Abstract
Muraenidae is a species-rich family, with relationships among genera and species and taxonomy that have not been completely clarified. Few cytogenetic studies have been conducted on this family, and all of them showed the same diploid chromosome number (2n=42) but with conspicuous karyotypic variation among species. The Mediterranean moray eel Gymnothorax unicolor was previously cytogenetically studied using classical techniques that allowed the characterization of its karyotype structure and the constitutive heterochromatin and argyrophilic nucleolar organizer regions (Ag-NORs) distribution pattern. In the present study, we describe two new repetitive elements (called GuMboI and GuDdeI) obtained from restricted genomic DNA of G. unicolor that were characterized by Southern blot and physically localized by in situ hybridization on metaphase chromosomes. As they are highly repetitive DNA sequences, they map in heterochromatic regions. However, while GuDdeI was localized in the centromeric regions, the GuMboI fraction was distributed on some centromeres and was co-localized with the nucleolus organizer region (NOR). Comparative analysis with other Mediterranean species such as Muraena helena pointed out that these DNA fractions are species-specific and could potentially be used for species discrimination. As a new contribution to the karyotype of this species, we found that the major ribosomal genes are localized on acrocentric chromosome 9 and that the telomeres of each chromosome are composed of a tandem repeat derived from a poly-TTAGGG DNA sequence, as it occurs in most vertebrate species. The results obtained add new information useful in comparative genomics at the chromosomal level and contribute to the cytogenetic knowledge regarding this fish family, which has not been extensively studied.
Key words: anguilliformes, muraenidae, heterochromatin, nucleolus organizer region, repetitive DNA, restriction enzymes.
Introduction
A large portion of eukaryotic genomes is composed of repetitive DNA sequences, that include tandem repeats such as satellites, minisatellites, microsatellites, and interspersed repeats as transposable elements.1,2 In particular, satellite DNA consists of the same sequence, ranging in length from a few base pairs (bp) to thousands of bp, repeated thousands or millions of times in tandem.3,4 Repetitive DNA makes up the predominant part of heterochromatin and is located mainly in pericentromeric and/or telomeric chromosome regions.3,5 This fraction of the genome seems to escape the selective pressure actings on the non-repetitive segments, representing good evolutionary marker in studies of species evolution, chromosome structure and function, and in the detection of chromosomal rearrangements, supernumerary and sex chromosomes.6–8 The molecular organization and chromosome location of repetitive DNAs have been analyzed in a large number of fish species.9–13 These studies have demonstrated the enormous potential that the investigation of repetitive DNAs offers in extending our knowledge of karyotype differentiation in fish.
Muraenidae is a species-rich family (185 species) of tropical and subtropical fish, distributed worldwide and especially common on coral reefs.14 The current classification is based mainly on morphological and anatomical characteristics, but the taxonomy remains incomplete; in particular, recent molecular phylogenetic and cytogenetic data do not support clear separation between the Muraena and Gymnothorax genera.14–18
Cytogenetically, only approximately 7% of the Muraenidae species has been studied, and for most of them, only karyotype morphology and heterochromatin distribution are known. They show a conserved chromosome number, genome size and AT-content, with the highest values among Anguilliformes.19–21 Chromosome numerical similarity, with a 42 diploid number reported for all species, is accompanied by great karyotype variation [fundamental numbers (FN) = 42 to 84]; in this group, pericentric inversions may have played an important role in karyotype evolution.22,23 The pattern of heterochromatin distribution is known for eight Muraenidae species; these few available data indicate the presence of centromeric heterochromatic blocks in almost all species; furthermore, large bands in pericentromeric and/or interstitial position are present in some species.20,22,24 The nucleolus organizer regions (NOR) were localized by silver staining on one chromosome pair in all species studied; furthermore, fluorescence in situ hybridization (FISH) mapping of major ribosomal genes and telomeric sequences was carried out in M. helena and G. tile.18,25,26 In addition, two repetitive DNA fractions from the M. helena genome, were isolated, physically mapped and compared to G. unicolor.25,27 Previous cytogenetic studies characterized the karyotype of the Mediterranean moray eel Gymnothorax unicolor (Delaroche, 1809) by heterochromatic (C-, restriction enzyme- and CMA3-) and replication- banding and Ag-staining.20,23,28 Cytogenetic comparison with the other Mediterranean species Muraena helena and with the congeneric species G. tile pointed out many chromosomal banding similarities, as well as the occurrence of pericentric inversions and changes in heterochromatin amount.18
In the present study, we carried out the isolation, molecular characterization and chromosomal mapping of two repeated DNA fractions in the G. unicolor genome and localized the major ribosomal genes and telomeric sequences using FISH. Furthermore, we investigated the presence of these DNA fractions in the M. helena genome.
