Abstract
Moderate chronic kidney disease (CKD) (defined by an estimated glomerular filtration rate of 30–60 ml/min) is associated with mild hypertriglyceridemia related to delayed catabolism of triglyceride-rich lipoprotein particles. Altered apolipoprotein C-III (apoC-III) metabolism may contribute to dyslipidemia in CKD. To further characterize the dyslipidemia of CKD, we investigated the kinetics of plasma apoC-III in 7 nonobese, nondiabetic, non-nephrotic CKD subjects and 7 age- and sex-matched healthy controls, using deuterated leucine ([5, 5, 5, 2H3]leucine), gas chromatography-mass spectrometry, and multicompartmental modeling. Compared with controls, CKD subjects had higher concentrations of plasma and VLDL triglycerides and plasma and VLDL apoC-III (P < 0.05). The increased plasma apoC-III concentration was associated with a decreased apoC-III fractional catabolic rate (FCR) (1.21 ± 0.15 vs. 0.74 ± 0.12 pools/day, P = 0.03). There were no differences between apoC-III production rates of controls and those of CKD subjects. In CKD subjects, plasma apoC-III concentration was significantly and negatively correlated with apoC-III FCR (r = −0.749, P = 0.05) but not with apoC-III production rate. Plasma apoC-III concentration was positively correlated with plasma and VLDL triglycerides and VLDL apoB concentrations and negatively correlated with VLDL apoB FCR (P < 0.05 for all). ApoC-III FCR was negatively correlated with plasma and VLDL triglycerides and VLDL apoB concentration and positively correlated with VLDL apoB FCR (P < 0.05 for all). Altered plasma apoC-III metabolism is a feature of dyslipidemia in moderate CKD. Modification of apoC-III catabolism may be an important therapeutic target for reducing cardiovascular disease risk in moderate CKD.
Keywords: kinetics , lipoprotein metabolism , triglycerides
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD) (1). While the precise mechanisms for increased CVD risk are unknown, both traditional and novel CVD risk factors have been implicated (1, 2). Dyslipidemia, a well-established risk factor for CVD in the general population, is highly prevalent in CKD (3, 4). The most frequent dyslipoproteinemic phenotype patterns are elevated plasma concentrations of triglycerides and increased numbers of atherogenic triglyceride-rich lipoprotein (TRL) particles, particularly VLDL and intermediate-density lipoprotein (IDL) (3, 4). The regulation of TRL metabolism in CKD, however, is poorly understood.
Apolipoprotein C-III (apoC-III) is an 8.8 kDa glycoprotein synthesized by the liver and intestines (5). ApoC-III is highly associated with hypertriglyceridemia and is a powerful independent predictor of CVD risk in subjects without renal disease (5). In the circulation, apoC-III is associated with TRL and HDL exchanging rapidly between these lipoproteins (6). In vitro studies demonstrate that apoC-III inhibits LPL and HL activities and the uptake of TRL and their remnants by hepatic lipoprotein receptors (5). ApoC-III may also stimulate apoB and triglyceride synthesis and, hence, drive hepatic overproduction of VLDL particles (7, 8). Elevated plasma apoC-III concentration, specifically its accumulation in TRL and their remnants, is a consistent feature of dyslipidemia in CKD (9). Of note, we recently reported that moderate CKD is associated with hypertriglyceridemia related to delayed catabolism of triglyceride-rich VLDL and IDL particles (10). In CKD subjects, plasma apoC-III concentration was significantly elevated and was an independent predictor of impaired VLDL catabolism (10). The underlying mechanism for the increased plasma apoC-III concentration in these CKD subjects, however, has not been examined. A better understanding of the metabolism of apoC-III may clarify the association between dyslipidemia and CVD, with implications for better therapeutic management in the CKD population.
In the present study, we investigated the kinetics of plasma apoC-III in predialysis, moderate CKD subjects (defined as those with an estimated glomerular filtration rate [eGFR] of 30–60 ml/min). We hypothesized that subjects with moderate CKD would exhibit catabolic defects in apoC-III metabolism. We also explored associations between the kinetics of plasma apoC-III and VLDL apoB and other markers of TRL metabolism, including apoA-V.
METHODS AND MATERIALS
Subjects
Seven CKD subjects (having an eGFR of 30–60 ml/min, according to modified Modification of Diet in Renal Disease Study equation) and 7 healthy controls were recruited from the department of nephrology (Royal Perth Hospital, Western Australia) and the community, respectively. Healthy controls were recruited to match the CKD subjects based on age, sex, and waist circumference. All subjects were nondiabetic, nonobese (body mass index [BMI] of <30 kg/m2) and did not have central adiposity (waist circumference of <102 cm for men and <88 cm for women, as defined by National Cholesterol Education Program Adult Treatment Panel III criteria) (11). All subjects underwent complete physical examinations and laboratory investigations. Healthy control subjects had no clinical or laboratory evidence of renal disease or any chronic disorder that required the use of regular medication. Patients with CKD who were smokers were included, as well as those using aspirin, angiotensin-converting enzyme inhibitors, and/or other antihypertensive agents. Patients with CKD receiving lipid-modifying therapy underwent a 6 week washout period, and those receiving antioxidant vitamin or fish oil therapy underwent a 2 week washout period before study participation. CKD subjects were excluded if they had nephrotic syndrome (or proteinuria >3 g/day), significant CVD, hypothyroidism, abnormal liver function test results, alcohol consumption of >30 g/day, and the presence of apolipoprotein E2/E2 genotype. ApoE genotyping was not performed in the control subjects, but none of these subjects exhibited a dyslipidemic phenotype. Secondary causes of dyslipidemia were excluded in CKD subjects. None of the subjects had a family history of premature CVD. Causes of renal disease included glomerulonephritis, interstitial nephritis, previous nephrectomy, adult polycystic kidney disease, vasculitis, and systemic lupus erythematosus. In one CKD subject, the cause of renal dysfunction was unknown. All subjects were consuming ad libitum, weight maintenance diets. All subjects provided informed written consent, and the study was approved by the Ethics Committee of Royal Perth Hospital.
