Abstract
Hypoxia affects vascular function and cell metabolism, survival, growth, and motility; these processes are partially regulated by prostanoids. We analyzed the effect of hypoxia and inflammation on key enzymes involved in prostanoid biosynthesis in human vascular cells. In human vascular smooth muscle cells (VSMC), hypoxia and interleukin (IL)-1β synergistically increased prostaglandin (PG)I2 but not PGE2 release, thereby increasing the PGI2/PGE2 ratio. Concomitantly, these stimuli upregulated cyclooxygenase-2 (COX-2) expression (mRNA and protein) and COX activity. Interestingly, hypoxia enhanced PGI-synthase (PGIS) expression and activity in VSMC and human endothelial cells. Hypoxia did not significantly modify the inducible microsomal-PGE-synthase (mPGES)-1. Hypoxia-inducible factor (HIF)-1α-silencing abrogated hypoxia-induced PGIS upregulation. PGIS transcriptional activity was enhanced by hypoxia; however, the minimal PGIS promoter responsive to hypoxia (-131 bp) did not contain any putative hypoxia response element (HRE), suggesting that HIF-1 does not directly drive PGIS transcription. Serial deletion and site-directed mutagenesis studies suggested several transcription factors participate cooperatively. Plasma levels of the stable metabolite of PGI2 and PGIS expression in several tissues were also upregulated in mice exposed to hypoxia. These data suggest that PGIS upregulation is part of the adaptive response of vascular cells to hypoxic stress and could play a role in counteracting the deleterious effect of inflammatory stimuli.
Keywords: cyclooxygenase-pathway , prostaglandin I-synthase , prostacyclin-synthase , prostacyclin , smooth muscle cells , endothelial cells
Hypoxia is the underlying condition in several human pathologies, including cardiovascular diseases, chronic renal disease, hematological and pulmonary disorders, and cancer (1). It triggers adaptive mechanisms aimed to increase local oxygen concentration to insure cell viability, and it is critical driving tissue remodeling (2, 3). In fact, hypoxia regulates a large number of genes involved in widespread biological activities, including regulation of vascular function, cell metabolism, cell survival, and cell growth and motility. The transcriptional response to hypoxia is mediated by a network of transcription factors (4–9), but particularly by the hypoxia-inducible factor (HIF). HIF-1, the prototype of this family, is a heterodimeric basic helix-loop-helix transcription factor composed of HIF-1β (constitutive subunit) and HIF-1α (oxygen-sensitive subunit) (10). In recent years, a growing number of genes potentially regulated by hypoxia have been identified (11). Nevertheless, the global picture of genes and proteins regulated by hypoxia in vascular cells is not completely understood.
Increasing evidence emphasizes the role of a prostanoid biosynthetic pathway in hypoxia-mediated biological effects. In several cell types, hypoxia upregulates the inducible isoform of prostaglandin (PG) endoperoxide H synthase (cyclooxygenase-2, COX-2) (4), one of the two isoenzymes that catalyzes the conversion of arachidonic acid to PGH2, the first committed step in the biosynthesis of a wide range of prostanoids. COX-2, rarely expressed in normal tissues, is an immediate/early gene transiently induced in response to hormones, growth factors, and cytokines (12). COX-2 has commonly been associated with pro-inflammatory/pro-atherogenic stages, due to its upregulation at inflammatory sites such as active atherosclerotic lesions. However, COX-2 is constitutively expressed in the endothelium (13, 14), is upregulated by atheroprotective lipoproteins in vascular cells (15–17), and seems to contribute to the innate defensive mechanism of the myocardium (18, 19). Indeed, the pathophysiological meaning of COX-2 induction in a particular setting depends on the activity of downstream enzymes that transform PGH2 into different prostanoids in a cell-type-specific manner (12, 20). PGH2 is transformed into PGI2 (prostacyclin), the main prostanoid synthesized in the mammalian vasculature, by PGI-synthase (PGIS, prostacyclin-synthase). PGI2 counteracts the biological effects of thromboxane (Tx) A2 and exerts a wide spectrum of vasoprotective functions (12). PGH2 is also the intermediate in the biosynthesis of PGE2 by the catalytic activity of PGE-synthases (PGES). PGE2 is a pro-inflammatory molecule that can act through four different receptor subtypes (EP1, EP2, EP3, and EP4), eliciting disparate effects (21). Although three PGES isoenzymes have been characterized so far, two microsomal isoforms (mPGES-1 and mPGES-2) and one cytosolic isoform (cPGES) (22), mPGES-1 seems to be the main isoenzyme involved in PGE2 biosynthesis in human vasculature (23).
Data from experimental and clinical studies suggest that COX-2 is the major source of vascular PGI2 (13, 14). This is in agreement with the increased risk of cardiovascular events, including myocardial infarction and stroke, reported for selective COX-2 inhibitors (20, 24). Nevertheless, COX-2 and mPGES-1 upregulation leads to large amounts of prothrombotic and pro-inflammatory prostanoids (untransformed PGH2 and PGE2) (23, 25, 26). At least in part, the balance between PGI2 versus PGE2 depends on the PGIS/PGES activity ratio (23, 26). Therefore, the induction of COX-2 could have a dissimilar impact on the prostanoid biosynthetic profile depending on the expression and activity of the COX-downstream enzymes present in vascular cells (23, 25, 26). As the effect of hypoxia on the final synthases of the COX-pathway in vascular cells is as yet unknown, we aimed to investigate the effect of hypoxia on the prostanoid biosynthesis and mPGES-1 and PGIS expression in resting vascular cells and under exposure to a strong COX-2 inducer such as interleukin (IL)-1β.
MATERIAL AND METHODS
Cell culture and treatment
Human VSMC were isolated from aorta of multi-organ donors by an explant procedure as previously described (26). Cells were maintained in medium supplemented with 1% fetal bovine serum (FBS) for 24 h prior to hypoxia treatment and/or to the addition of 50 U/ml of human recombinant IL-1β (Roche Applied Science). VSMC were maintained in these conditions for the indicated period of time until prostanoid, enzyme activity, or gene expression was determined. Cells were used between passages 3 and 7. Endothelial cells from human umbilical veins (HUVEC) were isolated and cultured as previously described (23). Cells in the first passage were cultured in 6-well plates in M199 medium containing 20 % fetal bovine serum (without heparin and EC growth factor) for 48 h prior to hypoxia treatment and/or the addition of IL-1β (10 U/ml). Treatments were performed in M199 supplemented with 1% FCS for the indicated period of time (shown in “Results” section) until prostanoid, enzyme activity, or enzyme expression (see below) was determined. Mouse lung endothelial cells (MLEC) were isolated from C57BL/6 mice by collagenase digestion, then selected by ICAM-2-coated magnetic beads and cultured as previously described (27). Cells were exposed to hypoxia (1% O2, 5% CO2, balanced with N2) in a Forma Series II hypoxic incubator (model 3141; Thermo Electron Corp.). To determine whether hypoxia affected PGIS transcription, cells were preincubated (30 min) with actinomycin D (4 µmol/l; Sigma) before hypoxia exposure. To assess the effect of hypoxia on PGIS mRNA half-life, cells were maintained under normoxic conditions or exposed to hypoxia for 24 h. Actinomycin D was then added to inhibit further transcription. Finally, PGIS mRNA levels were examined at different time points. All the procedures were approved by the Reviewer Institutional Committee on Human Research of the Hospital de la Santa Creu i Sant Pau and conformed to the Declaration of Helsinki.
Prostanoids released in the conditioned medium
Prostanoid levels were analyzed by specific enzyme immunoassays (EIA) following the manufacturer's instructions. 6-oxo-PGF1α (stable hydrolysis product of PGI2) and PGE2 were from GE Healthcare; TxB2 (stable metabolite of TxA2) and PGF2α were from Cayman.
Western blot analysis
Vascular cells were exposed to hypoxia and/or IL-1β for the indicated periods of time, and protein extracts were analyzed by Western blot as described (23). Briefly, cell cultures were washed twice with phosphate buffered saline (PBS) and lysed with lysis buffer (20 mM Tris-HCl, pH 7.4) containing protease inhibitor cocktail (Roche Diagnostics), 1 mM EDTA, and 0.1% Triton X-100. Protein concentration was determined by the Bradford method. Total protein equivalents were resolved by SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore). Membranes were incubated with antibodies against human PGIS (Cayman Chemical), COX-2 (PG27; Oxford Biomedical Research), mPGES-1 (PG15; Oxford Biomedical Research), and HIF-1α (NB100-449; Novus Biologicals). Bound antibody was detected using the appropriate horseradish peroxidase-conjugated antibody (Dako) and a chemiluminescent detection system (Amersham ECL Plus Western Blotting Detection Reagents; GE Healthcare). Results were normalized by β-actin (Sigma) used as a loading control.
