104[0030:SPCMBS]2.0.CO;2 (doi:10.1650/0010-5422(2002)104[0030:SPCMBS]2.0.CO;2) [DOI] [Google Scholar]
- 29.Lacava R. V., Brasileiro L., Maia R., Oliveira R. F., Macedo R. H. 2011. Social environment affects testosterone level in captive male blue-black grassquits. Horm. Behav. 59, 51–55 10.1016/j.yhbeh.2010.10.003 (doi:10.1016/j.yhbeh.2010.10.003) [DOI] [PubMed] [Google Scholar]
- 30.Reynolds E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 10.1083/jcb.17.1.208 (doi:10.1083/jcb.17.1.208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raška I., Shaw P. J., Cmarko D. 2006. New insights into nucleolar architecture and activity. Int. Rev. Cytol. 255, 177–235 10.1016/S0074-7696(06)55004-1 (doi:10.1016/S0074-7696(06)55004-1) [DOI] [PubMed] [Google Scholar]
- 32.Gregg K., Rogers G. E. 1986. Feather keratin: composition, structure and biogenesis. In Biology of the integument vol 2: vertebrates (eds Bereiter-Hahn J., Matoltsy A. G., Richards K. S.), pp. 666–694 Berlin, Germany: Springer [Google Scholar]
- 33.Alibardi L. 2005. Cell structure of developing barbs and barbules in downfeathers of the chick: central role of barb ridge morphogenesis for the evolution of feathers. J. Submicrosc. Cytol. Pathol. 37, 19–41 [PubMed] [Google Scholar]
- 34.Spearman R. I. 1966. The keratinization of epidermal scales, feathers and hairs. Biol. Rev. 41, 59–96 10.1111/j.1469-185X.1966.tb01538.x (doi:10.1111/j.1469-185X.1966.tb01538.x) [DOI] [PubMed] [Google Scholar]
- 35.Matulionis D. H. 1970. Morphology of the developing down feathers of chick embryos: a descriptive study at the ultrastructural level of differentiation and keratinization. Z. Anat. Entwicklungsgesch 132, 107–157 10.1007/BF00523275 (doi:10.1007/BF00523275) [DOI] [PubMed] [Google Scholar]
- 36.Lucas A., Stettenheim P. 1972. Avian anatomy—integument, part II. Washington, DC: US Department of Agriculture [Google Scholar]
- 37.Durrer H., Villiger W. 1967. Bildung der Schillerstruktur beim Glanzstar: Elektronenmikroskopische Untersuchungen der Entstehung Gasgefüllter Melaninkörner. Z. Zellforsch 81, 445–456 10.1007/BF00342767 (doi:10.1007/BF00342767) [DOI] [PubMed] [Google Scholar]
- 38.Haake A. R., König G., Sawyer R. H. 1984. Avian feather development: relationships between morphogenesis and keratinization. Dev. Biol. 106, 406–413 10.1016/0012-1606(84)90240-9 (doi:10.1016/0012-1606(84)90240-9) [DOI] [PubMed] [Google Scholar]
- 39.Asakura S., Oosawa F. 1958. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. 33, 183. 10.1002/pol.1958.1203312618 (doi:10.1002/pol.1958.1203312618) [DOI] [Google Scholar]
- 40.Yodh A., Lin K., Crocker J., Dinsmore A., Verma R., Kaplan P. 2001. Entropically driven self-assembly and interaction in suspension. Phil. Trans. R. Soc. Lond. A 359, 921–937 10.1098/rsta.2000.0810 (doi:10.1098/rsta.2000.0810) [DOI] [Google Scholar]
- 41.Herzfeld J. 1996. Entropically driven order in crowded solutions: From liquid crystals to cell biology. Acc. Chem. Res. 29, 31–37 10.1021/ar9500224 (doi:10.1021/ar9500224) [DOI] [PubMed] [Google Scholar]
- 42.Fraden S., Kamien R. D. 2000. Self-assembly in vivo. Biophys. J. 78, 2189–2190 10.1016/S0006-3495(00)76767-1 (doi:10.1016/S0006-3495(00)76767-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemsley A., Collinson M., Kovach W., Vincent B., Williams T. 1994. The role of self-assembly in biological systems: evidence from iridescent colloidal sporopollenin in Selaginella megaspore walls. Phil. Trans. R. Soc. Lond. B 345, 163–173 10.1098/rstb.1994.0095 (doi:10.1098/rstb.1994.0095) [DOI] [Google Scholar]