Materials and Methods
Chromosomal and genomic DNA preparation
Six individuals of both sexes (four males and two females) of G. unicolor were captured along the southwestern Sardinian coast and raised in an aquarium. Blood was collected from the dorsal artery of individuals using a heparinized syringe, and blood cells were used for all experiments. Sex was determined by histological analysis of the gonads of each individual. Metaphase chromosomes were obtained by lymphocyte cultures and chromosome morphology was classified according to arm ratios.28,29 Genomic DNA was obtained using standard phenol-chloroform procedure.30 For comparative purposes, M. helena metaphase chromosomes and genomic DNA were also used in the same experiments.
Characterization of genomic DNA: Southern hybridization, cloning, sequencing and sequence analysis
A search for repetitive DNAs was conducted using restriction enzyme digestion of the genomic DNA of G. unicolor with different restriction endonucleases (MboI, DdeI, HaeIII and AluI). The endonucleases MboI and DdeI revealed conspicuous bands of about 5 kb and 350 bp, respectively. Briefly, approximately 7 µg of genomic DNA was digested with 14 U of MboI or DdeI at 37°C for 16 h and subsequently analyzed by gel-electrophoresis using 1% agarose gels containing ethidium bromide (0.5 µg/mL) and TAE buffer (80.04 M Tris-acetate, 0.002 M EDTA) at 25 V for 16 h. The genomic organization of the isolated repetitive fragment was determined using Southern blot hybridization. Genomic DNA was digested with different restriction enzymes (MboI and DdeI), separated by electrophoresis in 1.5% agarose gel, and the DNA fragments were transferred onto a Hybond N+ nylon membrane (Amersham Biosciences, Uppsala, Sweden) by capillarity. The MboI purified band (called GuMboI family) was used as a probe and hybridized under conditions of high stringency, using the non-radioactive ECL method (Enhanced Chemioluminescent Kit, Amersham Bioscences) while the DdeI 350-bp isolated band (called GuDdeI family) was cloned in the One Shot Top 10 competent E. coli (Invitrogen Life Technologies, Carlsbad, CA, USA) using the Ready To Go pUC18 BAP+ligase kit (Amersham Biotech, Inc., Uppsala, Sweden) and following the manufacturer's recommendations. Five positive clones of the GuDdeI family were sequenced using the automated Alpha express DNA sequencer (Pharmacia, Uppsala, Sweden) and the sequences aligned in CLUSTAL W using the default parameters.31 MEGA version 4 was used to determine nucleotide composition and the genetic distance between sequences calculated using the Kimura 2-parameter (K2P) method.32 Moreover, a GenBank search was performed in order to compare DdeI satDNA with other sat DNAs in the database. One of the clones (called GuDdeI 313) was employed as a probe in filter experiments, following the same procedure described for GuMboI DNA hybridization.
Fluorescence in situ hybridization procedure and karyotype analysis
FISH procedure was effected using the same probes employed in the filter hybridization (GuMboI family and GuDdeI 313 clone), the GuDdeI family and Xenopus laevis 45S rDNA. The probes were labeled with biotin-16-dUTP (Roche Applied Science, Indianapolis, IN, USA) by nick-translation, using the Nick Translation kit (Roche Applied Science) and following the manufacturer's instructions. Hybridization was performed overnight at 37°C in 50% formamide/2xSSC. Post-hybridization washes were carried out at 37°C in 50% formamide/ 2xSSC for 15 min followed by 15 min washes in 2xSSC at 37°C. Detection was carried out using fluorescein isothiocyanate-conjugated avidin (Vector Laboratories, Inc., Burlingame, CA, USA), with two amplification steps using anti-avidin biotin coniugated (Vector Laboratories, Inc). Chromosomes were counterstained using propidium iodide (Sigma Aldrich, St. Louis, MO, USA) (1 µg/mL) and DAPI (4',6-diamidi-no-2-phenylindole) (Sigma Aldrich) (0.5 µg/mL). The same procedure was effected for all probes. Telomeric sequences (TTAGGG) were mapped by two-hour-FISH using a telomere PNA (peptide nucleic acid) probe conjugated with FITC (Telomere PNA FISH kit/FITC, DakoCytomation, Glostrup, Denmark), following the manufacturer's instructions.