COX and PGIS enzymatic activities
Cells were exposed to hypoxia in the presence or absence of IL-1β for the indicated periods of time. COX activity was assayed by incubating cells with 25 μmol/l [1-14C]arachidonic acid ([14C]AA, 55-58 mCi/mmol; GE Healthcare) for 10 min as previously reported (25). COX activity was evaluated as the sum of all HPLC peaks corresponding to eicosanoids formed through the COX pathway. PGIS activity was evaluated as noted previously (25). HPLC analysis of eicosanoids was performed as described (28).
Animal procedures
All procedures in the study were reviewed and approved by the Animal Care and Experimentation Committee of the Cardiovascular Investigation Centre. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male C57BL/6 mice (two months old; n = 16; Charles River) were acclimated to custom-made plexiglas chambers (260 × 120 × 170 mm) for 48 h. Mice were maintained on a 12-h light/12-h dark cycle and housed in groups of four animals, with unlimited access to water and chow (R04, SAFE). To prevent the accumulation of CO2, a CO2 absorber (Medisorb; GE Healthcare) was put inside the cage beyond the reach of mice. At the start of the experiment, mice were placed in the custom-made sealed plexiglas chambers and exposed to continuous hypoxia (Hyp; n = 8) or room air [Control group (Normoxia); n = 8]. Hypoxia exposure was performed by means of a gas delivery system, using a hypoxic gas mixture (10% O2, balanced with N2; Abelló Linde). The level of the inspiratory fraction of oxygen (FiO2) to 10% was decreased within 5 min by flushing the chamber with the hypoxic gas mixture at an appropriate high flow rate. The flow was then lowered to 1 l/min for the duration of the hypoxic exposure. The O2 concentration inside the chamber was monitored using a multi-gas analyzer (Anagas CD 98 Plus; Bacharach). Mice were kept at hypoxia or normoxia for 24 h. They were terminally anesthetized with ketamine (150 mg/kg; Merial Laboratories) and medetomidine (1 mg/kg; Divasa-Farmavic). Blood was immediately collected by heart puncture with a heparinized syringe. Plasma samples were collected from blood centrifuged (2,000 g for 10 min) and kept at –80°C until PGI2 determination (Cayman). Tissues were excised and cut into small pieces that were quickly frozen in liquid nitrogen and stored at −80°C for subsequent RNA extraction. All tissue harvesting was performed within 10 min of taking the mice out of the chambers.
Real-time PCR
Total RNA from vascular cells was isolated by chloroform/isopropanol precipitation, whereas RNA extraction from mouse tissues was performed by RNAeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. The quality and quantity of the RNA was determined using the Agilent's 2100 Bioanalyzer. cDNA was prepared by reverse transcribing 1 µg RNA with High-Capacity cDNA Archive Kit with random hexamers (Applied Biosystems, Foster City, CA) (23). mRNA levels were quantified by real-time PCR using TaqMan™ Gene Expression Assays (Applied Biosystems) for COX-2 (Hs00153133_m1), COX-1 (Hs00377721_m1), mPGES-1 (Hs00610420_m1), and PGIS (human: Hs00168766_m1; mouse: Mm00447271_m1). Levels of EPO were analyzed by real-time-PCR in mouse tissues using SYBR Green (Roche) and primers (Biomers.net) as follows: forward 5′-GATGGGGGTGCCCGAACGTC-3′, and reverse 5′- GTCGCAGATGAGGCGTGGGG-3′. RT-PCR data were analyzed for relative gene expression using the ΔΔCt method. Gene expression data were normalized by β-actin (Hs99999903_m1) or 18S rRNA (4319413E) used as endogenous controls (as stated).
siRNA transfection and knockdown experiments
Silencer predesigned siRNA (Ambion) targeting HIF-1α (ID# 42840) or Silencer™ Negative Control #1 siRNA (siControl; ID# 4390843) were used in knockdown experiments. HUVEC and VSMC were transfected with siRNAs using Nucleofector™ (Amaxa) and the corresponding kits according to the manufacturer's recommendations (9). Briefly, electroporation was carried out with 1 × 106 cells and 3 µg of siRNA with the U001 or the A33 program (for HUVEC and VSMC, respectively). After electroporation, cells were resuspended in 500 µl of prewarmed cell culture medium and seeded in complete medium for 24 h. Finally, cells were arrested and exposed to hypoxia. Gene knockdown was verified by real time-PCR and/or Western-blot as indicated.
Constructs of PGIS promoter
Deletions of human PGIS promoter were generated by PCR from the plasmid pGL3/PGIS-1963 as previously described (29). The putative AP2 site and the CACCC box present in PGIS promoter at positions -146 and -137, respectively, were mutated using the QuickChange II Site-directed Mutagenesis Kit (Stratagene Agilent Technologies) according to the manufacturer's instructions (9). The primers used were: forward AP-2 mut (5′-TCGGAGGGCCCCATaaC CCACCCCCCC-3′ and reverse (5′-GGGGGGGTGGGttATGGG GCCCTCCGA-3′; and forward CACCC box mut (5′-GAGGGCCCCATCCCC CAaaC CCCCCACC-3′) and reverse (5′-GGTGGGGG GttTG GGGGATGGGGCCCTC-3′) (putative sites are underlined, and changes are indicated in lowercase letters). Mutation was confirmed by DNA sequencing using the ABIPRISM Big Dye Terminator cycle sequencing kit v3.1 (Applied Biosystems).
Transient transfection assays
Vascular cells were transfected with luciferase reporter plasmids using Lipofectin™ Reagent (Invitrogen) (30). Briefly, transient transfections were performed in subclonfluent cells seeded in 6-well plates using 1 μg/well of the luciferase reporter plasmid, 0.3 μg/well of pSVβ-gal (Promega) as an internal control, and 3 μl of Lipofectin™. The complexes DNA/liposome were added to the cells for 6 h. Cells were washed once with arresting medium containing 1% FBS, without heparin or endothelial growth factor. They were arrested overnight and then exposed to hypoxia. Luciferase activity was measured in cell lysates using the Luciferase Assay Kit (Promega) and a luminometer (Orion I; Berthold Detection Systems) according to the manufacturer's instructions. Results were normalized by β-galactosidase activity using the Enzyme Assay System™ (Promega).
PGIS nitration
Nitration of PGIS in cell extracts was determined by immunoprecipitation and Western blot by a modification of the previously described method (31). HUVEC were cultured under normoxia or hypoxia in presence or absence of IL-1β for 48 h. Briefly, cells were washed, harvested, and homogenized in lysis buffer: 1 mM PMSF, 5 mM EDTA, 150 mM NaCl, 0.5% (v/v) Nonidet P40, 1× protease inhibitor cocktail set I (Calbiochem), and 50 mM Tris/HCl, pH 8.0. Solubilized extracts were sonicated twice for 30 s with a Bioruptor™ UCD-200 sonicator (Diagenode) set on medium power. Extracts were then centrifuged at 4°C for 5 min to remove cellular debris. Protein concentration was estimated by the BCA method, and an aliquot was saved as input protein. Protein extracts (150 μg) were incubated with 3 μg of a PGIS antibody (Cayman Chemical) for 18 h at 4°C. Specific immune complexes were incubated with 50 μl of Protein A/G PLUS-Agarose at 4°C for 18 h. After washing with lysis buffer, immune complexes were precipitated by centrifugation (10000 g, 3 min). Supernatants were resuspended in Laemmli sample buffer, boiled, resolved by 7.5 % SDS-PAGE, and blotted onto polyvinylidene difluoride membranes. Membranes were incubated with an anti-nitrotyrosine antibody (clone 1A6; Millipore). Bound antibody was detected using the Clean-Blot IP Detection Reagent (HRP) and the SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific) following the manufacturer's recommendations.
Statistical analysis
Sigma-Stat software was used for statistical analysis. Statistical significance between more than two groups was assessed using one-way ANOVA and the Student-Newman-Keuls test; Student's t-test was used to compare two groups. P < 0.05 was considered significant.
RESULTS
Hypoxia increases IL-1beta-induced release of PGI2 in VSMC
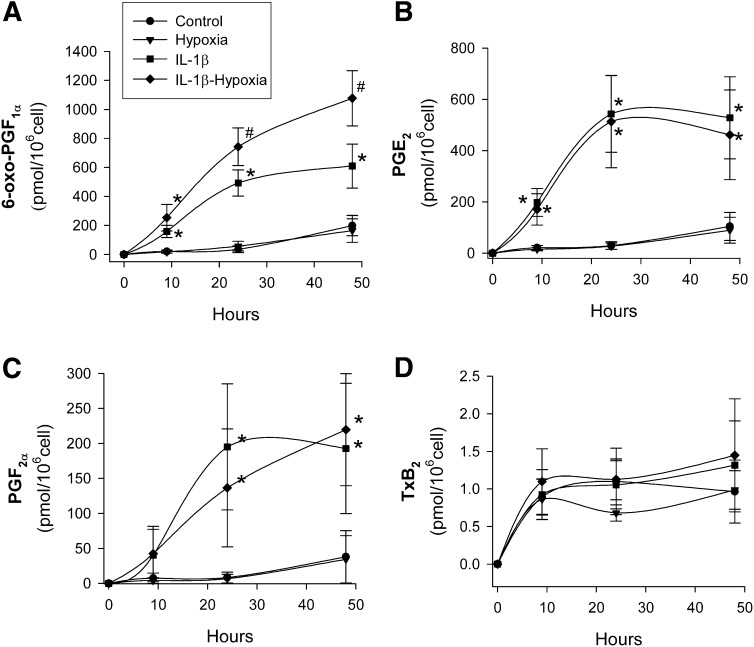
Fig. 1 shows the time course of IL-1β-induced release of prostanoids to the medium by VSMC, in normoxia and hypoxia (1% O2). IL-1β increased production of all the prostanoids analyzed: PGI2 (in terms of 6-oxo-PGF1α), PGE2, PGF2α, and TxA2 (in terms of TxB2), although the last was scarcely produced. Hypoxia caused a further increase in PGI2 accumulation in the culture medium in response to IL-1β, compared with that elicited by this cytokine under normoxic conditions. In contrast, hypoxia did not modify the release of the other prostanoids analyzed. Similar results were obtained in endothelial cells (data not shown). In the absence of IL-1β, the release of all prostanoids analyzed was scarce and not modified by hypoxia, suggesting that hypoxia per se was not able to mobilize arachidonic acid from cellular stores.
