Abstract
15-series prostaglandins (PGE2s) and isoprostanes (isoPGE2s) are robust biomarkers of oxidative stress, possess potent biological activity, and may be derived through cyclooxygenase or free radical pathways. Thus, their quantification is critical in understanding many biological processes where PG, isoPG, or oxidative stress are involved. LC/MS/MS methods allow a highly selective, sensitive, simultaneous analysis for prostanoids without derivatization. However, the LC/MS/MS methods currently used do not allow for simultaneous separation of the major brain PGE2/D2 and isoPGE2 without derivatization and multiple HPLC separations. The developed LC/MS/MS method allows for the major brain PGE2/PGD2/isoPGE2 such as PGE2, entPGE2, 8-isoPGE2, 11β-PGE2, PGD2, and 15(R)-PGD2 to be separated and quantified without derivatization. The method was validated by analyzing free and esterified isoPGE2 in mouse brains fixed with head-focused microwave irradiation before or after global ischemia. Using the developed method, we report for the first time the esterified isoPGE2 levels in brain tissue under basal conditions and upon global ischemia and demonstrate a nonreleasable pool of esterified isoPG upon ischemia. In addition, we demonstrated that PGE2s found esterified in the sn-2 position in phospholipids are derived from a free radical nonenzymatic pathway under basal conditions. Our method for brain PG analysis provides a high level of selectivity to detect changes in brain PG and isoPG mass under both basal and pathological conditions.
Keywords: prostaglandin, isoprostane, phospholipid, brain lipids, prostanoid analysis, ischemia, microwave irradiation, high performance liquid chromatography, tandem mass spectrometry
Prostaglandins (PGs) are important signaling molecules that are ubiquitously produced in many tissues and regulate many physiological functions under both normal and pathophysiological conditions. In the brain, they modulate synaptic plasticity through modulation of adrenergic, noradrenergic, and glutamatergic neurotransmission and regulation of membrane excitability (1–4). In addition, PGs are involved in a broad array of nervous system diseases including cancer, inflammation, neurodegenerative diseases, central nervous system injury, and neuropsychiatric conditions (5–12). The physiological importance of PGs in mediating pain, fever, and inflammation is highlighted by the use of a wide array of inhibitors of PG biosynthesis.
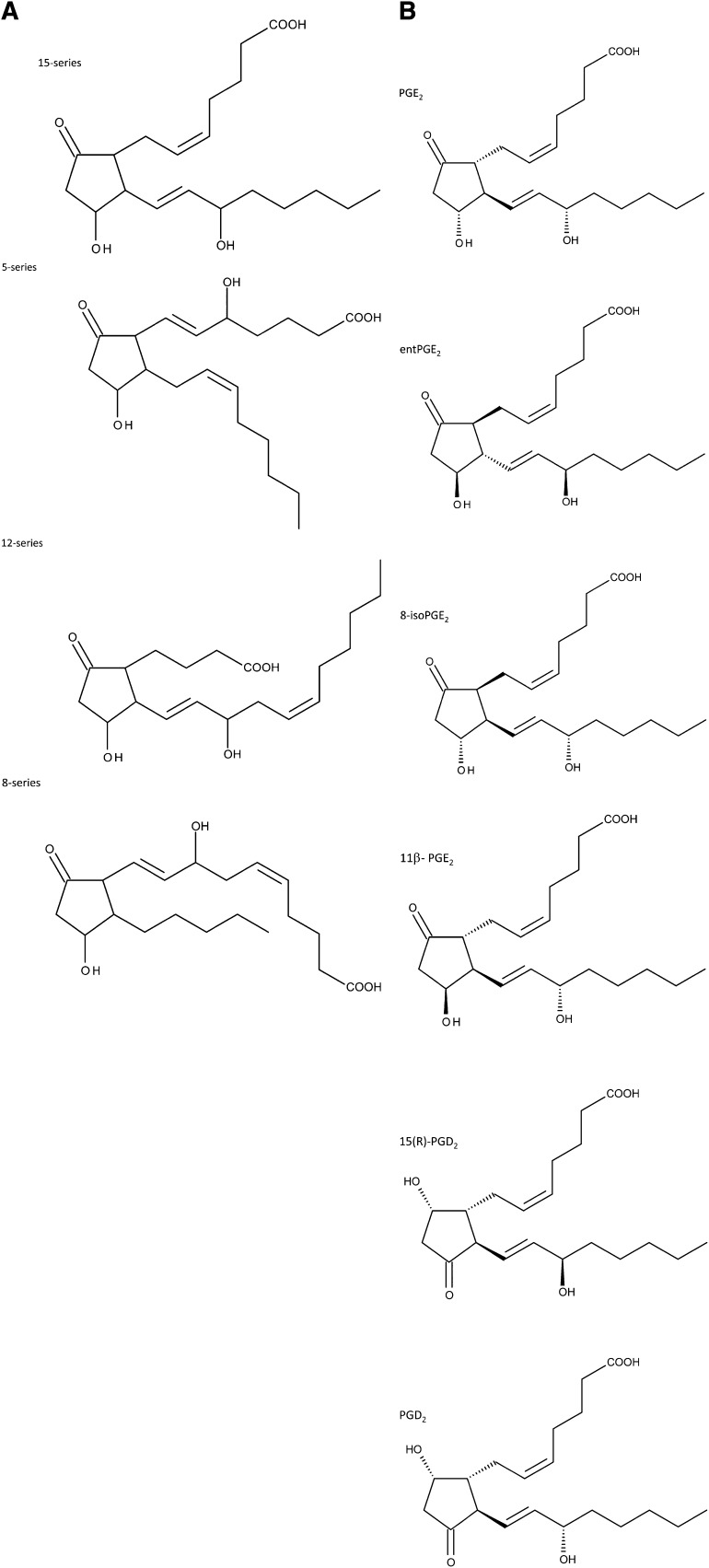
PGs are produced in two distinguished pathways: enzymatically through a cyclooxygenase (COX)1/2 dependent mechanism, and nonenzymatically through an isoprostane (isoPG) mechanism (13). In the isoPG pathway, PGs and isoPGs are formed in situ via the nonenzymatic oxidation of esterified arachidonic acid (20:4n-6) and remain in the esterified form on phospholipids (PLs) (13–17). Thus, PLs contain both PG and PG-like isoPG in the esterified form. There are four positional isoforms of isoPG that belong to 5-, 12-, 8-, and 15-series with COX1/2 derived PGE2 belonging to the 15-series (18) (Fig. 1A). Because isoPGE2/D2 have four chiral centers, there are 16 possible chiral isoforms within each group. Within the 15-series isoPGE2, the most thermodynamically stable are PGE2, PGE2 enantiomer (entPGE2), 8-isoPGE2, and 11β-PGE2 that are found in abundance in tissues (13, 19–21) (Fig. 1B). Although isoPGs are stable robust biomarkers of oxidative stress (22, 23), 15-series isoPG might also possess potent biological activity (15, 21). Esterified PGE2 and isoPGE2 might be released from PL by platelet activating factor acetylhydrolase II (24) that is activated at early stages of tissue stimulation (25). Because the initial rapid increase in PG mass triggers downstream phases of tissue response upon stimulation (26–28), the released PG and isoPG from PL at early stages of tissue stimulation may also have a critical role during the later phases of tissue response. Because of the important role for PGE2 and 15-series isoPGE2 in normal and pathophysiological processes, as well as biomarkers of oxidative stress, their quantification is critical in understanding many biological processes where PG, isoPG, or oxidative stress are involved.
Fig. 1.