Study design and clinical protocols
All subjects were admitted to the metabolic ward in the morning after a minimum of 12 h fast. They were studied in a semirecumbent position and were allowed only water for the initial 10 h of the study. Venous blood was collected for biochemical measurements. Body weight and height were measured, and arterial blood pressure was recorded using a Dinamap1846 SX/P monitor (Critikon, Tampa, FL). Subjects were advised to continue isocaloric diets and maintain a physical activity constant during the study. Dietary intake was assessed by using 24 h dietary diary records and DIET 4 nutrient calculation software (Xyris Software, Qld, Australia).
A single bolus (5 mg/kg body weight) of deuterated leucine ([5, 5, 5, 2H3]leucine) was administered intravenously into an antecubital vein via a Teflon cannula. Blood samples were collected at baseline and at 5, 10, 20, 30, and 40 min and at 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 10 h after isotope injection. Additional blood samples were collected in the mornings on the four following days (24, 48, 72, and 96 h) after a minimum 12 h fast.
Biochemical analyses
Laboratory methods for measurements of lipids, lipoproteins, and other biochemical analytes have been previously detailed (10). Insulin resistance was calculated using a homeostasis model assessment (HOMA) score. Plasma apoC-III was determined by using a turbidimetric immunoassay kit (Wako Pure Chemicals Industries, Osaka, Japan); interassay coefficients of variations (CVs) were <4.3%. Plasma VLDL and HDL apoC-III were determined by electroimmunodiffusion using a Hydragel LP CIII electroimmunodiffusion kit (Sebia, Moulineaux, France); interassay CVs were <5.0% (12). Plasma total apoA-I and apoA-II concentrations were determined by immunonephelometry (Dade Behring, IL). Plasma apoA-V concentration was determined using a dual-antibody sandwich ELISA (Linco Diagnostic Services, MO).
Isolation and measurement of isotopic enrichment of apoC-III
Early kinetic studies using radiolabeled apoC-III demonstrated exchange and equilibration of apoC-III between TRL and HDL particles in both normal and hyperlipidemic subjects (6, 13, 14). Furthermore, in vitro studies have demonstrated that the transfer of apoC-III between VLDL and HDL particles is bidirectional and that all of the apoC-III appears to be available for exchange such that equilibrium is acquired (15). Other radioisotope studies have suggested nonequilibrating pools of apoC-III that do not exchange between VLDL and HDL (16, 17). These studies, however, used exogenously labeled VLDL and HDL, which may not uniformly label all of the apoC-III present on the lipoprotein particles. Furthermore, the differences between the VLDL and HDL specific activity–time curves were small and may represent limitations in the measurement of apoC-III mass or in apoC-III isolation.
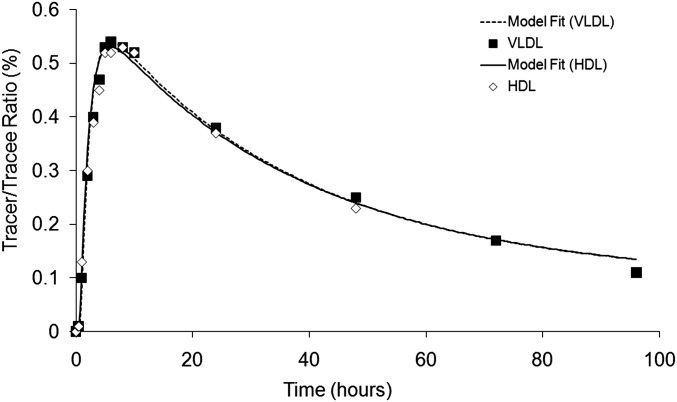
We have recently demonstrated that VLDL and HDL apoC-III have similar tracer enrichment curves and fractional catabolic rates (FCR) in both normolipidemic and overweight–obese subjects (12). Consistent with our earlier studies, we found that the VLDL and HDL apoC-III enrichment curves in CKD subjects were superimposable (Fig. 1) and that the FCRs of apoC-III in VLDL and HDL fractions were not significantly different in CKD subjects (VLDL vs. HDL, 0.74 ± 0.12 vs. 0.73 ± 0.11, pools/day, respectively, P = 0.574, n = 7 CKD subjects). Our tracer kinetic findings in these three different subject populations do not provide evidence of nonexchangeable pools of apoC-III. Therefore, our data support the rapid exchangeability of apoC-III between VLDL and HDL particles. We acknowledge, however, that there may be different pools of apoC-III on VLDL and/or HDL particles (15). Nonetheless, based upon the apoC-III tracer enrichment data that we have generated, the kinetics of apoC-III are not different in VLDL and HDL fractions. We have, therefore, used the VLDL apoC-III enrichment curve, in part because of its ease of isolation compared with plasma and HDL apoC-III, as a measure of leucine enrichment to represent plasma apoC-III.
Fig. 1.
Isotope enrichment of apoC-III of VLDL (squares) and HDL (diamonds), expressed as tracer-to-tracee ratios, is shown for up to 96 h after a single bolus injection of 3D-leucine in a representative CKD subject.
Briefly, 3 ml of plasma was used for isolation of 1 ml of VLDL (<1.006 kg/l) fractions by sequential ultracentrifugation at 40,000 rpm in a Ti 50.4 rotor (Optima LE-80K; Beckman Coulter, Australia). The VLDL samples were then prepared for isoelectric focusing (IEF) gel electrophoresis, as described previously (17). VLDL (200 μl) from each time point was then delipidated and reconstituted in 50 ml of IEF sample buffer (8 M urea; 0.001% w/v bromphenol blue). ApoC-III was isolated by preparative IEF gel electrophoresis (8 M urea; 7.5% acrylamide; 1.5% ampholytes [pH 4–6]; run for 16 h at 200 V at 4°C). Gels were electroblotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon; Millipore) at 700 mA for 1 h using a Hoefer TE 42 transfer unit (Amersham Biosciences, Australia) and stained with Coomassie Brilliant Blue R 250 (12).