Fig. 1.
Time course of prostanoid release by human VSMC as a function of the time of exposure to hypoxia. VSMC were cultured with/without IL-1β (50 U/ml) under normoxia or hypoxia for the indicated periods of time, and (A) PGI2 (measured as its stable metabolite 6-oxo-PGF1α, n = 8), (B) PGE2 (n = 8), (C) PGF2α (n = 4), and (D) TxA2 (measured as its stable metabolite TxB2, n = 4) were analyzed by EIA. Data are mean ± SEM. *P < 0.05 versus cells exposed to normoxia or hypoxia alone; #P < 0.05 versus cells exposed to normoxia, hypoxia alone, or treated with IL-1β alone. All comparisons refer to the same treatment time.
Hypoxia increases IL-1beta-induced expression of COX-2 in VSMC
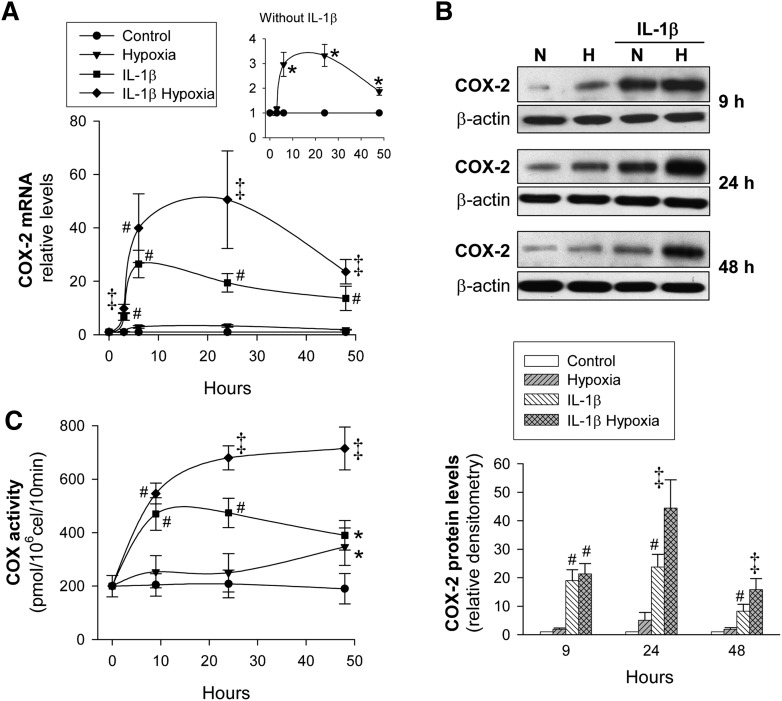
We analyzed the effect of hypoxia and inflammation on key enzymes involved in prostanoid synthesis. As expected, IL-1β was a strong inducer of COX-2 expression (Fig. 2A, B). Hypoxia also upregulated COX-2 in terms of mRNA (Fig. 2A, top inset), but this effect, comparatively lower than that of IL-1β, was not accompanied by a significant increase in COX-2 protein levels (Fig. 2B). However, a synergistic effect between IL-1β and hypoxia regarding COX-2 was observed. Indeed, in cells treated with IL-1β, hypoxia further increased COX-2, in terms of both mRNA and protein levels 24 h after challenge (Fig. 2A, B). This finding was also associated with a synergistic increase in COX activity (Fig. 2C). Neither IL-1β nor hypoxia modified the expression of COX-1 (the constitutive COX isoform; data not shown).
Fig. 2.
COX-2 expression and COX activity in human VSMC as a function of the time of exposure to hypoxia. VSMC were cultured with/without IL-1β (50 U/ml) under normoxia (N) or hypoxia (H) for the indicated periods of time. A: COX-2 mRNA levels were analyzed by real-time PCR and expressed relative to IL-1β-untreated cells in normoxia (Control) (n = 7). The top inset show the transient and early upregulation of COX-2 by hypoxia. B: COX-2 protein levels were analyzed by Western blot. A representative immunoblot is shown. Bar graph represents computer-assisted densitometry values normalized to Control (n = 6). C: COX activity was analyzed by incubating the cells with [1-14C]arachidonic acid as described (25, 28) (n = 5). Values are the mean ± SEM. *P < 0.05 versus cells exposed to normoxia; #P < 0.05 versus cells exposed to normoxia or hypoxia alone; ‡P < 0.05 versus cells exposed to normoxia, hypoxia alone, or IL-1β alone. All comparisons refer to the same treatment time.
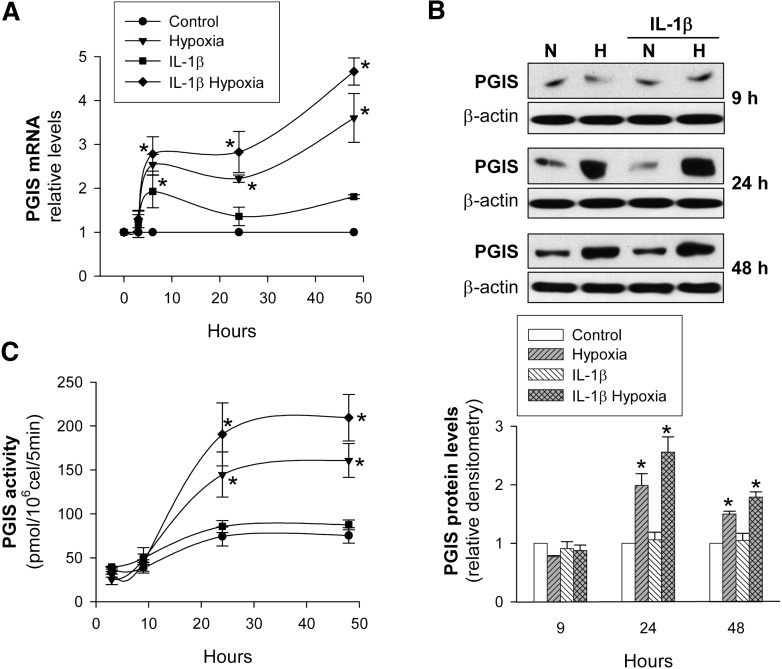
Hypoxia increases PGIS expression and activity in VSMC
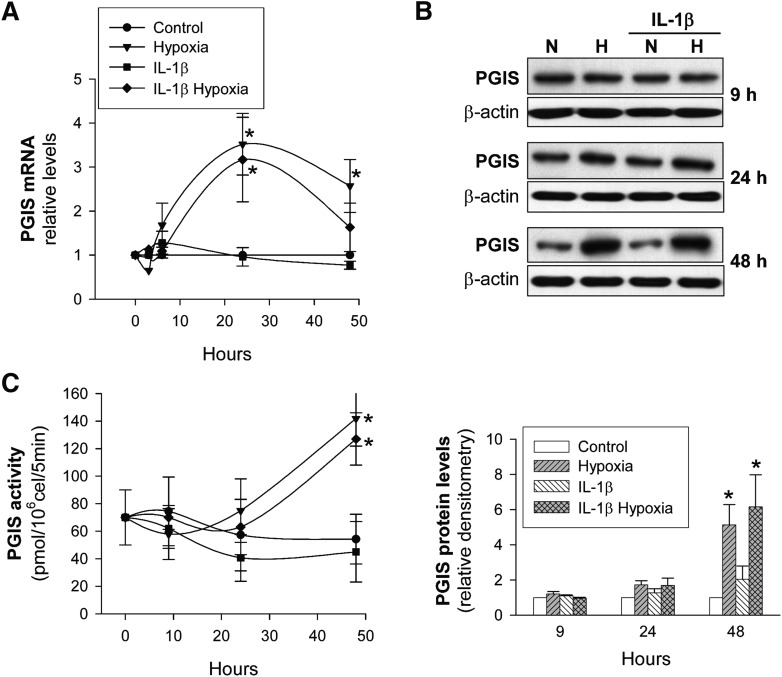
Next, we analyzed the effect of hypoxia and inflammation on the expression pattern of PGIS, the key enzyme for PGI2 synthesis. In VSMC, hypoxia increased PGIS mRNA levels, which reached a maximum at 24 h and then progressively declined (Fig. 3A). In contrast, PGIS protein levels (Fig. 3B) and PGIS activity (Fig. 3C) did not change significantly before 24 h, but they then increased markedly, reaching statistical significance at 48 h. As expected and in agreement with our previous report, IL-1β did not modify PGIS expression (25).