Positional and selected chiral isoforms of isoPGE2 and PGD2. A: Positional isoforms for isoPGE2. For simplicity, stereochemical orientation is not indicated in the figure. B: Selected isoPGE2 and isoPGD2 stereoisoforms used in the study.
A number of methods have been used for brain and other tissue-esterified isoPGE2 quantification. Liquid chromatography with tandem mass spectrometer detection (LC/MS/MS) methods are considered to be the most specific and less laborious for isoPG measurement as compared with enzyme immunoassay and radioimmunoassay methods, high performance liquid chromatography (HPLC) with ultraviolet or fluorescence detection, and gas chromatography with MS or flame ionization detection because of a better isomer separation and no derivatization or multi-step purification requirements (29, 30). However, the LC/MS/MS methods currently used do not allow for simultaneous separation of the major brain PGE2/D2 and isoPGE2 such as PGE2, entPGE2, PGD2, 8-isoPGE2, and 11β-PGE2 without derivatization and multiple HPLC separations (13, 20, 29, 31–36).
In the present study, we developed an LC/MS/MS method that allows for the major brain PGE2/D2 series molecules such as PGD2, 15(R)-PGD2, PGE2, entPGE2, 8-isoPGE2, and 11β-PGE2 to be separated and quantified without derivatization and validated the method by analyzing free and esterified isoPGE2 in brains fixed with head-focused microwave irradiation before or after global ischemia. Using the developed method, we report for the first time esterified isoPGE2 levels in brain tissue under basal conditions and upon global ischemia and demonstrate a nonreleasable pool of isoPG esterified onto brain PL. In addition, we demonstrate that PGE2s found esterified in the sn-2 position in PL are derived from free radical nonenzymatic pathway under basal conditions.
MATERIALS AND METHODS
Chemicals
9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic acid (PGE2), 9-oxo-11β,15R-dihydroxy-(8β,12α)-prosta-5Z,13E-dien-1-oic acid (entPGE2), 9-oxo-11β,15S-dihydroxyprosta-5Z,13E-dien-1-oic acid (11β-PGE2), 9-oxo-11α,15S-dihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid (8-isoPGE2), 9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic-3,3,4,4-2H4 acid (PGE2-d4), 9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic-17,17,18,18,19,19,20,20,20 -2H4 acid (PGE2-d9), 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid (PGD2), 9α,15R-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid (15(R)-PGD2), 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic-3,3,4,4-2H4 acid (PGD2-d4), and arachidonic acid (20:4n-6) were purchased from Cayman Chemical Co. (Ann Arbor, MI). Other chemicals of analytical or higher quality were from Merck KGaA (Darmstadt, Germany).
Animals
This study was conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication 80-23) and under an animal protocol approved by the IACUC at the University of North Dakota (Protocol #0903-1 and #0806-1). Male CD1 strain mice (30–35 g) were maintained on standard laboratory chow diet and water ad libitum. The ages of the mice used in this study were between 8-11 months.
Brain prostanoid extraction
To analyze PG under basal conditions, male mice were anesthetized with isoflurane (1-3%) and euthanized by head-focused microwave irradiation (3 kW, 1.33 s; Cober Electronics, Inc., Norwalk, CT) to heat denature enzymes in situ. To model global ischemia, mouse brains were subjected to head-focused microwave irradiation 5 min after decapitation (29, 37, 38). The whole brain was frozen in liquid nitrogen and pulverized under liquid nitrogen temperatures to a fine homogeneous powder.
Prostanoids were extracted with acetone using liquid/liquid extraction as previously described (29). This method demonstrates an ∼90% of extraction efficiency and demonstrates the greatest sensitivity during MS analysis as the result of reduction of basal noise (29). Briefly, pulverized tissue was homogenized in 3 ml of acetone/saline (2:1) containing 100 pg of PGE2-d4 as an internal standard and 0.005% of butylated hydroxytoluene (BHT) to prevent fatty acid oxidation using a Tenbroeck tissue grinder (Kontes Glass Co., Vineland, NJ). After 10 min of centrifugation (2000 g) at 4°C, the supernatant was washed using 3 × 2.0 ml of hexane, acidified with formic acid to pH = 3.5 (30 μl of 2M formic acid), and extracted with 2 ml of chloroform. Chloroform extract containing PG was transferred to silanized with Sigmacote® (Sigma Chemical Co., St. Louis, MO) tube, flushed with nitrogen, and cooled at −80°C for at least 15 min. This cooling allowed the separation of any residual upper phase, which was then removed and discarded before analysis.
Sample preparation for LC/MS/MS
Samples were prepared for LC/MS/MS analysis as previously described (29). After the residual upper phase was discarded, 200 μl of methanol was added to the extract and dried down under a stream of nitrogen. The dried extract was transferred to 300 μl silanized microvial inserts (National Scientific, Rockwoods, TN) using 2 × 0.1 ml of chloroform containing 10% methanol. The solvent in microvial inserts was dried down under a stream of nitrogen and redissolved in 30 μl of acetonitrile:water (1:2) for isoPG separation. For chiral chromatography of PGE2 and entPGE2, 12 μl of 35% acetonitrile was used to redissolve samples.
HPLC of isoPGE2 and PGE2
The separation of isoPGE2 and PGE2 was carried out using a Luna C-18(2) column (3 μm, 100 Å pore diameter, 150 × 2.0 mm, Phenomenex, Torrance, CA) with a stainless steel frit filter (0.5 μm) and security guard cartridge system (C-18) (Phenomenex). The HPLC system consisted of an Agilent 1100 series LC pump equipped with a wellplate autosampler (Agilent Technologies, Santa Clara, CA). The autosampler was set at 4°C. A 25 μl out of 30 μl sample was injected onto a chromatographic column.
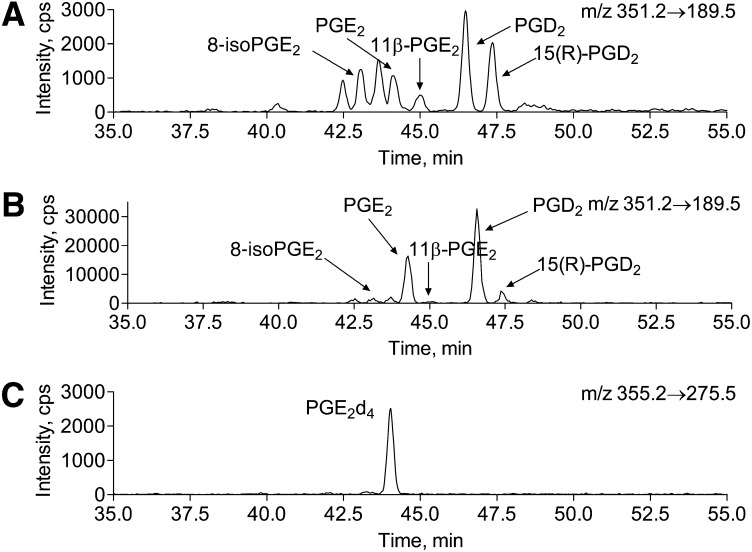
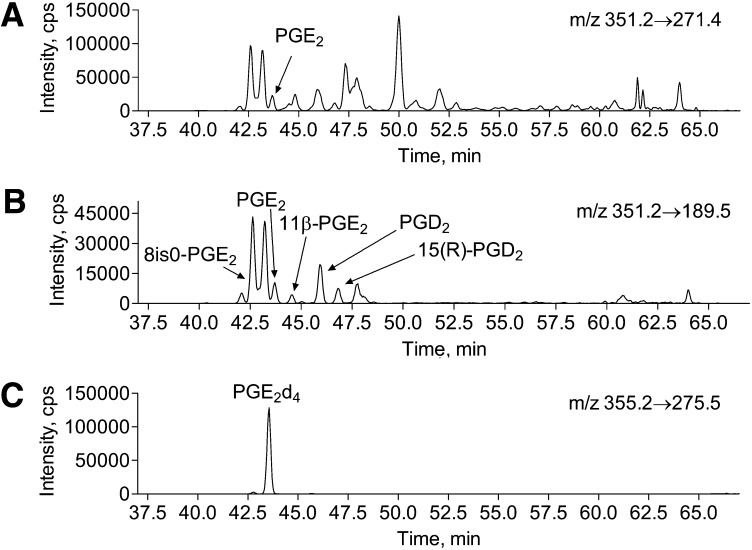
The solvent system was composed of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The flow rate was 0.2 ml/min. Solvent B was increased from 20% to 42.5% over 50 min, at 50 min was increased further to 90% over 10.5 min to wash the column, and at 65.5 min, it was returned to 20% over 1 min for column equilibration. Equilibration time between runs was 14 min. An example of brain PG LC/MS/MS analysis is presented in Fig. 8.