IEF resolves apoC-III into three isoforms, apoC-III0, apoC-III1, and apoC-III2. ApoC-III1 was investigated in this study because of its greater concentration in plasma, and observations from previous studies showed that the kinetics of apoC-III isoforms were similar (6). All references to apoC-III kinetics will correspond to the kinetics of apoC-III1. The apoCIII1 protein bands were excised from the PVDF membrane and hydrolyzed in 200 μl of 6 M HCl overnight at 110°C in pyrolysis-cleaned half-dram vials. Samples were dried at 110°C and derivatized using a modified oxazolinone method. The oxazolinone derivatives were analyzed by negative ion chemical ionization gas chromatography-mass spectrometry. The isotopic enrichment was determined as the tracer-to-tracee ratio of monitored selected ions at m/z ratios of 212 and 209. The average CV of apoC-III tracer measurement, including processes associated with isolation of apoC-III from plasma through to the measurement of isotopic enrichment, was 5.4% (12).
Kinetic analyses
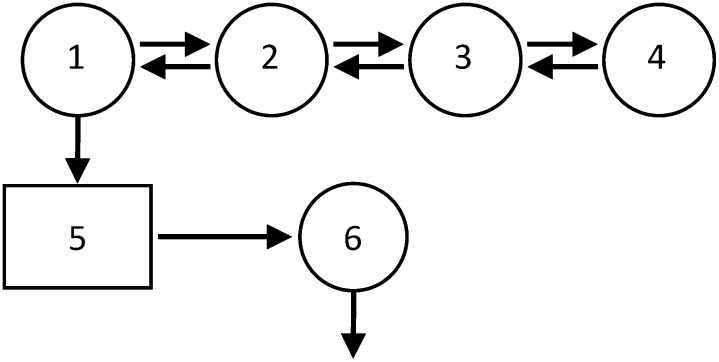
A model of plasma apoC-III metabolism (Fig. 2) was developed using Simulation, Analysis, and Modeling II software (SAAM; University of Washington, Seattle, WA) (18). The model consists of a four compartment subsystem (compartments 1–4) that describes plasma leucine kinetics. This subsystem is connected to an intrahepatic delay compartment (compartment 5) that accounts for the time required for the assembly, synthesis, and secretion of apoC-III into plasma. The kinetics of apoC-III are described by a plasma compartment (compartment 6). The FCR of plasma apoC-III, equivalent to the irreversible loss from compartment 6, was estimated after fitting the model to the apoC-III tracer data. The production rate (PR) of apoC-III was calculated as the product of the FCR and the pool size, which equals the plasma concentration multiplied by plasma volume; plasma volume was estimated as 4.5% of body weight.
Fig. 2.
Compartment model describes apoC-III tracer kinetics. Leucine tracer is injected into plasma (compartment 2) and distributes to extravascular compartments (compartments 1, 3, and 4). Compartments 1–4 are required to describe leucine tracer kinetics observed in plasma. Compartment 1 is connected to an intracellular delay compartment (compartment 5) that account for the synthesis, assembly, and secretion of apoC-III. Compartment 6 describes the kinetics of plasma apoC-III.
Statistical analyses
Skewed variables were logarithmically transformed where appropriate. Statistical analyses were performed using PASW Statistics version 17 software (SPSS Software, Chicago IL). Data are presented as means ± standard errors of the mean (SEM), unless stated otherwise. Group comparisons were performed using independent t-tests. Statistical associations were examined using simple and multiple linear regression methods. Pvalues are reported, with statistical significance set at the 5% level.
RESULTS
Clinical and biochemical characteristics of CKD subjects and healthy controls are shown in Table 1. The two groups were matched for age, body weight, BMI, and waist circumference. Blood pressure, plasma glucose, and insulin values and HOMA scores did not differ significantly between groups (Table 1). CKD subjects had significantly higher serum creatinine levels and lower eGFRs (P < 0.01, Table 1). None of the subjects had the apoE2/E2 genotype. The average daily energy intake amounts and the proportions of energy from protein, fat, carbohydrate, and alcohol were not different between groups (CKD mean total energy ± SEM values were 6,958 ± 617 kJ; protein, 20% ± 2.3%; fat, 39% ± 3.6% [saturated fat, 44% ± 1.8%; polyunsaturated fat, 15% ± 1.9%; monounsaturated fat, 41% ± 0.8%]; carbohydrates, 37% ± 1.9%; and alcohol, 4.5% ± 2.7%; whereas control values were total energy, 8,190 ± 936 kJ; protein, 21% ± 2.6%; fat, 32% ± 3.9% [saturated fat, 38% ± 3.5%; polyunsaturated fat, 17% ± 2.4%; monounsaturated fat, 41% ± 1.1%]; carbohydrates, 43% ± 4.9%; and alcohol, 3.6% ± 2.1%).
TABLE 1.