More than twenty metaphase plates were analyzed for each probe using a Zeiss Imager M1 fluorescence microscope; images were captured with a Hamamatsu digital camera C8484 and processed using a karyotyping-and FISH-dedicated image analysis system (Cromowin Plus, TESI Imaging, Pianiga (VE), Italy).
Results
Characterization of MboI- and DdeI- restricted DNA fractions, Southern hybridization and sequencing
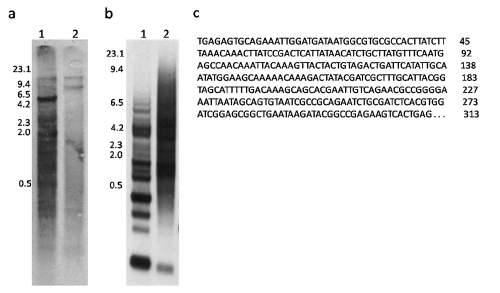
Genomic DNA of G. unicolor digested with either MboI or DdeI showed a high mw band of about 21 Kb (Figure 1). Moreover, MboI enzyme produced a band of about 5 Kb (GuMboI) and a smear composed of DNA fragments of about 4 kb to a few hundred base pairs (Figure 1). Cleavage of genomic DNA of the same species with DdeI produced an electrophoretic smear, where a band of about 350 bp (GuDdeI) was present (Figure 1). When GuMboI DNA was used as a probe in G. unicolor MboI- digested DNA, a band of about 5 Kb and other fainter bands of lower mw, ranging from 5 Kb to about 200 bp, were observed (Figure 2a). The Southern hybridization of GuMboI probe on M. helena genomic DNA digested with the same enzyme produced a band of about 10 Kb and other bands of lower mw (Figure 2a).
Figure 1.
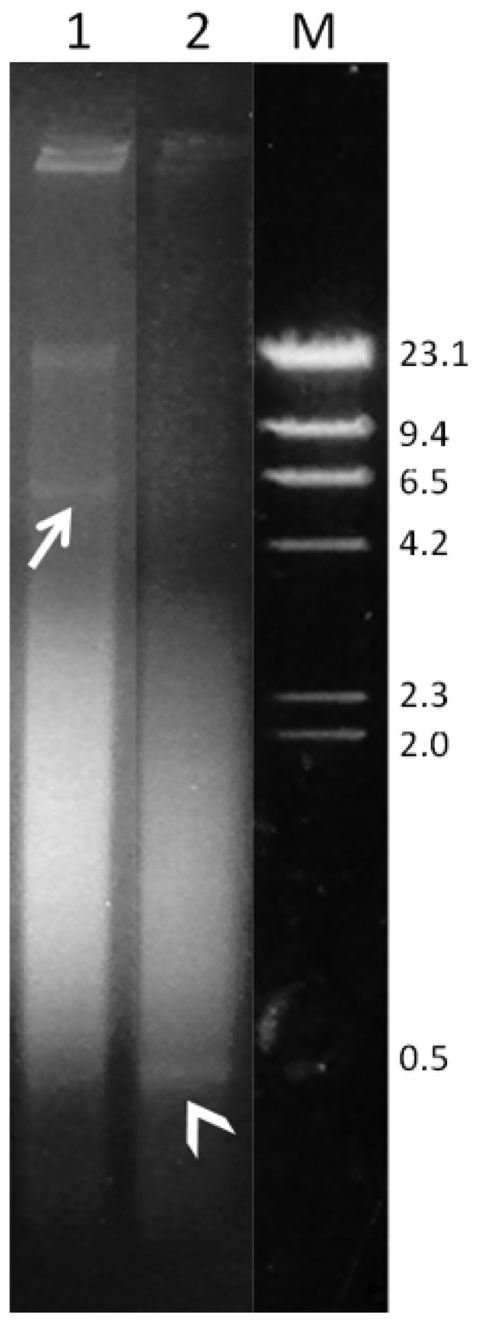
Agarose gel electrophoresis of Gymnothorax unicolor genomic DNA digested with restriction endonucleases MboI (lane 1) and DdeI (lane 2). The arrow indicates the 5kb band detected by MboI, and the arrowhead indicates the 350-bp band detected by DdeI. M, phage lambda DNA cleaved with HindIII, used as a molecular size marker.