Fig. 8.
HPLC of brain tissue total (free and esterified) isoPGE2/D2 under basal conditions and upon 5 min of global ischemia. Mouse brain total (free and esterified) PGs were extracted from fixed mouse brain tissue before or after 5 min of global ischemia. PG extracts were separated on C18(2) Luna column as described in the Materials and Methods. A: PG separation from brain tissue (20 mg) fixed with head-focused microwave irradiation under basal conditions. B: PG separation from brain tissue (10 mg) fixed with head-focused microwave irradiation after 5 min of global ischemia. C: Extracted ion chromatogram of internal standard PGE2d4.
Esterified PG determination
Brain samples (∼35.5 mg) were incubated in 200 μl of buffer (80 mM Hepes, pH 7.4, containing 300 mM sodium chloride, 20 mM CaCl2, 8 mM Triton X-100, 60% glycerol, and 2 mg/ml BSA) containing 100 pg of PGE2d4 with or without secretory (s)PLA2 (∼0.9 μmole/min of total activity). This enzyme was tested for contamination with isoPG and no contamination was detected in the quantities of enzyme used in the experiments. To validate completeness of PG hydrolysis from PL under these conditions, a time course between 1 to 240 min was built. Esterified PGs were completely released during 1 h of incubation (data not shown). In addition, there was no nonenzymatic hydrolysis during tissue incubation in the buffer without sPLA2. After 1 h of incubation at ambient temperature, PGs were extracted and quantified as described above. The difference between prostanoid levels with and without sPLA2 treatment represents prostanoids esterified onto the sn-2 position in PL.
Chiral HPLC of PGE2 enantiomers
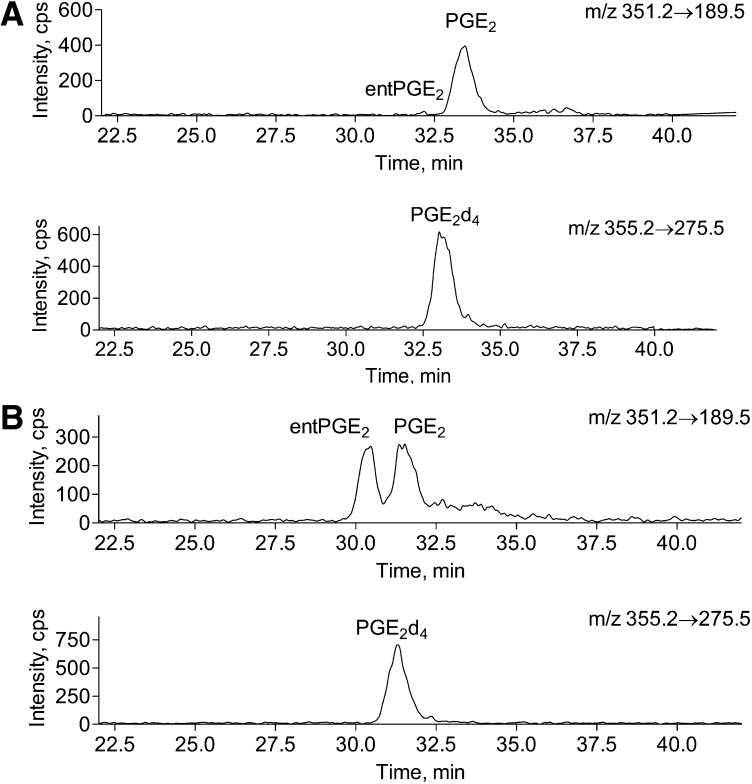
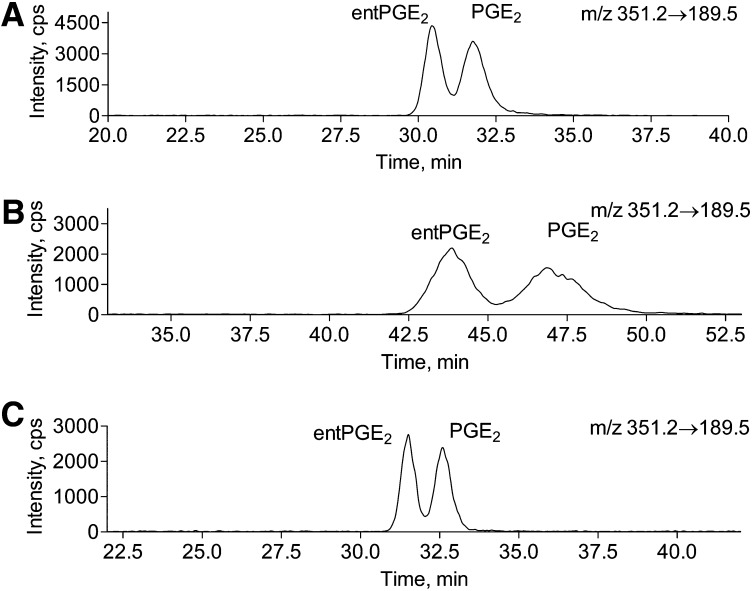
The separation of PGE2 and entPGE2 was carried out on two chiral Lux Amylose2 columns connected in tandem (3 μm, 100 Å pore diameter, 150 × 2.0 mm; Phenomenex). Tissue PG extracts were separated on a C18(2) Luna column as described above. PGE2, entPGE2, and coeluted internal standard PGE2d4 fractions from HPLC run (∼150 μl) were collected, dried down in rotary vacuum concentrator, redissolved in 12 μl of 35% acetonitrile in water, and 11 μl were loaded onto the column. PGs were eluted at 50 μl/min with isocratic 35% acetonitrile in 0.1% aqueous formic acid solvent system. An example of brain entPGE2 LC/MS/MS analysis is presented in Fig. 6.
Fig. 6.
Chiral HPLC of brain tissue PGE2 and entPGE2 under basal conditions and upon global ischemia. Brain PGs were extracted from fixed mouse brain tissue before or after 5 min of global ischemia. PG extracts were separated on a C18(2) Luna column as described in Materials and Methods. PGE2, entPGE2, and coeluted internal standard PGE2d4 fractions from an HPLC run (Fig. 8) were collected, dried down in a rotary vacuum concentrator, redissolved in 12 μl of 35% acetonitrile in water, and 11 μl were loaded onto two chiral Lux Amylose2 columns connected in tandem. PGs were eluted at 50 μl/min with 35% acetonitrile in 0.1% aqueous formic acid. Please note the difference in the sample size between A and B. A: Brain tissue (20 mg) fixed with head-focused microwave irradiation after 5 min of global ischemia. Upper panel: extracted ion chromatogram for isoPGE2. Lower panel: extracted ion chromatogram for internal standard PGE2d4. B: Brain tissue (whole brain, 300 mg) fixed with head-focused microwave irradiation under basal conditions. Upper panel: extracted ion chromatogram for isoPGE2. Lower panel: extracted ion chromatogram for internal standard PGE2d4.