Clinical and biochemical characteristics of CKD and control subjects
| CKD (n = 7) | Control (n = 7) | ||||
|---|---|---|---|---|---|
| Characteristic | Mean | SEM | Mean | SEM | P value |
| Age (years) | 58.6 | 5.1 | 63.6 | 2.3 | 0.39 |
| Gender (M/F) | 5/2 | 5/2 | 5/2 | 5/2 | |
| Weight (kg) | 69.7 | 6.2 | 69.4 | 4.7 | 0.98 |
| Waist (cm) | 90.9 | 3.2 | 89.0 | 3.1 | 0.67 |
| BMI (kg/m2) | 23.4 | 1.0 | 23.4 | 1.5 | 0.99 |
| Systolic blood pressure (mmHg) | 127.4 | 4.0 | 129.1 | 5.7 | 0.81 |
| Diastolic blood pressure (mmHg) | 72.6 | 0.8 | 73.3 | 1.5 | 0.68 |
| Serum creatinine (μmol/l) | 150.1 | 16.8 | 75.9 | 6.2 | <0.01 |
| Estimated glomerular filtration rate (ml/min) | 43.8 | 4.0 | 91.1 | 5.7 | <0.01 |
| Fasting glucose (mmol/l) | 4.9 | 0.1 | 5.1 | 0.2 | 0.31 |
| Fasting Insulin (μU/ml) | 6.5 | 0.7 | 5.7 | 1.1 | 0.56 |
| HOMA score | 1.4 | 0.1 | 1.3 | 0.3 | 0.78 |
Data are presented as means ± SEM. To convert eGFR from ml/min to ml/s, multiply by 0.0167; for conversion of glucose in mmol/l to mg/dl, divide by 0.0555; for conversion of insulin in μU/ml to pmol/l, multiply by 7.175.
Table 2 compares the lipid, lipoprotein, and apolipoprotein concentrations in CKD and control subjects. Compared with controls, CKD subjects had significantly higher plasma triglycerides, VLDL triglycerides, VLDL cholesterol, and apoB-48 and apoA-V concentrations (P < 0.05). Total cholesterol, LDL cholesterol, HDL cholesterol, non-HDL cholesterol, apoB, apoA-I, and apoA-II concentrations and LPL mass were not significantly different between CKD and control subjects.
TABLE 2.
Plasma lipid, lipoprotein, and apolipoprotein concentrations in CKD and control subjects
| CKD (n = 7) | Control (n = 7) | ||||
|---|---|---|---|---|---|
| Lipid | Mean | SEM | Mean | SEM | P value |
| Total cholesterol (mmol/l) | 5.59 | 0.46 | 5.06 | 0.30 | 0.35 |
| LDL cholesterol (mmol/l) | 2.99 | 0.34 | 2.93 | 0.20 | 0.89 |
| HDL cholesterol (mmol/l) | 1.43 | 0.16 | 1.74 | 0.19 | 0.25 |
| Non-HDL cholesterol (mmol/l) | 4.15 | 0.58 | 3.32 | 0.24 | 0.22 |
| Plasma triglycerides (mmol/l) | 2.49 | 0.73 | 0.83 | 0.10 | 0.01 |
| VLDL triglycerides (mmol/l) | 1.62 | 0.53 | 0.37 | 0.08 | <0.01 |
| VLDL cholesterol (mmol/l) | 1.14 | 0.46 | 0.18 | 0.04 | 0.01 |
| Non esterified free fatty acids (NEFA) | 0.29 | 0.05 | 0.33 | 0.03 | 0.58 |
| Plasma apoB (g/l) | 1.06 | 0.11 | 0.96 | 0.05 | 0.39 |
| Plasma apoB-48 (mg/l) | 5.11 | 0.53 | 2.86 | 0.28 | <0.01 |
| Plasma apoA-I (g/l) | 1.64 | 0.12 | 1.86 | 0.13 | 0.24 |
| Plasma apoA-II (g/l) | 0.30 | 0.02 | 0.34 | 0.02 | 0.19 |
| Plasma apoA-V (μg/l) | 105.82 | 49.31 | 13.36 | 1.23 | 0.04 |
| Lipoprotein lipase mass (ng/ml) | 48.76 | 8.04 | 58.45 | 13.88 | 0.56 |
Data are presented as means ± SEM. To convert cholesterol, HDL cholesterol, LDL cholesterol, and non-HDL cholesterol from mmol/l to mg/dl, divide by 0.0259; to convert triglycerides from mmol/l to mg/dl, divide by 0.0113.