Fig. 3.
PGIS expression and activity in human VSMC as a function of the time of exposure to hypoxia. VSMC were cultured with/without IL-1β (50 U/ml) under normoxia (N) or hypoxia (H) for the indicated periods of time. A: PGIS mRNA levels were analyzed by real-time PCR and expressed relative to IL-1β-untreated cells in normoxia (Control) (n = 5). B: PGIS protein levels were analyzed by Western blot. A representative immunoblot is shown. Bar graph represents computer-assisted densitometry values normalized to Control (n = 5). C: PGIS activity was analyzed by incubating the cells with [1-14C]PGH2 as described (25) (n = 4). Values are the mean ± SEM. *P < 0.05 versus cells exposed to normoxia or IL-1β alone. All comparisons refer to the same treatment time.
Hypoxia does not modify mPGES-1 expression in VSMC
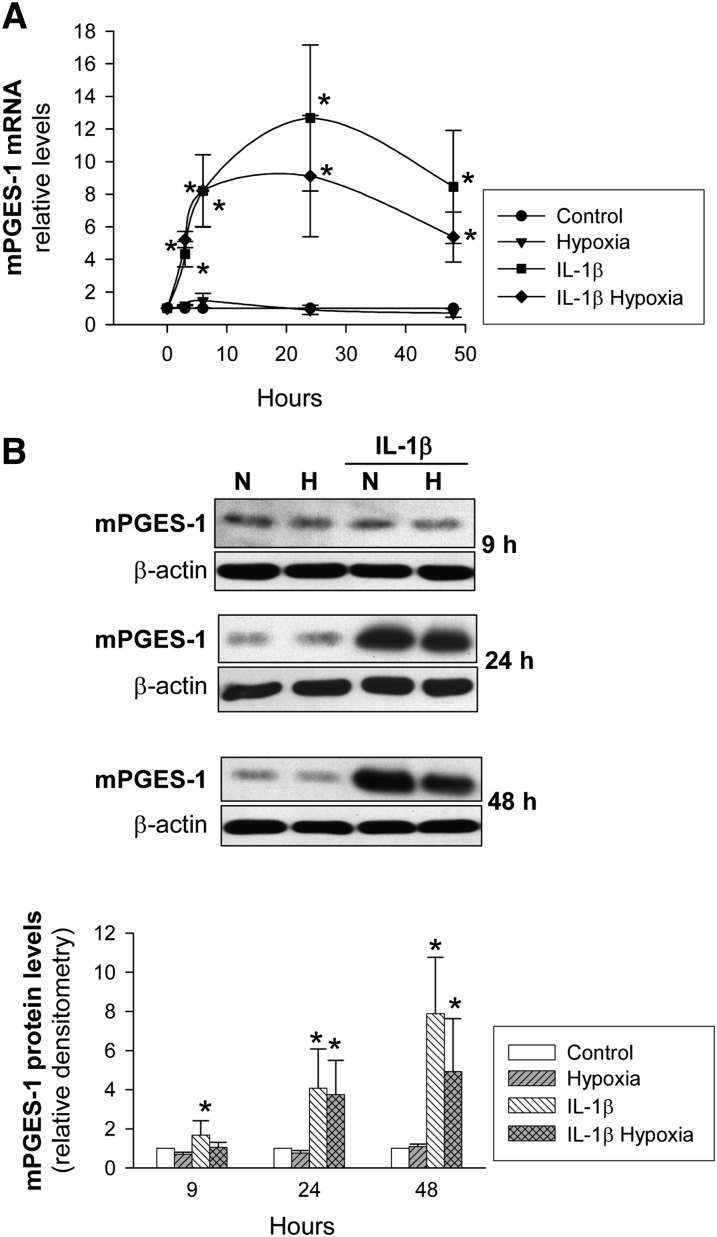
Regarding mPGES-1, IL-1β induced mPGES-1 mRNA levels in VSMC at an early stage and significantly increased mPGES-1 protein levels after a certain delay (Fig. 4). Hypoxia did not modify mPGES-1 expression, although in cells treated with IL-1β, it decreased in terms of mRNA and protein, although this effect failed to reach statistical significance.
Fig. 4.
mPGES-1 expression in human VSMC as a function of the time of exposure to hypoxia. VSMC were cultured with/without IL-1β (50 U/ml) under normoxia (N) or hypoxia (H) for the indicated periods of time. A: mPGES-1 mRNA levels were analyzed by real-time PCR and expressed relative to IL-1β-untreated cells in normoxia (Control) (n = 7). B: mPGES-1 protein levels were analyzed by Western blot. A representative immunoblot is shown. Bar graph represents computer-assisted densitometry values normalized to Control (n = 7). Values are the mean ± SEM. *P < 0.05 versus cells exposed to normoxia or hypoxia alone. All comparisons refer to the same treatment time.
In addition, we explored the effect of hypoxia on PGD-synthase (PGDS) expression. In agreement with previous reports (32), hematopoietic-type PGDS was not detected. Neither hypoxia nor IL-1β (or their combination) significantly modified the expression of the lipocalin-type PGDS (LPGDS) in either VSMC or HUVEC (data not shown).
Hypoxia upregulates PGIS mRNA, protein, and activity in endothelial cells
Next we addressed whether PGIS expression could also be modulated by hypoxia in human endothelial cells. Hypoxia increased PGIS expression in terms of mRNA, protein, and activity in HUVEC (Fig. 5.). It is noteworthy that the increase in mRNA in endothelial cells is more sustained than in VSMC and that the increase in both PGIS protein and activity occurred earlier (after 24 instead of 48 h).
Fig. 5.
PGIS expression and activity in HUVEC as a function of the time of exposure to hypoxia. HUVEC were cultured with/without IL-1β (10 U/ml) under normoxia (N) or hypoxia (H) for the indicated periods of time. A: PGIS mRNA levels were analyzed by real-time PCR and expressed relative to IL-1β-untreated cells in normoxia (Control) (n = 5). B: PGIS protein levels were analyzed by Western blot. A representative immunoblot is shown. Bar graph represents computer-assisted densitometry values normalized to Control (n = 5). C: PGIS activity was analyzed as indicated in methods (n = 4). Values are the mean ± SEM. *P < 0.05 when compared with normoxia or IL-1β alone.
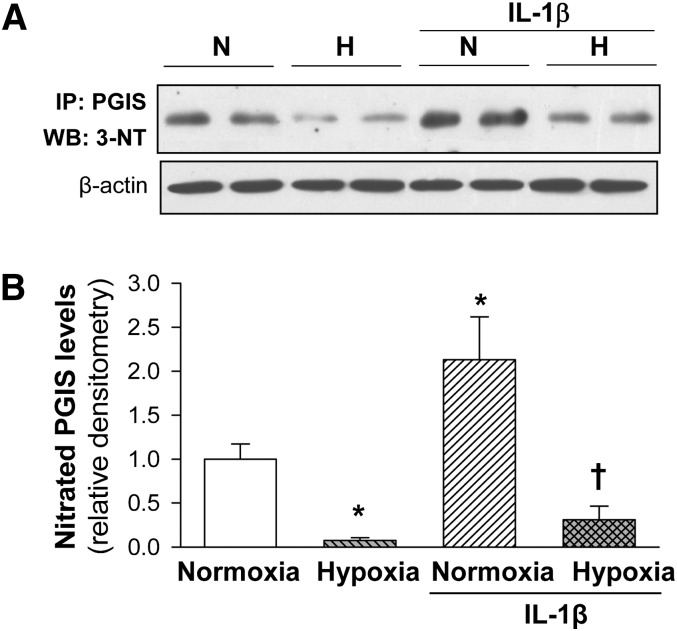
Hypoxia reduces basal and IL-1beta-induced PGIS nitration in HUVEC
Because IL-1β promotes tyrosine nitration-dependent PGIS inactivation in rat mesangial cells (31, 33), we assessed whether hypoxia could affect PGIS nitration in HUVEC. IL-1β increased the proportion of nitrated PGIS (approximately 2-fold; P < 0.05). As shown in Fig. 6, basal and IL-1β-induced PGIS nitration were significantly reduced under hypoxic conditions.
Fig. 6.