Electrospray ionization mass spectrometry
For PG quantification, a quadrapole mass spectrometer (API3000, AB Sciex, Foster City, CA) equipped with TurboIonSpray ionization source was used. Analyst software version 1.5.1 (Applied Biosystem) was used for instrument control, data acquisition, and data analysis. The mass spectrometer was optimized in the multiple reaction-monitoring mode. The source was operated in negative ion electrospray mode at 350°C, electrospray voltage was −4250 V, nebulizer gas was zero grade air at 8 L/min, and curtain gas was ultrapure nitrogen at 11 L/min. Declustering potential, focusing potential, and entrance potential were optimized individually for each analyte. The quadrupole mass spectrometer was operated at unit resolution. For high resolution exact mass analysis of PG product ions, API5500Q (AB Sciex) in product ion scan mode operated in electrospray negative ionization mode was used.
PGE2 and isoPGE2 were quantified using PGE2-d4 as the internal standard. For PGE2 and isoPGE2 monitoring in the MRM mode, 351.2/189.5 mass transition was used, and for PGE2d4, 355.2/275.5 was used.
Statistical analysis
All statistical comparisons were calculated using a two-way unpaired Student's t-test using GraphPad Prism 5 (Graphpad, San Diego, CA). Statistical significance was defined as <0.05. All values were expressed as mean ± SD.
RESULTS AND DISCUSSION
LC/MS/MS methods are considered to be the most specific and less laborious for PG and isoPG quantification as compared with other methods of analysis (29, 30). However, because PGE2, PGD2, and 15-series isoPGE2 have the same molecular weight and similar product ion spectrums, it is important to separate them in time before MS/MS detection. A number of HPLC conditions have been used to resolve PGE2/PGD2-like molecules (13, 20, 29, 31–36). However, to the best of our knowledge, none of the reported conditions allowed for PGE2, PGD2, and major isoPGE2 separation in a single run without derivatization.
Selection of MS/MS mass transition
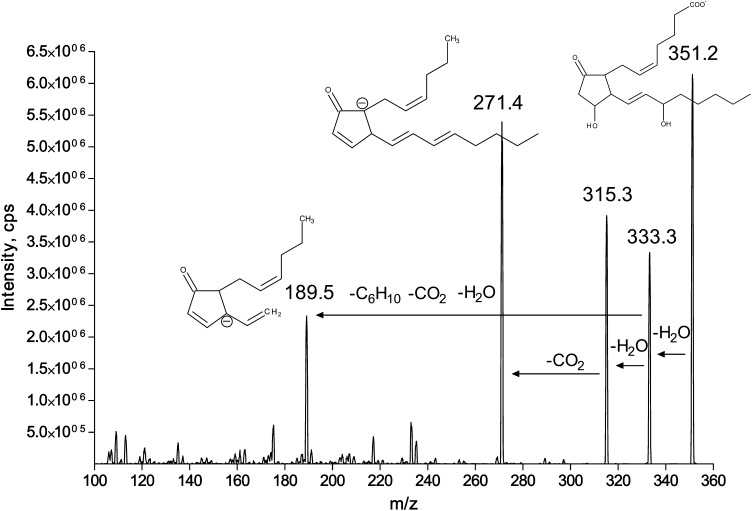
To resolve PGE2/PGD2-like molecules, we first optimized MS/MS parameters to achieve higher selectivity for 15-series isoPGE2. Most of the product ions produced from PGE2 are generated by water and carboxyl group loss (product ions 333.3, 315.3, and 271.4) that are not specific toward 15-series isoPGE2 (Fig. 2). However, a product ion with m/z = 189.5 is generated by the side chain loss (Fig. 2) that allows a more selective quantification for 15-series isoPG, thereby eliminating up to 48 possible isoforms from the analysis. The exact m/z of this product ion was determined using a high resolution mass spectrometer with linear ion trap (API5500Q) and appears to be m/z = 189.1279. This is consistent with the elemental composition of the presented structure (+1.2 mDa mass difference). The presented structure of the ion with m/z = 189 is also consistent with published data (39). In addition, PGE2 standards labeled with deuterium at 17, 17, 18, 18, 19, 19, 20, 20, 20 (PGE2d9) or at 3, 3, 4, 4 (PGE2d4) positions were used to confirm the pathway for an ion with m/z 189 generation from PGE2. Upon PGE2d9 collision, the ion with m/z = 189.5 was predominant over the 198.5 ion, whereas PGE2d4 produced mainly 193.5 rather than 189.5 product ions, thereby confirming the loss of side chain from PGE2 ω-end (data not shown). Because 5-, 12-, and 8-series isoPGE2s are not commercially available, we demonstrated the selectivity of the 189 versus 271 ion using 20:4n-6 peroxidation products. We analyzed 300 μg of 20:4n-6 standard using 351/271 and 351/189 mass transitions (Fig. 3). The 351/189 extracted ion chromatogram had less peaks indicating higher specificity of this mass transition as compared with 351/271. Although 351/271 mass transition was previously used for PGE2 and PGD2 quantification (29, 32, 34–36), the presented results indicate that 351/189 mass transition gains higher selectivity for the analysis. There were two unidentified peaks eluted before and after 8-isoPGE2 that were found in both 20:4n-6 standard and brain extracts (Figs. 3 and 8). The 315, 271, and 189 product ion ratio was similar to that of PGE2 and isoPGE2 (data not shown), indicating the possibility that these peaks are isoPGE2. Unfortunately, we were not able to identify them because none of the commercially available isoPG standards coeluted with these peaks.
Fig. 2.
MS/MS spectrum for PGE2.. Negative ion MS/MS spectrum of PGE2 standard was generated by direct infusion into API5500Q electrospray ion source.
Fig. 3.
Higher specificity of 351/189 over 351/271 mass transitions for LC/MS/MS analysis of arachidonic acid peroxidation products. Three hundred micrograms of arachidonic acid standard was extracted and analyzed after 6 months of storage in ethanol at −80°C as described in Materials and Methods. Ten nanograms of PGE2d4 was used as an internal standard. A: 351/271 extracted mass chromatogram. B: 351/189 extracted mass chromatogram. C: 355/275 extracted mass chromatogram for the internal standard PGE2d4.