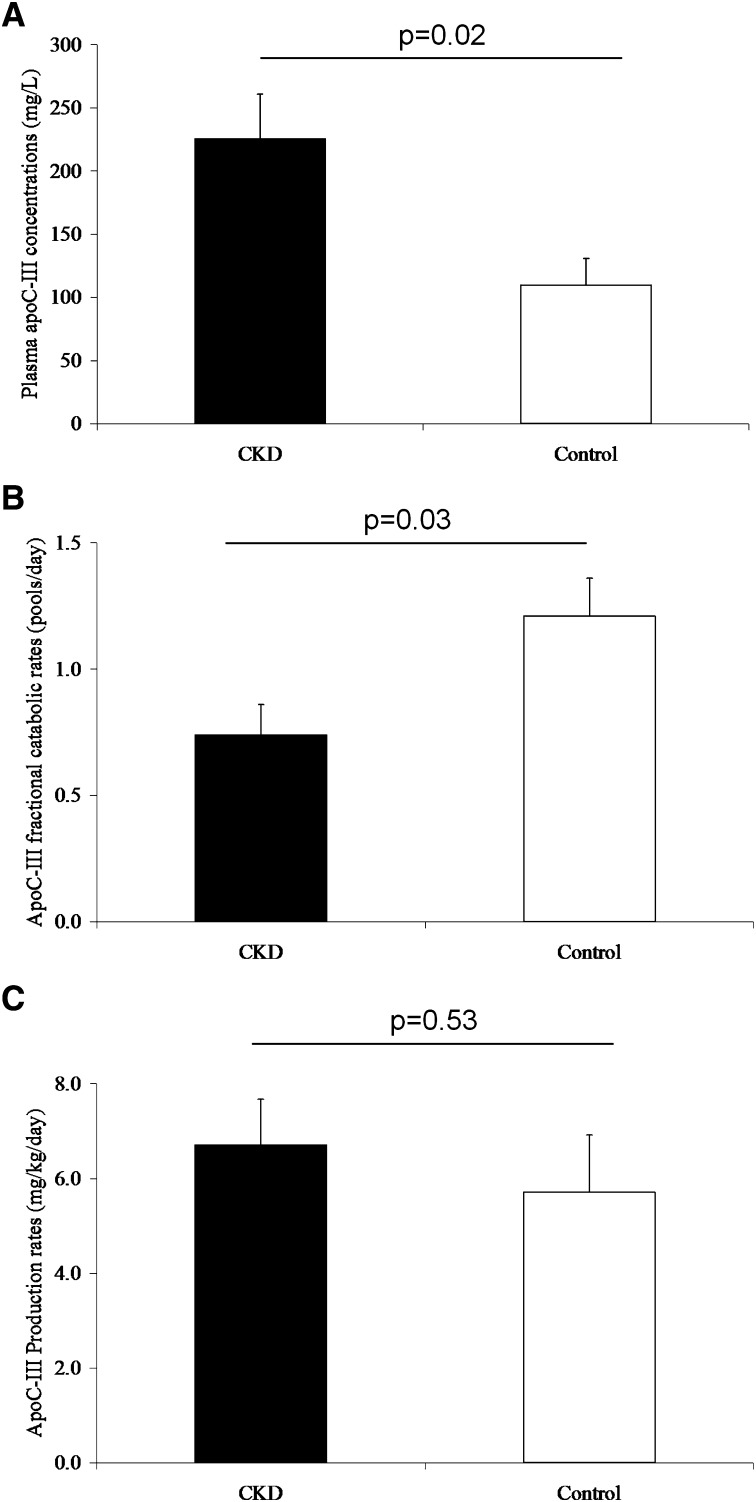
Figure 3 shows plasma concentration and kinetics of plasma apoC-III in CKD and control subjects. Compared with controls, CKD subjects had significantly higher plasma apoC-III concentrations (+106%, P = 0.02) and significantly lower plasma apoC-III FCRs (−39%, P = 0.03). There was no significant difference in plasma apoC-III PR. Compared with control subjects, CKD subjects had significantly higher VLDL apoC-III concentrations (CKD, 137 ± 40 mg/l, vs. control, 34 ± 9 mg/l, P = 0.04). No difference in HDL apoC-III concentration was observed (CKD, 88 ± 12 mg/l, vs. control, 76 ± 13 mg/l, P = 0.48).
Fig. 3.
Concentrations (A), fractional catabolic rates (B), and production rates (C) for plasma apoC-III in CKD and control subjects
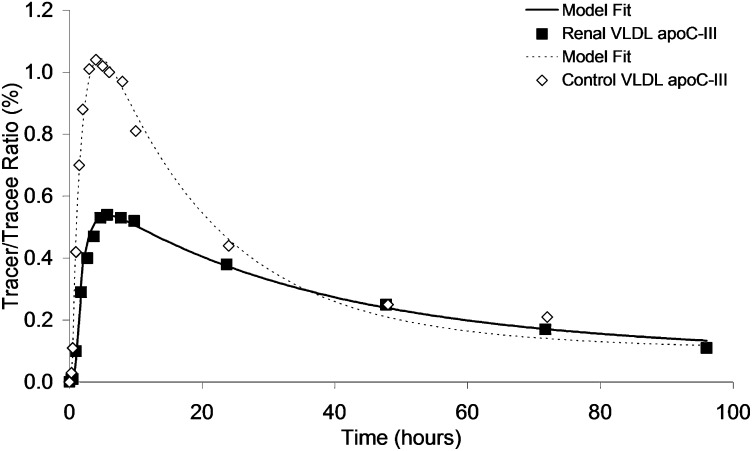
The isotopic tracer curves for plasma VLDL apoC-III after the administration of 3D-leucine in a representative CKD subject and a healthy control are shown in Fig. 4. In the CKD subject, the slower rate of appearance of tracer within the VLDL apoC-III fraction is consistent with the expansion of the apoC-III pool and the slower rate of catabolism.
Fig. 4.
VLDL apoC-III tracer enrichment curves for CKD (▪) and control (⋄) subjects.
In CKD subjects, plasma apoC-III concentration was negatively correlated with the plasma apoC-III FCR (r = −0.749, P = 0.05) but not the apoC-III PR (r = −0.491, P = 0.26). Plasma apoC-III concentration was significant and positively correlated with plasma and VLDL triglycerides (r = 0.842, P = 0.02 and r = 0.786, P = 0.04, respectively) and VLDL cholesterol (r = 0.833, P < 0.01). The plasma apoC-III FCR was significantly and negatively correlated with plasma and VLDL triglycerides (r = −0.782, P = 0.04, and r = −0.777, P = 0.04, respectively) and VLDL cholesterol (r = −0.802, P = 0.03).
Given the role of apoC-III in regulating TRL metabolism, associations between plasma apoC-III and VLDL apoB kinetic parameters in CKD subjects were explored. The kinetics of apoB-containing lipoproteins in the same CKD subjects were reported previously (10). Table 3 shows the associations between apoC-III concentration and FCR and PR and VLDL (total and subpopulations VLDL1 and VLDL2) apoB concentrations and kinetic parameters. Plasma apoC-III concentration was positively correlated with concentrations of total VLDL apoB, VLDL1 apoB, and VLDL2 apoB and negatively correlated with their respective FCRs. Plasma apoC-III FCR was negatively correlated with the concentrations of total VLDL apoB, VLDL1-apoB, and VLDL2 apoB and positively correlated with their respective FCRs. There was no association between plasma apoC-III concentration and FCR with VLDL apoB PR. No significant associations were observed between plasma apoC-III PR with VLDL apoB kinetic parameters.
TABLE 3.