Figure 2.
Southern blot of the genomic DNA samples of Gymnothorax unicolor (1) and Muraena helena (2) after digestion with the restriction enzyme MboI and hybridization to the GuMboI repetitive probe (a) and after digestion with the restriction enzyme DdeI and hybridization to the GuDdeI 313 repetitive probe (b). In (a), note the 5 Kb band in G. unicolor, and the 10 Kb faint band in M. helena. Base sequence of the GuDdeI 313 clone (c), its length correspond to 313 bp.
The cloning and sequencing of the GuDdeI band revealed DNA fragments of 248–340 bp. Analysis of the sequenced segment of the clones showed that the sequences were very different, and the GuDdeI 313 clone was selected for studies. Southern hybridization using GuDdeI 313 probe on G. unicolor genomic DNA cleaved with DdeI showed a ladder of bands, whose mw ranged from about 6.5 Kb to about 400 bp; furthermore, a large band of 313 bp (i.e. the GuDdeI 313 band) was observed (Figure 2b). These results indicate a tandem arrangement of repeating units typical of satellite DNA. When this probe was used on M. helena genomic DNA cleaved with DdeI, a series of bands ranging from about 6.5 Kb to about 600 bp and a band of about 310 bp was observed, the latter being thinner than that observed in G. unicolor (Figure 2b). The complete nucleotide sequence of the GuDdeI 313 clone is shown in Figure 2c; it was 313 bp long and composed of 58,1% AT bases. No similarity between the GuDdeI 313 and any sequence deposited in the nucleotide sequence databases was detected.
Cytogenetic mapping of repetitive DNA sequences
FISH using the GuMboI probe produced positive signals in the centromeric region of most chromosomes of G. unicolor, including a large acrocentric pair (Figure 3a), the latter region being DAPI negative (Figure 3c). 45S rDNA sites were located in the pericentromeric region of a single acrocentric pair (Figure 3b) and this region was also dull after DAPI staining (Figure 3d). The hybridization of GuDdeI 313 probe produced bright positive signals in the centromeric region of some G. unicolor chromosomes, mostly acrocentric ones (Figure 3e), while GuDdeI probe showed positive signals in all centromeres (Figure 3f). None of the probes (GuMboI, GuDdeI and GuDdeI 313) produced any positive signal on M. helena chromosomes (data not shown). FISH with the telomeric probe (TTAGGG)n revealed hybridization signals on each telomere of all chromosomes, and interstitial telomeric sites (ITS) were not detected (Figure 3g).
Figure 3.
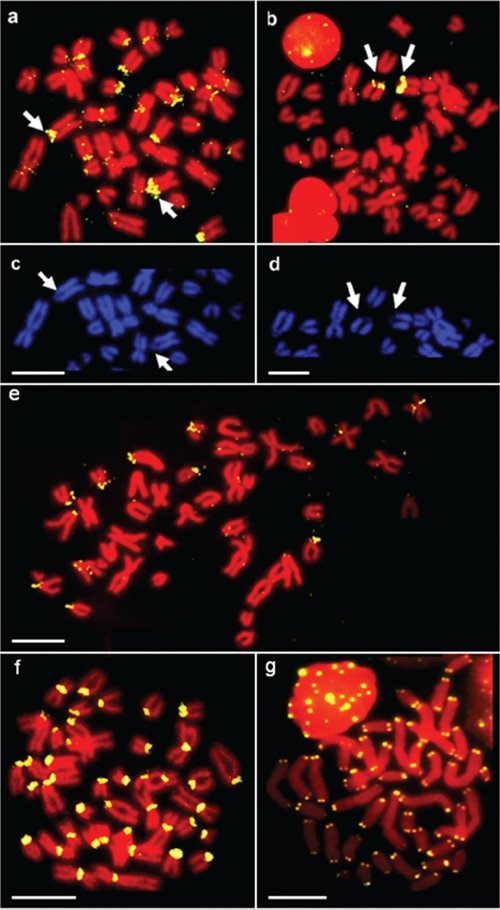
Fluorescence in situ hibridization of Gymnothorax unicolor metaphase chromosomes hybridized with different repetitive DNA sequences (yellow signals) and counter-stained with propidium iodide. They include GuMboI (a), 45S rDNA sites (b), the sequential DAPI counterstaining of the same metaphases (c) and (d), GuDdeI (e), GuDdeI 313 (f) and (TTAGGG)n sequences (g). The arrows indicate the DAPI-region in the chromosome no.9 (c,d), which corresponds to the NOR site (a,b). Scale bars: 10 µm.