Chiral chromatography for PGE2 and entPGE2 separation
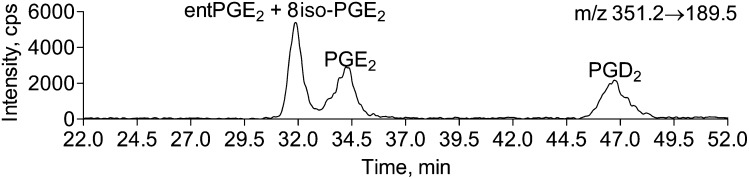
Next, to resolve 15-series isoPGE2 and PGD2, we tested a variety of columns: Luna C18(2) (Phenomenex), Luna phenyl-hexyl (Phenomenex), Lux cellulose1 (Phenomenex), Lux Cellulose2 (Phenomenex), Lux Amylose2 (Phenomenex), and Cheralpack AD-RH (Diacel Chemical) columns) and HPLC conditions (acetonitrile/water, methanol/acetonitrile, methanol/water, 2-propanol/water, 2-propanol/hexane, 2-propanol/hexane/methanol with formic acid or ammonium acetate). We first aimed to resolve PGE2 and entPGE2. Because PGE2 and entPGE2 are produced in equal quantities through free radical oxidation of 20:4n-6 (13, 19, 20), but only PGE2 is the major product of COX1/2 dependent pathway, the determination of PGE2/entPGE2 ratio might be important in determining PGE2 origin in biological samples. However, the LC/MS/MS methods used to date do not allow for separation of PGE2 and entPGE2 without derivatization and multiple HPLC separations (13, 19, 20). PGE2 and entPGE2 were not resolved on nonchiral HPLC even when the best conditions for the other isoPGE2 separation were used (Luna C18(2) column eluted with acetonitrile/water/0.1% formic acid gradient). We also could not resolve PGE2 and entPGE2 on CSP Chiralpak AD-RH column that was reported to separate enantiomers of trans-4-hydroxy-2-nonenoic acid (40). The only column that resolved PGE2 and entPGE2 was the Lux Amylose2 column when eluted with an acetonitrile/water/formic acid gradient (Fig. 4). Elution of the Lux Amylose2 column with methanol/water, or 2-propanol/water did not resolve these components. Both lower acetonitrile concentration (Fig. 4) and lower flow rate significantly increased separation. However, at a lower flow rate (50 μl/min) and acetonitrile concentration (25% in water with 0.1% formic acid) when the best separation was achieved, the peaks were broadened, resulting in reduction of a signal-to-noise ratio (Fig. 4B). The use of two columns in tandem improved peak sharpness while basal resolution was maintained (Fig. 4C). However, this HPLC method did not allow for entPGE2 and 8-isoPGE2 separation (Fig. 5). To separate PGE2 from 8-isoPGE2, preseparation on nonchiral C18(2) Luna column is required as indicated below.
Fig. 4.
The effect of acetonitrile concentration on chiral HPLC of PGE2 and entPGE2. PGE2 and entPGE2 standards (100 pg each) were loaded on one or two Lux Amylose2 columns connected in tandem and eluted using isocratic acetonitrile/water with 0.1% formic acid solvent composition at 50 μl/min. A: 27% acetonitrile, one column. B: 25% acetonitrile, one column. C: 35% acetonitrile, two columns connected in tandem.
Fig. 5.
8iso-PGE2 and entPGE2 are not resolved with chiral HPLC. PGE2, entPGE2, 8-isoPGE2, and PGD2 standards (100 pg each) were loaded on one Lux Amylose2 column and eluted with 27% acetonitrile in 0.1% formic acid aqueous solution at 50 μl/min.
PGE2 and entPGE2 determination in brain tissue
To demonstrate the application of the chiral HPLC method for biological samples, we quantified PGE2 and entPGE2 from brain tissue subjected to head-focused microwave irradiation and treated with bee venom sPLA2 to release esterified isoprostanes (15, 20). The PGE2 peak was collected from a HPLC run on the C18(2) Luna column and loaded onto the chiral Lux Amylose2 column. As indicated in Fig. 6, entPGE2 and PGE2 were generated in equal quantities (48.4 ± 3.5% and 51.7 ± 3.7%, respectively, n = 3), which is consistent with the presence of esterified isoPG onto PL (13, 19, 20). However, in brains subjected to ischemia followed by microwave irradiation, PGE2 mass was significantly higher as compared with entPGE2 (Fig. 6), which is consistent with COX1/2-dependent production of PGE2 upon ischemia (41, 42). These data indicate that PGE2 found esterified onto brain PL are derived from free radical oxidation pathway and demonstrate the appropriateness of the developed method for PGE2 and entPGE2 analysis.
Non-chiral HPLC for isoPGE2 separation
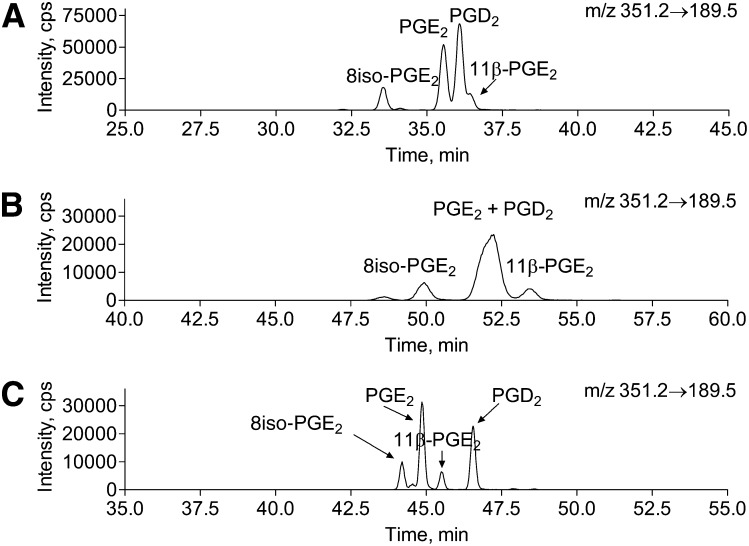
Next, because the developed chiral chromatography method did not allow for PGE2 and 8-isoPGE2 separation, we aimed to develop an HPLC method to separate major classes of 15-series of isoPGE2 and PGD2. The selection of the HPLC conditions was based on the reported methods for PG separation (13, 20, 29, 31–36). Although a number of LC/MS/MS methods have been developed, to the best of our knowledge, no methods have been reported to separate the major brain PGE2/D2 and isoPGE2 isoforms such as PGE2, PGD2, 8-isoPGE2, and 11β-PGE2 without derivatization and multiple HPLC separations (13, 20, 29, 31–36). One of the most promising columns was phenyl-hexyl column eluted with ammonium acetate/methanol gradient that has been reported to separate 8-isoPGE2 from PGE2 (35); however, we could not separate other isoPG using this approach. The only condition that we found to separate all major isoPGE2 was the 18(2) Luna column eluted with acetonitrile/water gradient with 0.1% formic acid (Figs. 7, 8). Interestingly, although addition of methanol or 2-propanol to the elution solvents increased resolving power for PG using other columns (31, 33, 40, 43–45), it decreased resolution when the C18(2) Luna column was used . For example, PGE2 and PGD2 were not separated in the presence of 2-propanol, and PGD2 and 11β-PGE2 were not separated in the presence of methanol in the elution solvents (Fig. 7).
Fig. 7.
Separation of a isoPGE2/D2 standard mixture on Luna C18(2) column using different elution solvents. One nanogram of PGE2 and 0.5 ng of PGD2, 8iso-PGE2, and 11β-PGE2 were injected on the column. The best separation conditions are presented. A: Methanol/water gradient with 0.1% formic acid. Methanol concentration was increased from 50% to 75% in 50 min. PGE2 and PGD2 were not well separated and PGD2 and 11β-PGE2 were not separated. B: 2-Propanol/water gradient with 0.1% formic acid. 2-Propanol concentration was increased from 25% to 35% in 40 min. PGE2 and PGD2 were not resolved. C: Acetonitrile/water with 0.1% formic acid gradient. Acetonitrile/water gradient was as described in Materials and Methods. All standards were well resolved at baseline.
Method validation
Limits of detection.