Association between plasma apoC-III and VLDL apoB concentrations and kinetic parameters in 7 CKD subjects
| Plasma apoC-III concentration | Plasma apoC-III FCR | Production rate | ||||
|---|---|---|---|---|---|---|
| Plasma | r | Pvalue | r | Pvalue | r | Pvalue |
| Concentration | ||||||
| Total VLDL apoB | 0.841 | 0.02 | −0.768 | 0.04 | 0.077 | 0.869 |
| VLDL1 apoB | 0.916 | <0.01 | −0.804 | 0.03 | 0.143 | 0.759 |
| VLDL2 apoB | 0.814 | 0.03 | −0.75 | 0.05 | 0.063 | 0.893 |
| Fractional catabolic rate | ||||||
| Total VLDL apoB | −0.832 | 0.02 | 0.909 | <0.01 | 0.176 | 0.705 |
| VLDL1 apoB | −0.763 | 0.05 | 0.887 | <0.01 | 0.196 | 0.674 |
| VLDL2 apoB | −0.800 | 0.03 | 0.894 | <0.01 | 0.119 | 0.799 |
| Production rate | ||||||
| Total VLDL apoB | 0.112 | 0.811 | 0.399 | 0.376 | 0.501 | 0.252 |
| VLDL1 apoB | 0.827 | 0.224 | 0.144 | 0.759 | 0.739 | 0.060 |
| VLDL2 apoB | 0.275 | 0.551 | 0.248 | 0.592 | 0.436 | 0.328 |
Additional analyses showed that in CKD subjects, plasma apoA-V was significantly and positively correlated with plasma and VLDL triglycerides (r = 0.861, P = 0.03; and r = 0.820, P = 0.05, respectively).
DISCUSSION
We demonstrate for the first time that elevated plasma apoC-III concentration in subjects with moderate CKD is chiefly a consequence of impaired catabolism of apoC-III. Moderate CKD was not associated with changes in apoC-III synthesis. We also demonstrate that elevated apoC-III concentration is associated with elevated VLDL cholesterol and VLDL triglyceride concentrations. These results add further to our work on the impact of CKD on apoB-containing lipoprotein metabolism (10).
Dysregulation of lipoprotein metabolism may develop early in CKD with altered apolipoprotein concentrations despite normal plasma lipid concentrations (4). Of note, Kimak and Solski (19) reported that elevated plasma apoC-III concentrations, particularly the accumulation of apoC-III in VLDL particles, occur in moderate CKD (19). Compositional changes in apoB-containing lipoprotein may render these particles less suitable as substrates for hepatic receptor-mediated uptake (20). Consistent with this notion, cellular studies report that apoC-III can abolish apoB- and apoE-mediated binding of lipoproteins to the LDL receptor, either by masking or altering the conformation of apoB and apoE (21, 22). The binding of chylomicrons and VLDL particles to the lipolysis-stimulated receptor is also significantly inhibited by apoC-III (23). We recently reported that a higher apoC-III concentration was associated with slower VLDL, VLDL1, and VLDL2 apoB catabolism in moderate CKD (10). Therefore, elevated apoC-III concentrations may explain the delayed catabolism and accumulation of TRL particles in CKD.
Elevated apoC-III concentration in moderate CKD was primarily a function of impaired apoC-III fractional catabolism. Holdsworth et al. (24) showed that renal impairment was associated with excess sialylation of apoC-III, which may render apoC-III-containing TRL particles less suitable as substrates for lipolytic degradation. Furthermore, the kidney is partly involved in the removal of apoC-III from plasma (24). Hence, the reduction in apoC-III fractional catabolism could partly be a consequence of moderate CKD. Pooled analysis of controls and CKD subjects showed a strong positive association between apoC-III catabolism and eGFR (r = 0.569, P < 0.01). Similar associations were observed in CKD subjects but failed to reach statistical significance. In addition, protein carbamoylation (25) and accumulation of advanced glycation end products (26) in the setting of CKD have been shown to alter protein structure, enzymatic activities, and binding to cell surface receptors. Hence, modification of apoC-III structure and binding could contribute to altered apoC-III catabolism in CKD.
Decreased apoC-III fractional catabolism was associated with decreased VLDL particle catabolism in moderate CKD. The potential coupling of apoC-III and VLDL apoB metabolism is consistent with the role of apoC-III as a key regulator of apoB transport, as shown by recent studies using anti-apoC-III immunoaffinity chromatography that demonstrates kinetic and structural heterogeneity of apoB-containing lipoproteins, based on apoC-III content (27). However, the fact that the FCR of apoC-III is one-fifth that of VLDL apoB suggests the presence of additional factors that may modulate the catabolism of apoC-III. It is well established that during LPL-mediated hydrolysis of VLDL triglycerides, apoC-III redistributes from VLDL to HDL and is subsequently transferred back to TRL particles (28). This may prolong the period of time apoC-III circulates in vivo and explains the difference between the kinetics of apoC-III and VLDL apoB.
The expression of the apoC-III gene is regulated in part by insulin via the effect of the promoter insulin response element (IRE) on the apoC-III gene (29). Transcription of the apoC-III gene is downregulated by insulin (30). The transcription of the apoC-III gene is also mediated by peroxisome proliferator-activated receptor (PPAR) (31). The induction of PPAR, principally the PPAR-α form, reduces apoC-III gene expression (32, 33). These experimental data suggest that in insulin-resistant states, such as the metabolic syndrome and type 2 diabetes, the apoC-III gene expression and secretion of apoC-III by the liver and intestines are likely to be dysregulated (34). In contrast, the production rates of apoC-III in moderate CKD subjects were not different from those observed in healthy normolipidemic subjects. We therefore propose that moderate CKD per se does not contribute to altered hepatic apoC-III secretion.
ApoA-V may regulate triglyceride metabolism, including VLDL assembly, LPL activity, and VLDL receptor binding (35). However, the association between apoA-V and plasma triglycerides remains unclear (35–37). Recent evidence suggests that apoA-V concentrations are elevated in hypertriglyceridemic subjects (36, 37). No studies have examined the impact of CKD on apoA-V concentration. We report for the first time that plasma apoA-V concentration is elevated in moderate CKD. Furthermore, this elevation was associated with elevated plasma and VLDL triglyceride concentrations. These observations suggest that moderate CKD could be associated with dysregulated apoA-V metabolism, which may have an impact on the metabolism of TRL (38, 39). Future studies using VLDL triglyceride kinetics, coupled with cellular studies are warranted to better understand the role of apoA-V in vivo.