Discussion
The digestion of G. unicolor DNA with the enzymes MboI and DdeI showed the presence of distinct bands in agarose gels, as observed in other fish species after genomic DNA digestion with restriction endonucleases.33–36 Analysis of membrane immobilized genomic DNA of G. unicolor hybridized to the GuMboI family and to the GuDdeI 313 clone indicates that they correspond to highly repetitive elements, predominantly clustered in the genome. The comparison of satellite GuDdeI DNA sequences with nucleic acid sequences available on databases revealed no similarities to any known DNA sequence. Comparative Southern hybridization revealed that these repeated DNAs are present in both G. unicolor and M. helena, with species-specific features. Among Muraenidae, repetitive DNA fractions were characterized only in M. helena, and in particular two MboI-restricted DNA fractions, called B2 and B3; Southern blotting comparative analysis on the G. unicolor genome pointed out the presence of these DNA fractions in both species, although showing characteristic features in each species.25,27
The two isolated repetitive elements are clustered in the centromeric heterochromatin of many chromosomes of the complement, previously identified by C- banding.20 The repetitive sequences are not randomly distributed in the genome, suggesting a pattern of compartmentalization on chromosomes. In particular, the GuMboI sequence was localized in two different types of C-positive areas: in many centromeres and the pericentromeric region of acrocentric pair no. 9, co-localized with the Ag-NOR localization previously obtained.28 FISH mapping of the major ribosomal gene family confirms the presence of only one NOR-bearing pair in the G. unicolor genome; this situation is also found in all the Muraenidae studied and is the most frequent condition among Anguilliformes.18,22,37 The GuMboI fraction might be a heterogeneous repetitive DNA associated with ribosomal sequences; this hypothesis could account for the lack of signals in cross-species GuMboI FISH on M. helena chromosomes. A NOR-related DNA fraction was also obtained in M. helena, and the association between ribosomal cistrons and repetitive DNA is commonly found in eukaryotes.25 The G. unicolor GuDdeI DNA fraction was localized in the centromeric region of all G. unicolor chromosomes, while the GuDdeI DNA 313 probe hybridized in only some centromeres, indicating that this satellite DNA family is composed of several types of centromeric repetitive DNAs, also confirmed by the great differences in nucleotide sequences found among clones. Repetitive DNAs are frequently localized in centromeric regions of chromosomes in both animals and plants and represent the main component of functional centromeres.38–42 Although their real function is still being debated, satellite DNA is required for centromere composition and organization.43 Centromeric location of repetitive DNAs is also the most frequent situation among fish, and the distribution pattern in all centromeres had already been found in M. helena and other fish species, like some species of the genus Sparus and some species of Cichlidae.25,44–48 In the Erythrinid fish, Hoplias malabaricus, 5SHindIII is a satellite DNA family specific to the H. malabaricus genome, and is located only in the centromeric region of some chromosome pairs. This satellite family has spread to the centromeric region of several chromosomes and has been favored during evolution due to a possible role of the repetitive sequences of the centromeric region in centromere structure and function.10,11,34 In order to establish the degree of conservation of the GuMboI and GuDdeI DNA fractions, FISH was performed on M. helena chromosomes; the lack of positive signals suggests that these sequences are species-specific and might have originated after the divergence of G. unicolor from the other Muraenidae species. These results agree with the heterochromatin heterogeneity detected in the two species by in situ restriction enzyme banding.20
Telomeric (TTAGGG)n sequences are present in the telomeres of the chromosomes of all vertebrates, and their study allows one to establish the presence of chromosomal rearrangements, such as Robertsonian fusions or inversions, involved in the evolution of the chromosomes.49 FISH with the telomeric probe (TTAGGG)n revealed hybridization signals on each telomere of all chromosomes of G. unicolor, and interstitial telomeric sites (ITS) were not detected, which could indicate that Robertsonian fusions or chromosomal translocations were probably not involved in the karyotypic differentiation of this species. The absence of ITS had already been reported for the other Muraenidae species M. helena and G. tile.18,26 On the other hand, two Anguilliform species studied (Anguilla anguilla and A. rostrata) presented telomeric sequences colocalized with the NOR region.26
In conclusion, the results obtained provide information useful in comparative genomics at the chromosomal level and add to the cytogenetic knowledge regarding this fish family, contributing to the molecular and cytogenetical characterization of G. unicolor for studying karyotype evolution in Muraenidae. A more extensive cytogenetic survey is needed to allow evaluation of Muraenidae cytotaxonomy.