Effect of hypoxia and IL-1β on tyrosine nitration of PGIS in HUVEC. Cells were cultured with/without IL-1β (10 U/ml) under normoxia (N) or hypoxia (H) for 48 h and then PGIS nitration was evaluated in cell lysates. PGIS was immunoprecipitated with a polyclonal antibody against PGIS (IP: PGIS) and then Western blotted with a monoclonal antibody against 3-nitrotyrosine (WB: 3-NT). β-actin levels were analyzed in the input protein to assess that equal amounts of total protein were subjected to immunoprecipitation. A: Representative immunoblot analysis. B: Graphs corresponding to protein levels of nitrated PGIS represent computer-assisted densitometry values normalized to Control (n = 4). Values are the mean ± SEM. *P < 0.05 versus normoxia; †P < 0.05 versus IL-1β alone.
Hypoxia increases PGIS transcriptional activity
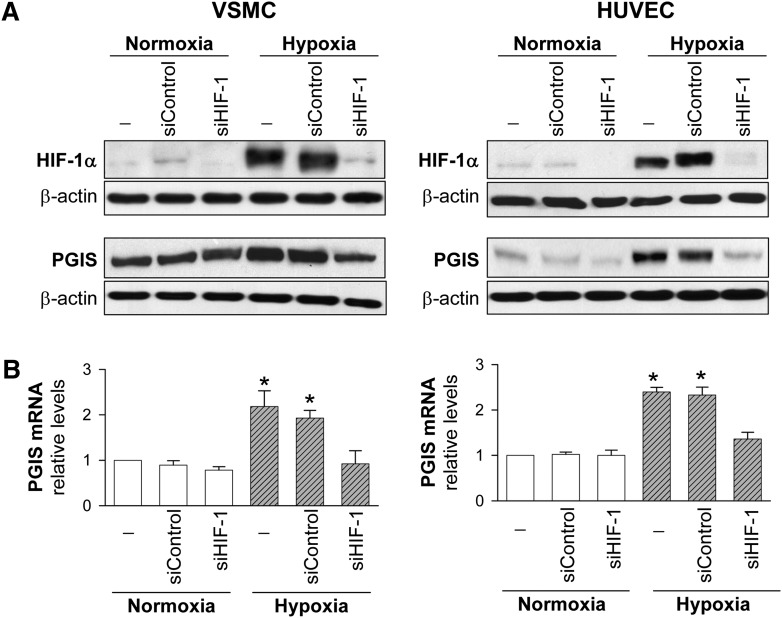
Actinomycin D completely prevented the upregulation of PGIS by hypoxia in both VSMC and HUVEC, suggesting the involvement of a transcriptional mechanism. Additionally, hypoxia did not modify PGIS mRNA stability (data not shown). Since the transcriptional response to hypoxia is primarily mediated by HIF-1α, we explored the implication of HIF-1α in PGIS expression induced by hypoxia. Hypoxia strongly increased HIF-1α protein levels, and when HIF-1α was inhibited by siRNA (siHIF-1), hypoxia-induced PGIS expression was completely suppressed in both VSMC and HUVEC (Fig. 7).
Fig.7.
Effect of HIF-1α knockdown on PGIS upregulation by hypoxia. VSMC and HUVEC were transfected with siRNA control (siControl) or siRNA against HIF-1α (siHIF-1), and then exposed to normoxia or hypoxia. Protein levels were analyzed by Western blot. A: Representative immunoblot analysis (one out of two) showing HIF-1α and PGIS protein levels in cells transfected with siControl or siHIF-1 and exposed to hypoxia for 6 h (HIF-1α protein levels) or 48 h (PGIS mRNA and protein levels). B: PGIS mRNA levels analyzed by real-time PCR in VSMC and HUVEC transfected with siControl or siHIF-1 and exposed to normoxia or hypoxia for 24 h (n = 4). Values are the mean ± SEM. *P < 0.05 compared with cells in normoxia or cells transfected with siHIF-1 and exposed to hypoxia.
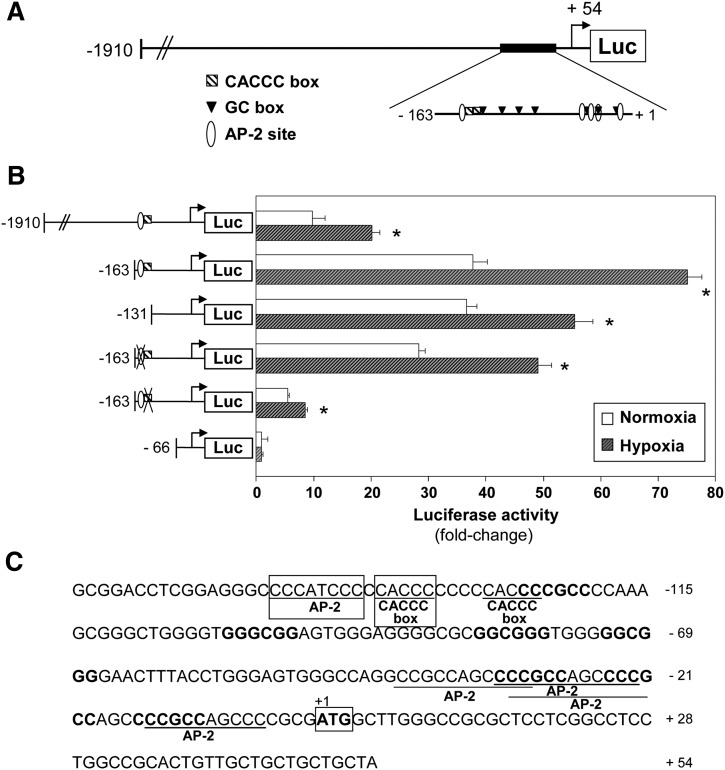
To gain further insight into the molecular mechanisms involved in hypoxia-induced PGIS upregulation, transient transfection experiments were performed in HUVEC using the luciferase reporter construct (pGL3/PGIS-1963) previously described (Fig. 8A) (29). Hypoxia significantly increased (2.01-fold) the transcriptional activity of this construct (Fig. 8B). As reported, serial deletion analysis showed that basal promoter activity increased as shorter constructs were assayed (34). A construct encompassing 163 bp of PGIS proximal promoter exhibited the maximal basal transcriptional activity and maintained hypoxia-dependent induction (1.99-fold). Further promoter deletion to -131 induced a partial loss of hypoxia responsiveness. The region located between promoter positions -163 and -131 contains a CACCC box and an AP-2 site (Fig. 8C). Basal promoter activity decreased significantly when the AP-2 site was mutated and dramatically when the CACCC box was deleted. In these constructs, the upregulation of PGIS transcriptional activity in response to hypoxia was partially prevented (1.72-fold and 1.56-fold in AP-2- and CACCC box-mutated constructs, respectively) (Fig. 5B). Subsequent deletion (to -66 bp) fully abolished basal transcriptional activity. Although PGIS proximal promoter contains several putative GC boxes recognized by Sp1 (Fig. 8C), specific inhibition of this transcription factor by siRNA did not affect PGIS induction by hypoxia (data not shown).
Fig.8.
Hypoxia induces PGIS expression through a transcriptional mechanism. A: Scheme showing the construct containing 1910 bp of PGIS promoter used in transfection experiments (pGL3/PGIS-1910). The locations of putative response elements in PGIS proximal promoter are indicated. B: HUVEC were transiently transfected with PGIS promoter constructs and maintained under normoxic (white bars) or exposed to hypoxia (shaded bars) for 18 h. Serial deletion studies were performed with different constructs named according to their length upstream of the translation-initiation site. Luciferase activity is expressed as fold-change using pGL3/PGIS-66 (in the absence of stimulus) as a reference value. Promoter activity of the pGL3/PGIS-163 construct mutated in the AP-2 site or in the CACCC box located at positions -146 and -137, respectively, is also shown. Results are the mean ± SEM (n = 9); *P < 0.05 compared with the same construct under normoxia. C: Nucleotide sequence corresponding to pGL3/PGIS-163. The translational initiation codon is numbered as +1. Putative AP-2 sites and CACCC boxes are underlined, and GC boxes are bolded.
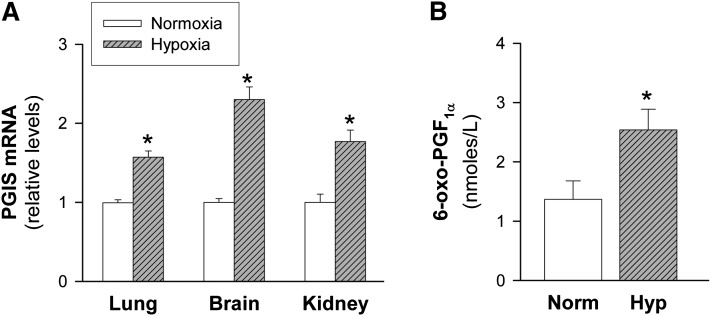
Hypoxia increases plasma PGI2 levels and PGIS expression in highly vascularized tissues
We tested whether hypoxic upregulation of PGIS could be extended to other species and other vascular beds. Our studies performed in MLEC confirmed that PGIS expression is also enhanced in these cells (data not shown), suggesting that regulation of PGIS by hypoxia is a general phenomenon in vascular cells. Next, we aimed to establish the occurrence of this effect in vivo. Mice were kept in a 10% O2 atmosphere for 24 h. Hypoxia significantly induced PGIS expression in highly vascularized tissues, including lung, brain, and kidney (Fig. 9A). Additionally, plasma levels of 6-oxo-PGF1α were significantly increased (about 2-fold) in mice exposed to hypoxia (Fig. 9B). These findings demonstrate that hypoxia stimulates the release of PGI2 in vivo and support the contribution of vascular PGIS to this effect.