We have previously reported a significant improvement of detection limits for PG analysis when acetone was used for extraction (0.6 ± 0.1 pg on column) (29). Importantly, a less selective 351/271 mass transition and HPLC separation was used for PGE2 and PGD2 quantification in this study. In the present study, we evaluated whether a more selective MS/MS mass transition, as well as an improved HPLC separation, might gain better limits of detection with acetone extraction. To determine detection limits, we used PG extract from ∼35 mg of brain tissue as a biological matrix for basal noise determination. A 3:1signal-to-noise ratio was used to calculate limits of detection. Although the 189 product ion has an ∼2-fold lower intensity as compared with the less selective 271 product ion (Fig. 2), the new improved method also reduced the basal noise, thus improving detection levels to 0.3 ± 0.1 pg on column for PGE2 and PGD2.
Method accuracy.
To determine the method accuracy in biologically relevant PG concentration range found in brain samples, 0.100 or 1.000 ng of PGE2 standard was spiked with microwaved brain samples (30.2 ± 0.7 mg, n = 6) and extracted with internal standard PGE2d4. A separate group of the same brain samples was analyzed without PGE2 standard to correct for endogenous brain PGE2 mass. The PGE2 standard mass determined in the spiked samples was 0.097 ± 0.004 and 1.029 ± 0.029 ng for 0.100 and 1.000 spiked standards, respectively, indicating 3.0 ± 2.9% method accuracy in the tested mass range.
Calibration curve, linearity, and dynamic range.
Dynamic range and linearity was determined using isotope dilution approach with a constant PGE2d4 mass (100 pg) as an internal standard, and variable concentrations of PGE2 and PGD2 standard mixture. For quantification, 351/189 mass transitions were used for PGE2 and PGD2 and 355/275 for PGE2d4 quantification. The instrument response was linear in the range between 1 pg to 100 ng with R2 1.0000 and 0.9999 for PGE2 and PGD2, respectively. The calibration curve was linear within the dynamic range with the fitting curve slopes 106.4 and 110.1 for PGD2 and 15(R)-PGD2, respectively, and 193.9 to 198.5 for PGE2 and isoPGE2.
Matrix effect.
To determine matrix effect, microwaved brain samples (36.3 ± 0.7 mg) were extracted with 100 pg of PGE2d4 internal standard. Three PG extracts were spiked with 50 pg of PGE2 and three other extracts were analyzed without PGE2 standard to correct for endogenous brain PGE2 mass. A separate set of PGE2 standard samples (100 pg) was analyzed without having been spiked with brain extracts. PGE2 peak area was determined after LC/MS/MS analysis. The matrix effect was determined as a percent difference between PGE2 standard peak area with and without spiking with brain extract after correction for endogenous PGE2 mass. The corrected integrated peak area for PGE2 spiked with tissue extract was 9783 ± 229, and for PGE2 standard alone was 9496 ± 127. Although the relative standard deviation was very low, in the range of 2%, these values were not statistically different between samples. In addition, no peaks interfering with PGE2d4 internal standard were detected on chromatograms (data not shown). These data indicate very little matrix effect from brain tissue samples used in the analysis and a high method precision for LC/MS/MS analysis of spiked standards.
Method precision and sample stability.
To determine the method precision for endogenous brain PGE2, we determined intra- and inter-day variability calculated as a relative standard deviation for brain samples. Two mouse brains subjected to microwave fixation were removed, frozen in liquid nitrogen, and pulverized together under liquid nitrogen temperatures to a fine homogeneous powder. Mixed brain powder samples (35.5 ± 0.6 mg) were incubated with or without sPLA2 as described in Materials and Methods and analyzed after extraction with acetone for free and esterified PG quantification. When PG analysis was performed on the same day, the average intra-day variability was 11 ± 5% (n = 10) for esterified PG and 31 ± 14% (n = 10) for free PG (Table 1). When the same brain powder was analyzed on the following day, the inter-day variability was 10 ± 5% for esterified PG and 22 ± 22% for free PG (Table 1). For most of the isoPG analyzed, the concentration was decreased when brain samples were analyzed on the following day. However, this decrease was not statistically different. Importantly, we have observed statistically significant 2- to 4-fold reduction in PG mass after 4 weeks of brain powder storage at −80°C, indicating significant instability of PG in brain samples (29). Free PG had higher intra-day variability that might be the results of their low concentrations under basal conditions (∼1 pg/sample) that were close to the limits of detection. This variability might be decreased by the increased sample mass used for the analysis. To address this possibility, we analyzed three samples (20.3 ± 0.2 mg, n = 3) from the same mouse brain subjected to 5 min of brain ischemia followed by head-focused microwave irradiation. The free PGE2 and PGD2 mass was 11.03 ± 0.33 and 25.54 ± 0.97ng/gram wet weight (gww), respectively, with ∼4% of variability. These data indicate a significant improvement of the method precision with increased PG mass in the samples. In addition, because isoPGs are products of free radical peroxidation that might be effected by sample handling, the sample handling might be also an important factor effecting method precision.
TABLE 1.
Intra- and inter-day precision for brain endogenous PG analysis
| Day 1 | Day 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Free | Esterified | Free | Esterified | Inter-day Difference | ||||||
| Mean ± SD | RSD | Mean ± SD | RSD | Mean ± SD | RSD | Mean ± SD | RSD | Free | Esterified | |
| ng/gww | % | ng/gww | % | ng/gww | % | ng/gww | % | % | ||
| PGE2 | 0.07 ± 0.01 | 11 | 1.67 ± 0.12 | 7 | 0.08 ± 0.01 | 13 | 1.39 ± 0.11 | 8 | 8 | −17 |
| 11β-PGE2 | 0.02 ± 0.01 | 43 | 0.50 ± 0.07 | 14 | 0.03 ± 0.01 | 43 | 0.47 ± 0.07 | 15 | 10 | −6 |
| 8-isoPGE2 | 0.05 ± 0.02 | 45 | 1.57 ± 0.19 | 12 | 0.05 ± 0.13 | 26 | 1.36 ± 0.23 | 17 | −8 | −13 |
| PGD2 | 0.06 ± 0.01 | 22 | 1.81 ± 0.11 | 6 | 0.06 ± 0.01 | 19 | 1.71 ± 0.7 | 4 | −12 | −5 |
| 15(R)-PGD2 | 0.05 ± 0.02 | 48 | 1.31 ± 0.15 | 12 | 0.04 ± 0.17 | 43 | 1.18 ± 0.08 | 7 | −19 | −20 |
Two mouse brains were fixed with head-focused microwave irradiation and pulverized together under liquid nitrogen temperatures to a fine homogeneous powder. The mixed brain powder samples (∼35 mg) were analyzed in triplicate on the same day (Day 1) and the following day (Day 2). Samples were incubated with or without sPLA2 as described in Materials and Methods and analyzed after extraction with acetone. PGE2d4 was added before incubation as an internal standard. The difference between prostanoid levels with and without sPLA2 treatment represents PG esterified onto the sn-2 position in PL. Inter-day difference was calculated as percent difference between average values for Day 1 and Day 2. SD, standard deviation; RSD, relative standard deviation.