Acknowledgements:
the authors wish to thank the anonymous referees for helpful suggestions and critical review of the manuscript and Mrs. Mary Ann Groeneweg for checking the English style.
References
- 1.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–7. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- 2.Biémont C, Vieira C. Junk DNA as an evolutionary force. Nature. 2006;443:521–4. doi: 10.1038/443521a. [DOI] [PubMed] [Google Scholar]
- 3.Sumner AT. Repetitive DNA – sequences with a function, or just junk? In: Sumner AT, editor. Chromosomes: organization and function. Blackwell Science Ltd; Malden, MA, USA: 2003. pp. 25–31. [Google Scholar]
- 4.Palomeque T, Lorite P. Satellite DNA in insects: a review. Heredity. 2008;100:564–73. doi: 10.1038/hdy.2008.24. [DOI] [PubMed] [Google Scholar]
- 5.Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371:215–20. doi: 10.1038/371215a0. [DOI] [PubMed] [Google Scholar]
- 6.Archidiacono N, Antonacci R, Marzella R, Finelli P, Lonoce A, Rocchi M. Comparative mapping of human alphoid sequences in great apes using fluorescence in situ hybridization. Genomics. 1995;25:477–84. doi: 10.1016/0888-7543(95)80048-q. [DOI] [PubMed] [Google Scholar]
- 7.Martins C. Chromosomes and repetitive DNAs: a contribution to the knowledge of the fish genome. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish Cytogenetics. Science Publishers; Enfield, NH, USA: 2006. pp. 421–52. [Google Scholar]
- 8.Gosalvez J, Crespo F, Vega-Pla JL, López-Fernández C, Cortés-Gutiérrez EI, Mezzanotte R. Shared Y chromosome repetitive DNA sequences in stallion and donkey as visualized using whole-genomic comparative hybridization. Eur J Histochem. 2010;54 doi: 10.4081/ejh.2010.e2.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volff JN, Korting C, Sweeney K, Schartl M. The Non-LTR retrotransposon Rex3 from the fish Xiphophorus is widespread among teleosts. Mol Biol Evol. 1999;16:1427–38. doi: 10.1093/oxfordjournals.molbev.a026055. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi MB, Martins C, Bertollo LAC. Comparative chromosome mapping of repetitive sequences. Implications for genomic evolution in the fish, Hoplias malabaricus. BMC Genetics. 2009;10:34. doi: 10.1186/1471-2156-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cioffi MB, Martins C, Bertollo LAC. Chromosome spreading of associated transposable elements and ribosomal DNA in the fish Erythrinus erythrinus. Implications for genome change and karyoevolution in fish. BMC Evol Biol. 2010;10:271. doi: 10.1186/1471-2148-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicari MR, Artoni RF, Moreira-Filho, Bertollo LAC. Colocalization of repetitive DNAs and silencing of major rRNA genes. A case report of the fish Astyanax janeiroensis. Cytogenet Genome Res. 2008;122:67–72. doi: 10.1159/000151318. [DOI] [PubMed] [Google Scholar]
- 13.Pazza R, Frehner Kavalko K, Bertollo LA. Chromosome polymorphism in Astianax fasciatus (Teleostei, Characidae). 2-- Chromosomal location of a satellite DNA. Cytogenet Genome Res. 2008;122:61–6. doi: 10.1159/000151317. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JS. Order Anguilliformes. In: Nelson JS, editor. Fishes of the world. John Wiley and Sons, Inc.; New york, NY, USA: 2006. pp. 114–124. [Google Scholar]
- 15.Böhlke EB, Smith DG. Type catalogue of Indo-Pacific Muraenidae. P Acad Nat Sci Phila. 2002;152:89–172. [Google Scholar]
- 16.Takai A, Ojima Y. Karyotype studies of five species of Anguilliformes (Pisces) P Jpn Acad B-Phys. 1985;61:253–6. [Google Scholar]