Fig.9.
PGIS expression in highly vascularized tissues and plasma levels of PGI2 in animals exposed to hypoxia. C57BL/6 mice were maintained under normoxia (Norm, n = 8) or exposed to hypoxia for 24 h (Hyp [10% O2], n = 8). A: Tissue PGIS mRNA levels in mice exposed to normoxia or hypoxia for 24 h were analyzed by real-time PCR. Data were normalized to 18S rRNA and expressed relative to normoxia. B: Levels of PGI2 (measured as its stable metabolite 6-oxo-PGF1α) in plasma were determined by EIA. Results are the mean ± SEM. *P < 0.05 versus normoxia.
DISCUSSION
In recent years, a growing number of genes whose expression is modulated by hypoxia have been identified. Among them is COX-2, the putative rate-limiting enzyme in the inducible biosynthesis of eicosanoids (4, 5, 11). However, the potential regulation by hypoxia of downstream enzymes involved in this pathway is not completely understood. In this work, we investigated the modulation of the enzymes involved in the biosynthesis of PGI2 and PGE2, two prostanoids with dissimilar effects on vascular function, under both hypoxic and hypoxic/inflammatory conditions. PGI2 levels were synergistically modulated by hypoxia and inflammation in vascular cells. This finding was associated with a concomitant upregulation of COX-2 by hypoxia and IL-1β. More interestingly, the induction of PGIS, but not mPGES-1, by hypoxia favors the conversion of COX-2-derived PGH2 to PGI2, increasing the PGI2/PGE2 ratio.
PGI2 and PGE2 are the main prostaglandins produced by human vascular cells (12). PGI2 plays an important role in vascular biology in preventing thrombosis, acting as a potent vasorelaxant agent, and preserving endothelial cell viability (35–37). PGE2 is a pro-inflammatory molecule that is able to act through four different receptor subtypes. It elicits disparate effects (21), including vasoconstriction, at least at high concentrations (38). The production of PGI2 and untransformed PGH2 depends on the PGIS/COX activity ratio (25), and the biosynthesis of PGI2 versus PGE2 depends on the relative expression/activity of PGIS and mPGES-1 (23). We here show that the upregulation of COX-2 expression/activity elicited by IL-1β was further increased by hypoxia, and that concomitantly, hypoxia significantly induced PGIS.
Our data support the involvement of a transcriptional mechanism in the upregulation of PGIS by hypoxia. Although it has recently been shown that PGIS is induced by retinoids in vascular cells (29), PGIS appears to be constitutively expressed in different tissues, and few studies have focused on the characterization of its promoter region. Transcriptional response to hypoxia is primarily mediated by HIF-1α. HIF-1 regulates a wide array of genes containing functional HRE, but it can also trigger secondary transcriptional programs through other transcription factors, including Sp1 (4–9). Data from our knockdown experiments supported the involvement of HIF-1 on hypoxia PGIS regulation; however, by serial deletion studies, we delimited hypoxia responsiveness to a proximal promoter region of -163 bp that does not exhibit any putative HRE. This sequence contains several Sp1-recognized GC boxes that are well-conserved among mouse and human PGIS genes (34). Sp1 is activated by hypoxia in HUVEC and is involved in hypoxia-mediated COX-2 regulation (5); however, Sp1 silencing had no consequences on PGIS induction by hypoxia (data not shown). The regulation of PGIS transcriptional activity by hypoxia could be the result of the cooperative binding of several transcription factors as has been reported for other hypoxia target genes, such as PAI-1 or COX-2 (4, 5, 8). Accordingly, deletion of the PGIS promoter region between -163 and -131 bp partially prevented the induction of PGIS transcriptional activity elicited by hypoxia. By site-directed mutagenesis, we identified a CACCC box that seemed to be partially responsible for this effect, although suppression of this element did not completely prevent PGIS activation by hypoxia. Further promoter analysis was hampered by the null transcriptional activity exhibited by shorter constructs, in agreement with previous studies that identified this region as a minimal promoter needed to retain basal PGIS transcriptional activity (34). As induction of PGIS was only seen after long exposure to hypoxia, significantly lagging behind changes in HIF-1, we assessed whether PGIS would be modulated by vascular endothelial growth factor (VEGF), a cytokine regulated by this transcription factor and induced by hypoxia in vascular cells at an early stage (9). However, VEGF was unable to modulate PGIS expression. We thus ruled out this cytokine acting in an autocrine manner (data not shown). Epigenetic mechanisms have been related with PGIS downregulation in pathologies such as lung cancer (39), and in the last few years modulation of chromatin methylation has been reported to underlie some of the cellular responses triggered by hypoxia (40). Furthermore, hypoxia-induced histone eviction contributes to hypoxic responses in endothelial cells (41). Transcriptional reprogramming under hypoxia is, therefore, a more complex process than the mere regulation of genes containing functional HIF binding sites. Further efforts are required to gain insight into the mechanisms by which hypoxia regulates PGIS transcriptional activity.
We show that PGIS expression was significantly enhanced in highly vascularized tissues (brain, lung, and kidney) from mice exposed to hypoxia. The increment in brain PGIS after 24 h of hypoxia conformed to the time-course reported in a model of cerebral ischemia (42). Interestingly, systemic hypoxia increased PGI2 plasma levels, as had been reported in previous studies in animals exposed to hypoxia (43, 44). Pidgeon et al. (43) reported an increase in the urinary excretion of 6-oxo-PGF1α in mice chronically exposed to hypoxia. This increase was associated with the upregulation of COX-2 observed in pulmonary tissues of these animals. Blumberg et al. (44) also reported an increase in plasma 6-oxo-PGF1α levels in rats chronically exposed to hypoxia, associated with the induction of lung PGIS mRNA levels. The increase was attributed to the hemodynamic changes triggered by pulmonary hypertension. Our in vivo studies further confirm the modulation of PGI2 synthesis by systemic hypoxia, while our in vitro results emphasize the role of hypoxia per se as a major driving force regulating PGIS expression. However, there was an apparent discrepancy between in vivo and in vitro experiments regarding release of PGI2 caused by hypoxia per se, because in cell cultures, hypoxia alone did not increase PG release. This finding could be explained by the fact that hypoxia alone was not able to mobilize arachidonic acid from cell membranes when vascular cells were in culture. In vivo, the scenario is more complex. Likely with the cooperation of inflammatory cells, hypoxia could induce the release of pro-inflammatory mediators. These in turn would activate phospholipases, releasing free arachidonic acid available for the COX catalysis. Indeed, in cultured cells, the upregulation of PGIS contributed to further increase PGI2 release when arachidonic acid was released and COX-2 was induced by the action of IL-1β. Conversely, hypoxia did not modify basal mPGES-1 expression and tended to downregulate mPGES-1 expression/activity in the presence of IL-1β, further contributing to increase the PGI2/PGE2 ratio.
Accumulative evidence supports the concept that PGIS/PGI2 plays a protective role in pathologies with an ischemic substrate. Deficient pulmonary PGI2 synthesis seems to underlie the development of pulmonary hypertension in humans. Indeed, PGI2 administration improves patient survival and is currently the most effective treatment for this disease (45). Accordingly, overexpression of PGIS protects mice against pulmonary hypertension triggered by chronic hypoxia (46). PGIS gene transfer protects against ischemic cerebral infarction (42, 47), and intravenous PGI2 administration also prevents ischemic brain damage in experimental models (48). In cell cultures, PGI2 exerts an anti-apoptotic effect (49), and PGIS overexpression improves cell survival and protects cells against cytokine toxicity (35, 37). Therefore, PGI2 seems to play a cytoprotective role under ischemic and inflammatory conditions. The stabilization of HIF-1α by PGI2 under prolonged hypoxia (50) could further contribute to the survival response induced by hypoxic stress. Despite the fact that COX activity in normal condition is the limiting step in PGI2, upregulation of PGIS could be relevant for PGI2 production when PGIS activity becomes limiting for the PGI2 biosynthesis, as could occur in an inflammatory environment. Under inflammatory conditions, PGIS could be more prone to be inactivated by several mechanisms. It is well known that PGIS undergoes substrate-dependent suicide inactivation (51). We have shown that PGIS catalytically inactivates faster than COX-2 when the latter enzyme is strongly upregulated, yielding high amounts of PGH2 (25, 52). Other mechanisms of PGIS inactivation involve tyrosine nitration at the catalytic center by nitrating agents derived from nitric oxide, which are produced in abundance under inflammatory conditions (25, 31, 33, 53). This nitric oxide-dependent inactivation of PGIS has been observed in systemic inflammatory syndromes, atherosclerosis, hypoxia-reoxygenation injury, and diabetes (36, 54–58). An oxygen tension-dependent downregulation of nitrotyrosine-containing protein levels has been reported under hypoxic conditions (59, 60), and we consistently observed that hypoxia reduces IL-1β-induced nitration of PGIS. PGIS modulation by hypoxia could thus contribute to preserving vascular biosynthesis of PGI2 under pathological conditions involving inflammation and hypoxic-ischemic insult.