Brain tissue isoPG separation and quantification under basal conditions and upon ischemia
To demonstrate the application of the method for biological sample analysis, we analyzed free isoPGE2 and isoPGE2 esterified onto PL in brain tissue fixed with microwave irradiation before (basal conditions) or 1 min after ischemia (29, 37). Although free isoPGE2/D2 levels are reported upon decapitation-induced ischemia (38), to the best of our knowledge, no previous studies addressed esterified isoPG levels in fixed or ischemic brain tissue. To release esterified isoPG from the sn-2 position of PL, tissue samples were treated with bee venom sPLA2 as described in Materials and Methods (15, 20). The difference between prostanoid levels with and without sPLA2 treatment represents prostanoids esterified onto the sn-2 position in PL. Under basal conditions, esterified PG and isoPG were 10- to 32-fold higher as compared with free isoPG (Table 2). Upon ischemia, free PGE2 and PGD2 were significantly increased with a more profound increase in PGD2 mass (Table 2), which is consistent with previous studies (29, 37, 38, 46). Total (free plus esterified) isoPGs were significantly increased ∼2-fold, which is consistent with increased oxidative stress upon ischemia (47, 48) and consistent with the increase in free isoPGE2/D2 levels in decapitated rat brains (38). The found values for brain isoPG are in the range of the previously reported values for rat liver (15). The increase in total isoPG was mainly accounted for by the increase in free isoPG levels that were increased 15- to 43-fold. However, the esterified pool was not decreased upon ischemia (Table 2). This is intriguing because PLA2 are activated upon ischemia (49, 50), and at least one PLA2 platelet activating factor acetylhydrolase II has been reported to hydrolyze isoPG from PL (24). It remains to be elucidated if the stability of the esterified isoPG pool upon ischemia is the result of reesterification of free isoPG, a lack of PLA2 activity toward esterified isoPG upon ischemia, or a balance between release and esterification processes.
TABLE 2.
Brain PG alterations upon decapitation induced ischemia
| Basal Conditions | Ischemia | |||||
|---|---|---|---|---|---|---|
| Free | Esterified | Total | Free | Esterified | Total | |
| PGE2 | 0.06 ± 0.01 | 1.55 ± 0.13 | 1.62 ± 0.43 | 14.52 ± 0.44 | 0.87 ± 0.37 | 15.39 ± 0.35 |
| 11β-PGE2 | 0.09 ± 0.04 | 0.87 ± 0.31 | 0.97 ± 0.29 | 1.36 ± 0.15* | 0.52 ± 0.33 | 1.88 ± 0.28* |
| 8-isoPGE2 | 0.08 ± 0.02 | 1.22 ± 0.4 | 1.30 ± 0.41 | 1.93 ± 0.47* | 0.73 ± 0.31 | 2.66 ± 0.28* |
| PGD2 | 0.06 ± 0.01 | 1.92 ± 0.10 | 1.98 ± 0.12 | 31.21 ± 0.53 | 1.02 ± 0.22 | 32.23 ± 0.62 |
| 15(R)-PGD2 | 0.05 ± 0.02 | 1.25 ± 0.17 | 1.51 ± 0.20 | 2.13 ± 0.32 | 1.65 ± 0.85 | 3.78 ± 0.75 |
Mouse brain was fixed with head-focused microwave irradiation before and after 5 min of global ischemia as described in Materials and Methods and pulverization under liquid nitrogen temperature. Samples of ∼20 mg tissue were incubated with or without sPLA2 and extracted with acetone as described in Materials and Methods. PGE2d4 was added before incubation as an internal standard. The difference between prostanoid levels with and without sPLA2 treatment represents prostanoids esterified onto the sn-2 position in PL. Data are mean (ng/gww) ± SD, n = 3. * indicates statistically different values as compared with basal conditions.
In summary, the developed method allows for a complete separation and quantification of the major 15-series isoPGE2 and PGD2 without derivatization that works sufficiently when a standard mixture or endogenous brain tissue isoPGs are analyzed. Using the developed LC/MS/MS method, we demonstrated that PGE2s found esterified in the sn-2 position in PL are derived from a free radical nonenzymatic pathway under basal conditions. We report for the first time values for esterified isoPGE2 levels in brain tissue under basal conditions and upon global ischemia and demonstrated a nonreleasable pool of isoPG esterified onto brain PL upon ischemia.
Acknowledgments
The authors thank Dr. Johnie Brown (AB Sciex) for assistance with MS/MS spectrum generation on API5500Q mass spectrometer.
Footnotes
Abbreviations:
- 20:4n-6
- arachidonic acid
- BHT
- butylated hydroxytoluene
- COX
- cyclooxygenase
- entPGE2
- 9-oxo-11β,15R-dihydroxy-(8β,12α)-prosta-5Z,13E-dien-1-oic acid
- isoPG
- isoprostane
- PG
- prostaglandin
- PGD2
- 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid
- 15(R)-PGD2
- 9α,15R-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid
- PGE2
- 9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic acid
- 11β-PGE2
- 9-oxo-11β,15S-dihydroxyprosta-5Z,13E-dien-1-oic acid
- 8-isoPGE2
- 9-oxo-11α,15S-dihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid
- PGE2-d4
- 9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic-3,3,4,4-2H4 acid
- PGE2-d9
- 9-oxo-11α,15S-dihydroxyprosta-5Z,13E-dien-1-oic-17,17,18,18,19,19,20,20,20 -2H4 acid
- PL
- phospholipid
- PLA2
- phospholipase A2
- sPLA2
- secreted phospholipase A2
This work was supported by the National Institutes of Health Grant 5R21NS064480-02 (M.Y.G.), North Dakota Experimental Program to Stimulate Competitive Research (ND EPSCoR) grant EPS-0814442 (M.Y.G.), University of North Dakota New Faculty Scholar Award (M.Y.G.), and the National Institutes of Health-funded Centers of Biomedical Research Excellence (COBRE) Mass Spec Core Facility Grant 5P20RR017699. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Phillis J. W., Horrocks L. A., Farooqui A. A. 2006. Cycloxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Rev. 52: 201–243. [DOI] [PubMed] [Google Scholar]
- 2.Bazan N. G., Colangelo V., Lukiw W. J. 2002. Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat. 68–69: 197–210. [DOI] [PubMed] [Google Scholar]
- 3.Chen C., Bazan N. G. 2005. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 77: 65–76. [DOI] [PubMed] [Google Scholar]
- 4.Minghetti L., Pocchiari M. 2007. Cyclooxygenase-2, prostaglandin E 2, and microglial activation in prison diseases. Int. Rev. Neurobiol. 82: 265–275. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe L. S. 1982. Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J. Neurochem. 38: 1–14. [DOI] [PubMed] [Google Scholar]
- 6.Demediuk P., Saunders R. D., Anderson D. K., Means E. D., Horrocks L. A. 1985. Membrane lipid changes in laminectomized and traumatized cat spinal cord. Proc. Natl. Acad. Sci. USA. 82: 7071–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E. J., Behrmann D. L., Bates C. M., Horrocks L. A. 1994. Lipid alterations following impact spinal cord trauma in the rat. Mol. Chem. Neuropathol. 23: 13–26. [DOI] [PubMed] [Google Scholar]
- 8.Backlund M. G., Mann J. R., DuBois R. N. 2005. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E 2. Oncology. 69: 28–32. [DOI] [PubMed] [Google Scholar]
- 9.Hata A. N., Breyer R. M. 2004. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103: 147–166. [DOI] [PubMed] [Google Scholar]
- 10.Rocha P. N., Plumb T. J., Coffman T. M. 2003. Eicosanoids: lipid mediators of inflammation in transplantation. Springer Semin. Immunopathol. 25: 215–227. [DOI] [PubMed] [Google Scholar]