- 17.Jiménez S, Schönhuth S, Lozano J, González A, Sevilla RG, Diez A, et al. Morphological, ecological, and molecular analyses separate Muraena augusti from Muraena helena as a valid species. Copeia. 2007;2007:101–13. [Google Scholar]
- 18.Coluccia E, Deiana AM, Libertini A, Salvadori S. Cytogenetic characterization of the moray eel Gymnothorax tile and chromosomal banding comparison in Muraenidae (Anguilliformes) Mar Biol Res. 2010;6:106–11. [Google Scholar]
- 19.Ronchetti E, Salvadori S, Deiana AM. Genome size and AT-content in Anguilliformes. Eur J Histochem. 1995;39:259–64. [PubMed] [Google Scholar]
- 20.Salvadori S, Deiana AM, Coluccia E, Cannas R, Cau A, Milia A. Heterochromatin distribution and structure in Gymnothorax unicolor (Anguilliformes, Muraenidae) Ital J Zool. 1997;64:125–29. [Google Scholar]
- 21.Gregory TR. Animal Genome Size Database, Release 2.0. 2008 Available from: http://www.genomesize.com.
- 22.Vasconcelos AJM, Molina WF. Cytogenetical studies in five Atlantic Anguilliformes fishes. Genet Mol Biol. 2009;32:83–90. doi: 10.1590/S1415-47572009005000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvadori S, Coluccia E, Cannas R, Cau A, Deiana AM. Replication banding in Mediterranean moray eels: chromosomal characterization and comparison. Genetica. 2003;119:253–8. doi: 10.1023/b:gene.0000003649.64247.5b. [DOI] [PubMed] [Google Scholar]
- 24.Porto-Foresti F, Oliveira C, Foresti F. First chromosome characterization in the Neotropical eel, Gymnothorax ocellatus (Pisces, Muraenidae) Cytologia. 2005;70:283–6. [Google Scholar]
- 25.Pichiri G, Nieddu M, Mezzanotte R, Coni P, Salvadori S, Deiana M, et al. The molecular characterization of the genome of M. helena L. I. Isolation and hybridisation of two MboI-restricted DNA fractions. Genome. 1995;38:809–13. doi: 10.1139/g95-103. [DOI] [PubMed] [Google Scholar]
- 26.Salvadori S, Deiana AM, Coluccia E, Floridia G, Rossi E, Zuffardi O. Colocalization of (TTAGGG)n telomeric sequences and ribosomal genes in Atlantic eels. Chromosome Res. 1995;3:54–58. doi: 10.1007/BF00711162. [DOI] [PubMed] [Google Scholar]
- 27.Pichiri G, Coni P, Deiana AM, Nieddu M, Mezzanotte R. On the variability of MboI repeated sequences and 5S rDNA in Muraena helena and Gymnothorax unicolor (Anguilliformes, Muraenidae) Chromosome Res. 2000;8:443–5. doi: 10.1023/a:1009271121778. [DOI] [PubMed] [Google Scholar]
- 28.Deiana AM, Salvadori S, Cau A. The characterization of somatic chromosomes of Gymnothorax unicolor (Delaroche, 1809) by C-banding and NOR staining (Osteichthyes, Anguilliformes) Genetica. 1990;81:17–20. [Google Scholar]
- 29.Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:201–20. [Google Scholar]
- 30.Sambrook J, Russell DW. Spring Harb. 3rd ed. Lab. Press, Cold; New York, NY, USA: 2001. Molecular Cloning. A Laboratory Manual. [Google Scholar]
- 31.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalities and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 33.Koehler MR, Haaf T, Guttenbach M, Schartl M, Schmid M. Cytogenetics of the genus Leporinus (Pisces, Anostomidae). II. Molecular cytogenetics, organization and evolutionary conservation of a chromosome-specific satellite DNA from Leporinus obtusidens. Chromosome Res. 1997;5:325–31. doi: 10.1023/B:CHRO.0000038763.52875.48. [DOI] [PubMed] [Google Scholar]
- 34.Martins C, Ferreira IA, Oliveira C, Foresti F, Galetti PM., Jr A tandemly repetitive centromeric DNA sequence of the fish Hoplias malabaricus is derived from 5S rDNA. Genetica. 2006;127:133–41. doi: 10.1007/s10709-005-2674-y. [DOI] [PubMed] [Google Scholar]