- 44.Kaplan P., Rouke J., Yodh A., Pine D. 1994. Entropically driven surface phase separation in binary colloidal mixtures. Phys. Rev. Lett. 72, 582–585 10.1103/PhysRevLett.72.582 (doi:10.1103/PhysRevLett.72.582) [DOI] [PubMed] [Google Scholar]
- 45.Kim S.-C., Suh S.-H., Seong B.-S. 2007. Effect of polymer size and chain length on depletion interactions between two colloids. J. Chem. Phys. 127, 114903. 10.1063/1.2776260 (doi:10.1063/1.2776260) [DOI] [PubMed] [Google Scholar]
- 46.Verma R., Crocker J., Lubensky T., Yodh A. 1998. Entropic colloidal interactions in concentrated DNA solutions. Phys. Rev. Lett. 81, 4004–4007 10.1103/PhysRevLett.81.4004 (doi:10.1103/PhysRevLett.81.4004) [DOI] [Google Scholar]
- 47.Verma R., Crocker J., Lubensky T., Yodh A. 2000. Attractions between hard colloidal spheres in semiflexible polymer solutions. Macromolecules 33, 177–186 10.1021/ma990362v (doi:10.1021/ma990362v) [DOI] [Google Scholar]
- 48.Dinsmore A., Wong D., Nelson P., Yodh A. 1998. Hard spheres in vesicles: curvature-induced forces and particle-induced curvature. Phys. Rev. Lett. 80, 409–412 10.1103/PhysRevLett.80.409 (doi:10.1103/PhysRevLett.80.409) [DOI] [Google Scholar]
- 49.Trokhymchuk A., Henderson D., Nikolov A., Wasan D. 2001. Entropically driven ordering in a binary colloidal suspension near a planar wall. Phys. Rev. E 64, 012401. 10.1103/PhysRevE.64.012401 (doi:10.1103/PhysRevE.64.012401) [DOI] [PubMed] [Google Scholar]
- 50.Dinsmore A., Warren P., Poon W., Yodh A. 1997. Fluid-solid transitions on walls in binary hard-sphere mixtures. Europhys. Lett. 40, 337–342 10.1209/epl/i1997-00468-4 (doi:10.1209/epl/i1997-00468-4) [DOI] [Google Scholar]
- 51.Lekkerkerker H. N. W., Poon W. C.-K., Pusey P. N., Stroobants A., Warren P. B. 1992. Phase behaviour of colloid + polymer mixtures. Europhys. Lett. 20, 559–564 10.1209/0295-5075/20/6/015 (doi:10.1209/0295-5075/20/6/015) [DOI] [Google Scholar]
- 52.Marenduzzo D., Finan K., Cook P. R. 2006. The depletion attraction: an underappreciated force driving cellular organization. J. Cell. Biol. 175, 681–686 10.1083/jcb.200609066 (doi:10.1083/jcb.200609066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brush A. 1978. Feather keratins. In Chemical Zoology (eds Florkin M., Brush A.), pp. 1–23 New York, NY: Academic Press [Google Scholar]
- 54.Filshie B. K., Rogers G. E. 1962. An electron microscope study of the fine structure of feather keratin. J. Cell. Biol. 13, 1–12 10.1083/jcb.13.1.1 (doi:10.1083/jcb.13.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee E., Tanaka H., Wakamatsu K., Sugita S. 2009. Melanin-based iridescent feather color in the jungle crow. J. Vet. Med. Sci. 71, 1261–1263 10.1292/jvms.71.1261 (doi:10.1292/jvms.71.1261) [DOI] [PubMed] [Google Scholar]
- 56.Uy J. A. C., Moyle R. G., Filardi C. E., Cheviron Z. A. 2009. Difference in plumage color used in species recognition between incipient species is linked to a single amino acid substitution in the Melanocortin-1 Receptor. Am. Nat. 174, 244–254 10.1086/600084 (doi:10.1086/600084) [DOI] [PubMed] [Google Scholar]
- 57.Clarke J. A., Ksepka D. T., Salas-Gismondi R., Altamirano A. J., Shawkey M. D., D'Alba L., Vinther J., DeVries T. J., Baby P. 2010. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957 10.1126/science.1193604 (doi:10.1126/science.1193604) [DOI] [PubMed] [Google Scholar]
- 58.Li Q., Gao K.-Q., Vinther J., Shawkey M. D., Clarke J. A., D'Alba L., Meng Q., Briggs D. E. G., Prum R. O. 2010. Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372 10.1126/science.1186290 (doi:10.1126/science.1186290) [DOI] [PubMed] [Google Scholar]