Our studies have several limitations. Observational design limits the inference of causal relationships. Measurement of LPL activity in post-heparin plasma may better clarify the changes in apoC-III metabolism and in vivo LPL activity in CKD. Although methodological differences, including the method used for tracer administration, may have an impact on kinetic parameter estimation, the apoC-III kinetic parameters determined in our study are concordant with those of previous studies (34, 40). Furthermore, we studied subjects in the postabsorptive state, and further studies are required to examine the impact of CKD on apoC-III metabolism in the fed state. A key strength of our study, however, is the exclusion of overweight–obese subjects with insulin resistance/diabetes and proteinuria. This provided the opportunity to analyze the contributions of CKD alone to the regulation of apoC-III metabolism.
Elevated apoC-III concentration is a common feature of dyslipidemia in CKD subjects. In addition, it is increasingly recognized as an important risk factor for CVD in the general population and may be an important target in CKD subjects. Moderate CKD subjects have elevated plasma apoC-III concentrations, a consequence of delayed apoC-III catabolism. Altered apoC-III catabolism in moderate CKD may also impair apoB metabolism, resulting in decreased catabolism and accumulation of TRL and their remnants in vivo. Modification of apoC-III catabolism may be a new therapeutic target for reducing CVD risk in moderate CKD. Future studies to assess the effects of statins and/or fibrates on apoC-III metabolism will provide better understanding of the cardiovascular benefits of such interventions in moderate CKD (41).
Acknowledgments
The authors thank J. F. Foo for laboratory assistance and M. A. Powell for nursing support. We also thank all Royal Perth Hospital nephrologists for clinical support.
Footnotes
Abbreviations:
- deuterated leucine
- ([5, 5, 5, 2H3]leucine)
- apo
- apolipoprotein
- apoC-III
- apolipoprotein C-III
- BMI
- body mass index
- CKD
- chronic kidney disease
- CV
- coefficient of variation
- CVD
- cardiovascular disease
- FCR
- fractional catabolic rate
- IDL
- intermediate intermediate-density lipoprotein
- IEF
- isoelectric focusing
- PR
- production rate
- PVDF
- polyvinylidene fluoride
- SEM
- standard error of the mean
- TRL
- triglyceride-rich lipoprotein
This study was funded in part by a research grant from the University of Western Australia. E.M.M.O. and T.W.K.N. are supported by National Health and Medical Research Council (NHMRC) postdoctoral research fellowships. D.C.C. is supported by an NHMRC career development award. P.H.R.B. is an NHMRC Senior Research Fellow.
REFERENCES
- 1.Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Busch M., Franke S., Muller A., Wolf M., Gerth J., Ott U., Niwa T., Stein G. 2004. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int. 66: 338–347. [DOI] [PubMed] [Google Scholar]
- 3.Kaysen G. A. 2007. Hyperlipidemia in chronic kidney disease. Int. J. Artif. Organs. 30: 987–992. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson O., Attman P. O., Knight-Gibson C., Kron B., Larsson R., Mulec H., Weiss L., Alaupovic P. 1994. Lipoprotein abnormalities without hyperlipidaemia in moderate renal insufficiency. Nephrol. Dial. Transplant. 9: 1580–1585. [PubMed] [Google Scholar]
- 5.Ooi E. M., Barrett P. H., Chan D. C., Watts G. F. 2008. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin. Sci. (Lond.). 114: 611–624. [DOI] [PubMed] [Google Scholar]
- 6.Huff M. W., Fidge N. H., Nestel P. J., Billington T., Watson B. 1981. Metabolism of C-apolipoproteins: kinetics of C-II, C-III1 and C-III2, and VLDL-apolipoprotein B in normal and hyperlipoproteinemic subjects. J. Lipid Res. 22: 1235–1246. [PubMed] [Google Scholar]
- 7.Sundaram M., Zhong S., Khalil M. B., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., Yao Z. 2010. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn J. S., Tremblay M., Batal R., Jacques H., Rodriguez C., Steiner G., Mamer O., Davignon J. 2004. Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis. 177: 137–145. [DOI] [PubMed] [Google Scholar]
- 9.Chan D. T., Irish A. B., Dogra G. K., Watts G. F. 2008. Dyslipidaemia and cardiorenal disease: mechanisms, therapeutic opportunities and clinical trials. Atherosclerosis. 196: 823–834. [DOI] [PubMed] [Google Scholar]
- 10.Chan D. T., Dogra G. K., Irish A. B., Ooi E. M., Barrett P. H., Chan D. C., Watts G. F. 2009. Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J. Lipid Res. 50: 2524–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001;285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen M. N., Chan D. C., Dwyer K. P., Bolitho P., Watts G. F., Barrett P. H. 2006. Use of Intralipid for kinetic analysis of HDL apoC-III: evidence for a homogeneous kinetic pool of apoC-III in plasma. J. Lipid Res. 47: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 13.Malmendier C. L., Lontie J. F., Grutman G. A., Delcroix C. 1988. Metabolism of apolipoprotein C-III in normolipemic human subjects. Atherosclerosis. 69: 51–59. [DOI] [PubMed] [Google Scholar]
- 14.Fidge N. H., Nestel P. J. 1986. Metabolism of apolipoprotein C. Methods Enzymol. 129: 443–457. [DOI] [PubMed] [Google Scholar]
- 15.Boyle K. E., Phillips M. C., Lund-Katz S. 1999. Kinetics and mechanism of exchange of apolipoprotein C-III molecules from very low density lipoprotein particles. Biochim. Biophys. Acta. 1430: 302–312. [DOI] [PubMed] [Google Scholar]