- 35.de Jesus CM, Galetti PM, Valentini SR, Moreira-Filho O. Molecular characterization and chromosomal localization of two families of satellite DNA in Prochilodus lineatus (Pisces, Prochilodontidae), a species with B chromosomes. Genetica. 2003;118:25–32. doi: 10.1023/a:1022986816648. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho de Azevedo MF, Oliveira C, Martins C, Pinto Wasko A, Foresti F. Isolation and characterization of a satellite DNA family in Achirus lineatus (Teleostei: Pleuronectiformes: Achiridae) Genetica. 2005;125:205–10. doi: 10.1007/s10709-005-8419-0. [DOI] [PubMed] [Google Scholar]
- 37.Deiana AM, Coluccia E, Cannas R, Pesci P, Fonnesu A, Salvadori S. Colocalization of the ribosomal gene families in Conger conger (Anguilliformes, Congridae) Ital J Zool. 2006;73:1–5. [Google Scholar]
- 38.Sun X, Wahlstrom J, Karpen G. Molecular structure of a functional Drosophila centromere. Cell. 1997;91:1007–19. doi: 10.1016/s0092-8674(00)80491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schueler MG, Higgins AW, Rudd MK, Gustashaw K, Willard HF. Genomic and genetic definition of a functional human centromere. Science. 2001;294:109–15. doi: 10.1126/science.1065042. [DOI] [PubMed] [Google Scholar]
- 40.Nagaki K, Song J, Stupar RM, Parokonny AS, Yuan Q, Ouyang S, et al. Molecular and cytological analyses of large tracks of centromeric DNA reveal the structure and evolutionary dynamics of maize centromeres. Genetics. 2003;163:759–70. doi: 10.1093/genetics/163.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Wing RA, Bennetzen JL, Jackson SA. Plant centromere organization: a dynamic structure with conserved functions. Trends Genet. 2007;23:134–9. doi: 10.1016/j.tig.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Ugarkovic D, Plohl M. Variation in satellite DNA profiles--causes and effects. EMBO J. 2002;21:5955–9. doi: 10.1093/emboj/cdf612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293:1098–102. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- 44.Martins C, Cabral-de-Mello DC, Targino Valente GT, Mazzucchelli J, Oliveira SG. Cytogenetic mapping and contribution to the knowledge of animal genomes. In: Urbano KV, editor. Advances in genetics research. Vol. 4. Nova Science Publishers, Inc.; New York, NY, USA: 2010. pp. 1–81. [Google Scholar]
- 45.Garrido-Ramos MA, Jamilena M, Lozano R, Ruiz Rejón C, Ruiz Rejón M. Cloning and characterization of a fish centromeric satellite DNA. Cytogenet Cell Genet. 1994;65:233–7. doi: 10.1159/000133637. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira C, Wright J. Molecular cytogenetic analysis of heterochromatin in the chromosomes of tilapia, Oreochromis niloticus (Teleostei: Cichlidae) Chromosome Res. 1998;6:205–11. doi: 10.1023/a:1009211701829. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira IA, Martins C. Physical chromosome mapping of repetitive DNA sequences in Nile tilapia Oreochromis niloticus: evidences for a differential distribution of repetitive elements in the sex chromosomes. Micron. 2008;39:411–8. doi: 10.1016/j.micron.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Mazzucchelli J, Martins C. Genomic organization of repetitive DNAs in the cichlid fish Astronotus ocellatus. Genetica. 2009;136:461–9. doi: 10.1007/s10709-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 49.Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA. 1989;89:7049–53. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]