Our results are also consistent with the fact that hypoxia increases production of vasorelaxant eicosanoids other than prostanoids. Hypoxia enhances expression of endothelial 15-lipoxygenase in several animal models (61, 62) and human retinal microvascular endothelial cells (63). 15-lipoxygenase metabolizes AAc to 11,12,15-trihydroxyeicosatrienoic acids and 15-hydroxy-11,12-epoxyeicosatrienoic acids, which act as relaxing factors (64).
In summary, our results indicate that the delayed upregulation of PGIS by hypoxia in vascular cells, both in culture and animal tissues in vivo, could be critical for the hypoxia-increased production of PGI2, a potent anti-thrombotic agent with a wide range of pro-angiogenic, vasculoprotective, and cytoprotective properties. This mechanism could contribute to the adaptive response of vascular cells to hypoxic stress, counteracting the deleterious effect induced by concomitant inflammatory stimuli.
Acknowledgments
The authors thank S. Aguiló for help with animal studies.
Footnotes
Abbreviations:
- COX-2
- cyclooxygenase-2
- EIA
- enzyme immunoassay
- HIF
- hypoxia-inducible factor
- HRE
- hypoxia response element
- HUVEC
- human umbilical vein endothelial cell
- IL
- interleukin
- LPGDS
- lipocalin-type PGDS
- MLEC
- mouse lung endothelial cell
- mPGES
- microsomal-PGE-synthase
- PG
- prostaglandin
- PGDS
- PGD-synthase
- PGES
- PGE-synthases
- PGIS
- PGI-synthase
- siRNA
- silencer RNA
- Tx
- thromboxane
- VEGF
- vascular endothelial growth factor
- VSMC
- vascular smooth muscle cell
This work was supported by Ministerio de Ciencia e Innovación Grants SAF2008-01777, SAF2009-09598, and SAF2009-11949; Red Temática de Investigación Cardiovascular (RECAVA) Grants RD06/0014/0027 and RD06/0014/1005; and Instituto de Salud Carlos III Grant PS09/01797.
REFERENCES
- 1.Semenza G. L., Agani F., Feldser D., Iyer N., Kotch L., Laughner E., Yu A. 2000. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv. Exp. Med. Biol. 475: 123–130. [DOI] [PubMed] [Google Scholar]
- 2.Semenza G. L. 2000. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J. Clin. Invest. 106: 809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestre J. S., Mallat Z., Tedgui A., Lévy B. I. 2008. Post-ischaemic neovascularization and inflammation. Cardiovasc. Res. 78: 242–249. [DOI] [PubMed] [Google Scholar]
- 4.Schmedtje J. F., Jr.,, Ji Y. S., Liu W-L., DuBois R. N., Runge M. S. 1997. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272: 601–608. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q., Ji Y. S., Schmedtje J. F., Jr 2000. Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J. Biol. Chem. 275: 24583–24589. [DOI] [PubMed] [Google Scholar]
- 6.Alfranca A., Gutiérrez M. D., Vara A., Aragonés J., Vidal F., Landázuri M. O. 2002. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol. Cell. Biol. 22: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummins E. P., Taylor C. T. 2005. Hypoxia-responsive transcription factors. Pflugers Arch. 450: 363–371. [DOI] [PubMed] [Google Scholar]
- 8.Liao H., Hyman M. C., Lawrence D. A., Pinsky D. J. 2007. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 21: 935–949. [DOI] [PubMed] [Google Scholar]
- 9.Martorell L., Gentile M., Rius J., Rodríguez C., Crespo J., Badimon L., Martínez-González J. 2009. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol. Cell. Biol. 29: 5828–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1). 2006. Mol. Pharmacol. 70: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 11.Scheurer S. B., Rybak J. N., Rösli C., Neri D., Elia G. 2004. Modulation of gene expression by hypoxia in human umbilical cord vein endothelial cells: a transcriptomic and proteomic study. Proteomics. 4: 1737–1760. [DOI] [PubMed] [Google Scholar]
- 12.Vila L. 2004. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med. Res. Rev. 24: 399–424. [DOI] [PubMed] [Google Scholar]
- 13.Topper J. N., Cai J., Falb D., Gimbrone M. A., Jr 1996. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively upregulated by steady laminar shear stress. Proc. Natl. Acad. Sci. USA. 93: 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdam B. F., Castella-Lawson F., Mardini I. A., Kapoor S., Lawson J. A., Fitzgerald G. A. 1999. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA.. 96: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudero I., Martínez-González J., Alonso R., Mata P., Badimon L. 2003. Experimental and interventional dietary study in humans on the role of HDL fatty acid composition in PGI2 release and Cox-2 expression by VSMC. Eur. J. Clin. Invest. 33: 779–786. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-González J., Escudero I., Badimon L. 2004. Simvastatin potenciates PGI2 release induced by HDL in human VSMC: effect on Cox-2 up-regulation and MAPK signalling pathways activated by HDL. Atherosclerosis. 174: 305–313. [DOI] [PubMed] [Google Scholar]
- 17.González-Díez M., Rodríguez C., Badimon L., Martínez-González J. 2008. Prostacyclin induction by high-density lipoprotein (HDL) in vascular smooth muscle cells depends on sphingosine 1-phosphate receptors: effect of simvastatin. Thromb. Haemost. 100: 119–126. [DOI] [PubMed] [Google Scholar]
- 18.Dowd N. P., Scully M., Adderley S. R., Cunningham A. J., Fitzgerald D. J. 2001. Inhibition of cyclooxygenase-2 aggravates doxorubicin-mediated cardiac injury in vivo. J. Clin. Invest. 108: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inserte J., Molla B., Aguilar R., Través P. G., Barba I., Martín-Sanz P., Boscá L., Casado M., Garcia-Dorado D. 2009. Constitutive COX-2 activity in cardiomyocytes confers permanent cardioprotection: constitutive COX-2 expression and cardioprotection. J. Mol. Cell. Cardiol. 46: 160–168. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Gonzalez J., Badimon L. 2007. Mechanisms underlying the cardiovascular effects of Cox-inhibition: benefits and risks. Curr. Pharm. Des. 13: 2215–2227. [DOI] [PubMed] [Google Scholar]
- 21.Breyer R. M., Bagdassarian C. K., Myers S. A., Breyer M. D. 2001. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41: 661–690. [DOI] [PubMed] [Google Scholar]
- 22.Murakami M., Nakatani Y., Tanioka T., Kudo I. 2002. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 68–69: 383–399. [DOI] [PubMed] [Google Scholar]
- 23.Camacho M., Gerbolés E., Escudero J. R., Anton R., García-Moll X., Vila L. 2007. Microsomal-PGE synthase-1, which is not coupled to a particular COX-isoenzyme, is essential for PGE2 biosynthesis in vascular smooth muscle cells. J. Thromb. Haemost. 5: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 24.Grosser T., Yu Y., Fitzgerald G. A. 2010. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu. Rev. Med. 61: 17–33. [DOI] [PubMed] [Google Scholar]
- 25.Camacho M., López-Belmonte J., Vila L. 1998. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and PGI-synthase activity. Circ. Res. 83: 353–365. [DOI] [PubMed] [Google Scholar]
- 26.Soler M., Camacho M., Escudero J. R., Iñiguez M. A., Vila L. 2000. Human vascular smooth muscle cells but not endothelial cells express prostaglandin E synthase. Circ. Res. 87: 504–507. [DOI] [PubMed] [Google Scholar]
- 27.Orbe J., Rodríguez J. A., Calvayrac O., Rodríguez-Calvo R., Rodríguez C., Roncal C., Martínez de Lizarrondo S., Barrenetxe J., Reverter J. C., Martínez-González J., et al. 2009. Matrix metalloproteinase-10 is up-regulated by thrombin in endothelial cells and increased in patients with enhanced thrombin generation. Arterioscler. Thromb. Vasc. Biol. 29: 2109–2116. [DOI] [PubMed] [Google Scholar]
- 28.Solá J., Godessart N., Vila L., Puig L., de Moragas J. M. 1992. Epidermal cell-polymorphonuclear leukocyte cooperation in the formation of leukotriene B4 by transcellular biosynthesis. J. Invest. Dermatol. 98: 333–339. [DOI] [PubMed] [Google Scholar]
- 29.Camacho M., Rodríguez C., Salazar J., Martínez-González J., Ribalta J., Escudero J. R., Masana L. L., Vila L. 2008. Retinoic acid induces PGI-synthase expression in human endothelial cells. J. Lipid Res. 49: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez C., Raposo B., Martínez-González J., Llorente-Cortés V., Vilahur G., Badimon L. 2003. Modulation of ERG25 expression by LDL in vascular cells. Cardiovasc. Res. 58: 178–185. [DOI] [PubMed] [Google Scholar]