- 59.Stavenga D. G., Leertouwer H. L., Marshall N. J., Osorio D. 2010. Dramatic colour changes in a bird of paradise caused by uniquely structured breast feather barbules. Proc. R. Soc. B 277, 2098–2104. (doi:10.1098/rspb.2010.2293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durrer H. 1986. The skin of birds: coloration. In Biology of the integument 2: vertebrates (eds Matoltsy A., Richards K.). Berlin, Germany: Springer [Google Scholar]
- 61.Dinsmore A., Yodh A., Pine D. 1995. Phase diagrams of nearly-hard-sphere binary colloids. Phys. Rev. E 52, 4045–4057 10.1103/PhysRevE.52.4045 (doi:10.1103/PhysRevE.52.4045) [DOI] [PubMed] [Google Scholar]
- 62.Griffith S. C., Parker T., Olson V. 2006. Melanin-versus carotenoid-based sexual signals: is the difference really so black and red? Anim. Behav. 71, 749–763 10.1016/j.anbehav.2005.07.016 (doi:10.1016/j.anbehav.2005.07.016) [DOI] [Google Scholar]
- 63.Stoehr A. 2006. Costly melanin ornaments: the importance of taxon? Funct. Ecol. 20, 276–281 10.1111/j.1365-2435.2006.01090.x (doi:10.1111/j.1365-2435.2006.01090.x) [DOI] [Google Scholar]
- 64.Boswell T., Takeuchi S. 2005. Recent developments in our understanding of the avian melanocortin system: its involvement in the regulation of pigmentation and energy homeostasis. Peptides 26, 1733–1743 10.1016/j.peptides.2004.11.039 (doi:10.1016/j.peptides.2004.11.039) [DOI] [PubMed] [Google Scholar]
- 65.Gangoso L., Grande J. M., Ducrest A. L., Figuerola J., Bortolotti G. R., Andrés J. A., Roulin A. 2011. MC1R-dependent, melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora's falcon. J. Evol. Biol. 10.1111/j.1420-9101.2011.02336.x (doi:10.1111/j.1420-9101.2011.02336.x) [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi S., Takahashi S., Okimoto R., Schiöth H. B., Boswell T. 2003. Avian melanocortin system: α-MSH may act as an autocrine/paracrine hormone. Ann. N. Y. Acad. Sci. 994, 366–372 10.1111/j.1749-6632.2003.tb03201.x (doi:10.1111/j.1749-6632.2003.tb03201.x) [DOI] [PubMed] [Google Scholar]
- 67.Brush A. H. 1983. Self-assembly of avian φ-keratins. J. Protein Chem. 2, 63–75 10.1007/bf01025168 (doi:10.1007/bf01025168) [DOI] [Google Scholar]
- 68.Powell B. C., Kemp D. J., Partington G. A., Gibbs P. E. M., Rogers G. E. 1976. Control of feather keratin synthesis by the availability of keratin mRNA. Biochem. Biophys. Res. Commun. 68, 1263–1271 10.1016/0006-291x(76)90333-8 (doi:10.1016/0006-291x(76)90333-8) [DOI] [PubMed] [Google Scholar]
- 69.Blumenberg M. 2006. Transcriptional regulation of keratin gene expression. In Intermediate filaments (ed. Paramio J. M.), pp. 93–109 Berlin, Germany: Springer US [Google Scholar]
- 70.Dawson A., Hinsley S., Ferns P., Bonser R., Eccleston L. 2000. Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proc. R. Soc. Lond. B 267, 2093–2098 10.1098/rspb.2000.1254 (doi:10.1098/rspb.2000.1254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DesRochers D. W., Reed J. M., Awerman J., Kluge J. A., Wilkinson J., van Griethuijsen L. I., Aman J., Romero L. M. 2009. Exogenous and endogenous corticosterone alter feather quality. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 152, 46–52 10.1016/j.cbpa.2008.08.034 (doi:10.1016/j.cbpa.2008.08.034) [DOI] [PubMed] [Google Scholar]
- 72.Lattin C. R., Reed J. M., DesRochers D. W., Romero L. M. 2011. Elevated corticosterone in feathers correlates with corticosterone-induced decreased feather quality: a validation study. J. Avian Biol. 42, 247–252 10.1111/j.1600-048X.2010.05310.x (doi:10.1111/j.1600-048X.2010.05310.x) [DOI] [Google Scholar]