- 16.Bukberg P. R., Le N. A., Ginsberg H. N., Gibson J. C., Rubinstein A., Brown W. V. 1985. Evidence for non-equilibrating pools of apolipoprotein C-III in plasma lipoproteins. J. Lipid Res. 26: 1047–1057. [PubMed] [Google Scholar]
- 17.Tornoci L., Scheraldi C. A., Li X., Ide H., Goldberg I. J., Le N. A. 1993. Abnormal activation of lipoprotein lipase by non-equilibrating apoC-II: further evidence for the presence of non-equilibrating pools of apolipoproteins C-II and C-III in plasma lipoproteins. J. Lipid Res. 34: 1793–1803. [PubMed] [Google Scholar]
- 18.Ooi E. M., Watts G. F., Chan D. C., Chen M. M., Nestel P. J., Sviridov D., Barrett P. H. 2008. Dose-dependent effect of rosuvastatin on VLDL-apolipoprotein C-III kinetics in the metabolic syndrome. Diabetes Care. 31: 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimak E., Solski J. 2002. ApoA- and apoB-containing lipoproteins and Lp(a) concentration in non-dialyzed patients with chronic renal failure. Ren. Fail. 24: 485–492. [DOI] [PubMed] [Google Scholar]
- 20.Saland J. M., Ginsberg H. N. 2007. Lipoprotein metabolism in chronic renal insufficiency. Pediatr. Nephrol. 22: 1095–1112. [DOI] [PubMed] [Google Scholar]
- 21.Clavey V., Lestavel-Delattre S., Copin C., Bard J. M., Fruchart J. C. 1995. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler. Thromb. Vasc. Biol. 15: 963–971. [DOI] [PubMed] [Google Scholar]
- 22.Sehayek E., Eisenberg S. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 23.Mann C. J., Troussard A. A., Yen F. T., Hannouche N., Najib J., Fruchart J. C., Lotteau V., Andre P., Bihain B. E. 1997. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated receptor. J. Biol. Chem. 272: 31348–31354. [DOI] [PubMed] [Google Scholar]
- 24.Holdsworth G., Stocks J., Dodson P., Galton D. J. 1982. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J. Clin. Invest. 69: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus L. M., Kraus A. P., Jr 2001. Carbamoylation of amino acids and proteins in uremia. Kidney Int. Suppl. 78: S102–S107. [DOI] [PubMed] [Google Scholar]
- 26.Miyata T., Sugiyama S., Saito A., Kurokawa K. 2001. Reactive carbonyl compounds related uremic toxicity (“carbonyl stress”). Kidney Int. Suppl. 78: S25–S31. [DOI] [PubMed] [Google Scholar]
- 27.Zheng C., Khoo C., Furtado J., Sacks F. M. 2010. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 121: 1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glangeaud M. C., Eisenberg S., Olivecrona T. 1976. Very low density lipoprotein. Dissociation of apolipoprotein C during lipoprotein lipase induced lipolysis. Biochim. Biophys. Acta. 486: 23–35. [PubMed] [Google Scholar]
- 29.Li W. W., Dammerman M. M., Smith J. D., Metzger S., Breslow J. L., Leff T. 1995. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J. Clin. Invest. 96: 2601–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M., Breslow J. L., Li W., Leff T. 1994. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J. Lipid Res. 35: 1918–1924. [PubMed] [Google Scholar]
- 31.Kliewer S. A., Xu H. E., Lambert M. H., Willson T. M. 2001. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog. Horm. Res. 56: 239–263. [DOI] [PubMed] [Google Scholar]
- 32.Hertz R., Bishara-Shieban J., Bar-Tana J. 1995. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J. Biol. Chem. 270: 13470–13475. [DOI] [PubMed] [Google Scholar]
- 33.Staels B., Vu-Dac N., Kosykh V. A., Saladin R., Fruchart J. C., Dallongeville J., Auwerx J. 1995. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J. Clin. Invest. 95: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan D. C., Nguyen M. N., Watts G. F., Barrett P. H. 2008. Plasma Apolipoprotein C-III Transport in central obese men: associations with very-low density lipoprotein apolipoprotein B and high-density lipoprotein apolipoprotein A-I metabolism. J. Clin. Endocrinol. Metab. 93: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong K., Ryan R. O. 2007. Characterization of apolipoprotein A-V structure and mode of plasma triacylglycerol regulation. Curr. Opin. Lipidol. 18: 319–324. [DOI] [PubMed] [Google Scholar]
- 36.Schaap F. G., Nierman M. C., Berbee J. F., Hattori H., Talmud P. J., Vaessen S. F., Rensen P. C., Chamuleau R. A., Kuivenhoven J. A., Groen A. K. 2006. Evidence for a complex relationship between apoA-V and apoC-III in patients with severe hypertriglyceridemia. J. Lipid Res. 47: 2333–2339. [DOI] [PubMed] [Google Scholar]
- 37.Talmud P. J., Cooper J. A., Hattori H., Miller I. P., Miller G. J., Humphries S. E. 2006. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 49: 2337–2340. [DOI] [PubMed] [Google Scholar]
- 38.Schaap F. G., Rensen P. C., Voshol P. J., Vrins C., van der Vliet H. N., Chamuleau R. A., Havekes L. M., Groen A. K., van Dijk K. W. 2004. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279: 27941–27947. [DOI] [PubMed] [Google Scholar]
- 39.Shu X., Nelbach L., Ryan R. O., Forte T. M. 2010. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim. Biophys. Acta. 1801: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohn J. S., Patterson B. W., Uffelman K. D., Davignon J., Steiner G. 2004. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 89: 3949–3955. [DOI] [PubMed] [Google Scholar]
- 41.Sniderman A. D., Solhpour A., Alam A., Williams K., Sloand J. A. 2010. Cardiovascular death in dialysis patients: lessons we can learn from AURORA. Clin. J. Am. Soc. Nephrol. 5: 335–340. [DOI] [PubMed] [Google Scholar]