- 31.Zou M. H., Klein T., Pasquet J. P., Ullrich V. 1998. Interleukin-1beta decreases prostacyclin synthase activity in rat mesangial cells via endogenous peroxynitrite formation. Biochem. J. 336: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taba Y., Sasaguri T., Miyagi M., Abumiya T., Miwa Y., Ikeda T., Mitsumata M. 2000. Fluid shear stress induces lipocalin-type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ. Res. 86: 967–973. [DOI] [PubMed] [Google Scholar]
- 33.Zou M., Yesilkaya A., Ullrich V. 1999. Peroxynitrite inactivates prostacyclin synthase by heme-thiolate- catalyzed tyrosine nitration. Drug Metab. Rev. 31: 343–349. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama C., Yabuki T., Inoue H., Tone Y., Hara S., Hatae T., Nagata M., Takahashi E. I., Tanabe T. 1996. Human gene encoding prostacyclin synthase (PTGIS): genomic organization, chromosomal localization, and promoter activity. Genomics. 36: 296–304. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa A., Makino H., Aoki M., Miyake T., Shiraya S., Nakamura T., Ogihara T., Kimata Y., Morishita R. 2007. Improvement of survival of skin flaps by combined gene transfer of hepatocyte growth factor and prostacyclin synthase. J. Gene Med. 9: 1087–1094. [DOI] [PubMed] [Google Scholar]
- 36.Pakrasi P. L., Jain A. K. 2008. Cyclooxygenase-2-derived endogenous prostacyclin reduces apoptosis and enhances embryo viability in mouse. Prostaglandins Leukot. Essent. Fatty Acids. 79: 27–33. [DOI] [PubMed] [Google Scholar]
- 37.Gurgul-Convey E., Lenzen S. 2010. Protection against cytokine toxicity through ER and mitochondrial stress prevention by prostacyclin synthase overexpression in insulin-producing cells. J. Biol. Chem. 285: 11121–11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang E. H., Jensen B. L., Skott O., Leung G. P., Feletou M., Man R. Y., Vanhoutte P. M. 2008. The role of prostaglandin E and thromboxane-prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc. Res. 78: 130–138. [DOI] [PubMed] [Google Scholar]
- 39.Stearman R. S., Grady M. C., Nana-Sinkam P., Varella-Garcia M., Geraci M. W. 2007. Genetic and epigenetic regulation of the human prostacyclin synthase promoter in lung cancer cell lines. Mol. Cancer Res. 5: 295–308. [DOI] [PubMed] [Google Scholar]
- 40.Krieg A. J., Rankin E. B., Chan D., Razorenova O., Fernandez S., Giaccia A. J. 2010. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol. Cell. Biol. 30: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fish J. E., Yan M. S., Matouk C. C., St Bernard R., Ho J. J., Jr, Gavryushova A., Srivastava D., Marsden P. A. 2010. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J. Biol. Chem. 285: 810–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang Y. C., Wu J. S., Chen J. J., Cheung W. M., Tseng P. H., Tam K. B., Shyue S. K., Chen J. J., Lin T. N. 2006. Induction of prostacyclin/PGI2 synthase expression after cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 26: 491–501. [DOI] [PubMed] [Google Scholar]
- 43.Pidgeon G. P., Tamosiuniene R., Chen G., Leonard I., Belton O., Bradford A., Fitzgerald D. J. 2004. Intravascular thrombosis after hypoxia-induced pulmonary hypertension. regulation by cyclooxygenase-2. Circulation. 110: 2701–2707. [DOI] [PubMed] [Google Scholar]
- 44.Blumberg F. C., Lorenz C., Wolf K., Sandner P., Riegger G. A., Pfeifer M. 2002. Increased pulmonary prostacyclin synthesis in rats with chronic hypoxic pulmonary hypertension. Cardiovasc. Res. 55: 171–177. [DOI] [PubMed] [Google Scholar]
- 45.Barst R. J., Rubin L. J., Long W. A., McGoon M. D., Rich S., Badesch D. B., Groves B. M., Tapson V. F., Bourge R. C., Brundage B. H. 1996. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med. 334: 296–302. [DOI] [PubMed] [Google Scholar]
- 46.Geraci M. W., Gao B., Shepherd D. C., Moore M. D., Westcott J. Y., Fagan K. A., Alger L. A., Tuder R. M., Voelkel N. F. 1999. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Invest. 103: 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin H., Lin T. N., Cheung W. M., Nian G. M., Tseng P. H., Chen S. F., Chen J. J., Shyue S. K., Liou J. Y., Wu C. W., et al. 2002. Cyclooxygenase-1 and bicistronic cyclooxygenase-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation. 105: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda S., Wen T. C., Karasawa Y., Araki H., Otsuka H., Ishihara K., Sakanaka M. 1997. Protective effect of a prostaglandin I2 analog, TEI-7165, on ischemic neuronal damage in gerbils. Brain Res. 769: 321–328. [DOI] [PubMed] [Google Scholar]
- 49.Liou J. Y., Lee S., Ghelani D., Matijevic-Aleksic N., Wu K. K. 2006. Protection of endothelial survival by peroxisome proliferator-activated receptor-δ mediated 14-3-3 upregulation. Arterioscler. Thromb. Vasc. Biol. 26: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 50.Chang T. C., Huang C. J., Tam K., Chen S. F., Tan K. T., Tsai M. S., Lin T. N., Shyue S. K. 2005. Stabilization of hypoxia-inducible factor1α by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J. Biol. Chem. 280: 36567–36574. [DOI] [PubMed] [Google Scholar]
- 51.Wade M. L., Voelkel N. F., Fitzpatrick F. A. 1995. “Suicide” inactivation of prostaglandin I2 synthase: characterization of mechanism-based inactivation with isolated enzyme and endothelial cells. Arch. Biochem. Biophys. 321: 453–458. [DOI] [PubMed] [Google Scholar]
- 52.Camacho M., Vila L. 2000. Transcellular formation of thromboxane A2 in mixed incubations of endothelial cells and aspirin-treated platelets strongly depends on the prostaglandin I-synthase activity. Thromb. Res. 99: 155–164. [DOI] [PubMed] [Google Scholar]
- 53.Zou M. H., Ullrich V. 1996. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 382: 101–104. [DOI] [PubMed] [Google Scholar]
- 54.Soler M., Camacho M., Molins-Pujol A. M., Vila L. 2001. Effect of an imidazolineoxyl N-oxide on prostaglandin synthesis in experimental shock: possible role of nitrogen dioxide in prostacyclin-synthase inactivation. J. Infect. Dis. 183: 105–112. [DOI] [PubMed] [Google Scholar]
- 55.Zou M., Jendral M., Ullrich V. 1999. Prostaglandin endoperoxide-dependent vasospasm in bovine coronary arteries after nitration of prostacyclin synthase. Br. J. Pharmacol. 126: 1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou M. H., Leist M., Ullrich V. 1999. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am. J. Pathol. 154: 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou M. H., Bachschmid M. 1999. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J. Exp. Med. 190: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie H., Wu J-L., Zhang M., Xu J., Zou M. H. 2006. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes. 55: 3133–3141. [DOI] [PubMed] [Google Scholar]
- 59.Aulak K. S., Koeck T., Crabb J. W., Stuehr D. J. 2004. Dynamics of protein nitration in cells and mitochondria. Am. J. Physiol. Heart Circ. Physiol. 286: H30–H38. [DOI] [PubMed] [Google Scholar]
- 60.Koeck T., Fu X., Hazen S. L., Crabb J. W., Stuehr D. J., Aulak K. S. 2004. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J. Biol. Chem. 279: 27257–27262. [DOI] [PubMed] [Google Scholar]
- 61.Zhu D., Medhora M., Campbell W. B., Spitzbarth N., Baker J. E., Jacobs E. R. 2003. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ. Res. 92: 992–1000. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal N. T., Pfister S. L., Gauthier K. M., Chawengsub Y., Baker J. E., Campbell W. B. 2009. Chronic hypoxia enhances 15-lipoxygenasemediated vasorelaxation in rabbit arteries. Am. J. Physiol. Heart Circ. Physiol. 296: H678–H688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Bajpai A. K., Blaskova E., Pakala S. B., Zhao T., Glasgow W. C., Penn J. S., Johnson D. A., Rao G. N. 2007. 15(S)-HETE production in human retinal microvascular endothelial cells by hypoxia: novel role for MEK1 in 15(S)-HETE induced angiogenesis. Invest. Ophthalmol. Vis. Sci. 48: 4930–4938. [DOI] [PubMed] [Google Scholar]
- 64.Pfister S. L., Spitzbarth N., Nithipatikom K., Edgemond W. S., Falck J. R., Campbell W. B. 1998. Identification of the 11,14,15- and 11,12, 15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J. Biol. Chem. 273: 30879–30887. [DOI] [PubMed] [Google Scholar]