- 11.Patrignani P., Tacconelli S., Schiulli M. G., Capone M. L. 2005. New insights into COX-2 biology and inhibition. Brain Res. Brain Res. Rev. 48: 352–359. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberger T. A., Villacreses N. E., Hovda J. T., Boestti F., Weerasinghe G., Wine R. N., Harry G. J., Rapoport S. I. 2004. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J. Neurochem. 88: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 13.Gao L., Zackert W. E., Hasford J. J., Danekis M. E., Milne G. L., Remmert C., Reese J., Yin H., Tai H-H., Dey S. K., et al. 2003. Formation of prostaglandins E2 and D2 via the isoprostane pathway. J. Biol. Chem. 278: 28479–28489. [DOI] [PubMed] [Google Scholar]
- 14.Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., Ii 1990. A series of prostaglandin F 2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalized mechanism. Proc. Natl. Acad. Sci. USA. 87: 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow J. D., Minton T. A., Mukundan C. R., Campbell M. D., Zackert W. E., Daniel V. C., Badr K. F., Blair I. A., Roberts L. J., Ii 1994. Free radical-induced generation of isoprostanes in vivo: evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 269: 4317–4326. [PubMed] [Google Scholar]
- 16.Rokach J., Kim S., Bellone S., Lawson J. A., Praticò D., Powell W. S., FitzGerald G. A. 2004. Total synthesis of isoprostanes: discovery and quantitation in biological systems. Chem. Phys. Lipids. 128: 35–56. [DOI] [PubMed] [Google Scholar]
- 17.Morrow J. D., Awad J. A., Boss H. J., Blair I. A., Roberts L. J., Ii 1992. Non-cyclooxygenase-derived prostanoids (F 2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 89: 10721–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow J. D. 2006. The isoprostanes - unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr. Pharm. Des. 12: 895–902. [DOI] [PubMed] [Google Scholar]
- 19.Morrow J. D., Roberts L. J., Daniel V. C., Awad J. A., Mirochnitchenko O., Swift L. L., Burk R. F. 1998. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanesin vitro and in vivo–effects of oxygen tension and glutathione. Arch. Biochem. Biophys. 353: 160–171. [DOI] [PubMed] [Google Scholar]
- 20.Morrow J. D., Scruggs J., Chen Y., Zackert W. E., Roberts L. J., II 1998. Evidence that the E2-isoprostane, 15–E2t-isoprostane (8-iso-prostaglandin E2) is formed in vivo. J. Lipid Res. 39: 1589–1593. [PubMed] [Google Scholar]
- 21.Longmire A. W., Roberts L. J., Morrow J. D. 1994. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/endoperoxide receptor in humans and rats: additional evidence for the existence of a unique isoprostane receptor. Prostaglandins. 48: 247–256. [DOI] [PubMed] [Google Scholar]
- 22.Roberts L. J., II, Milne G. L. 2009. Isoprostanes. J. Lipid Res. 50: S219–S223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne G. L., Yin H., Brooks J. D., Sanchez S., Jackson Roberts Ii L., Morrow J. D. 2007. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. In Methods in Enzymology. H. A. Brown, editor. Academic Press. 113–126. [DOI] [PubMed] [Google Scholar]
- 24.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., Ii 2006. Release of free F 2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281: 4616–4623. [DOI] [PubMed] [Google Scholar]
- 25.Meyer M. C., Rastogi P., Beckett C. S., McHowat J. 2005. Phospholipase A 2 inhibitors as potential anti-inflammatory agents. Curr. Pharm. Des. 11: 1301–1312. [DOI] [PubMed] [Google Scholar]
- 26.Tzeng S. F., Hsiao H. Y., Mak O. T. 2005. Prostaglandins and cycloxygenases in glial cells during brain inflammation. Curr. Drug Targets Inflamm. Allergy. 4: 335–340. [DOI] [PubMed] [Google Scholar]
- 27.Engblom D., Ek M., Saha S., Ericsson-Dahlstrand A., Jakobsson P. J., Blomqvist A. 2002. Prostaglandins as inflammatory messengers across the blood-brain barrier. J. Mol. Med. 80: 5–15. [DOI] [PubMed] [Google Scholar]
- 28.Strokin M., Sergeeva M., Reiser G. 2004. Role of Ca 2+ -independent phospholipase A 2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int. J. Dev. Neurosci. 22: 551–557. [DOI] [PubMed] [Google Scholar]
- 29.Golovko M. Y., Murphy E. J. 2008. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J. Lipid Res. 49: 893–902. [DOI] [PubMed] [Google Scholar]
- 30.Taylor A. W., Bruno R. S., Frei B., Traber M. G. 2006. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F2-isoprostanes. Anal. Biochem. 350: 41–51. [DOI] [PubMed] [Google Scholar]
- 31.Masoodi M., Nicolaou A. 2006. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20: 3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao H., Xiao L., Park G., Wang X., Azim A. C., Christman J. W., van Breemen R. B. 2008. An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Anal. Biochem. 372: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider C., Boeglin W. E., Prusakiewicz J. J., Rowlinson S. W., Marnett L. J., Samel N., Brash A. R. 2002. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and -2. A critical role for serine 530 and valine 349. J. Biol. Chem. 277: 478–485. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R., Coste O., Geisslinger G. 2005. LC-MS/MS-analysis of prostaglandin E2 and D2 in microdialysis samples of rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 826: 188–197. [DOI] [PubMed] [Google Scholar]
- 35.Yang P., Chan D., Felix E., Madden T., Klein R. D., Shureiqi I., Chen X., Dannenberg A. J., Newman R. A. 2006. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot. Essent. Fatty Acids. 75: 385–395. [DOI] [PubMed] [Google Scholar]
- 36.Kempen E. C., Yang P., Felix E., Madden T., Newman R. A. 2001. Simultaneous quantification of arachidonic acid metabolites in cultured tumor cells using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Biochem. 297: 183–190. [DOI] [PubMed] [Google Scholar]
- 37.Golovko M. Y., Murphy E. J. 2008. Brain prostaglandin formation is increased by alpha-synuclein gene-ablation during global ischemia. Neurosci. Lett. 432: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farias S. E., Basselin M., Chang L., Heidenreich K. A., Rapoport S. I., Murphy R. C. 2008. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J. Lipid Res. 49: 1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy R. C., Barkley R. M., Zemski Berry K., Hankin J., Harrison K., Johnson C., Krank J., McAnoy A., Uhlson C., Zarini S. 2005. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal. Biochem. 346: 1–42. [DOI] [PubMed] [Google Scholar]
- 40.Brichac J., Honzatko A., Picklo M. J. 2007. Direct and indirect high-performance liquid chromatography enantioseparation of trans-4-hydroxy-2-nonenoic acid. J. Chromatogr. A. 1149: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anton R. F., Wallis C., Randall C. L. 1983. In vivo regional levels of PGE and thromboxane in mouse brain: effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 26: 421–429. [DOI] [PubMed] [Google Scholar]
- 42.Bosisio E., Galli C., Galli G., Nicosia S., Spagnuolo C., Tosi L. 1976. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex cerebellum. Prostaglandins. 11: 773–781. [DOI] [PubMed] [Google Scholar]
- 43.Saenger A. K., Laha T. J., Edenfield M. J., Sadrzadeh S. M. H. 2007. Quantification of urinary 8-iso-PGF 2 alpha using liquid chromatography-tandem mass spectrometry and association with elevated troponin levels. Clin. Biochem. 40: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 44.Schneider C., Yu Z., Boeglin W. E., Zheng Y., Brash A. R., Brown H. A. 2007. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. In Methods in Enzymology. Academic Press. 145–157. [DOI] [PubMed] [Google Scholar]
- 45.Schneider C., Boeglin W. E., Brash A. R. 2000. Enantiomeric separation of hydroxy eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 287: 186–189. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Halim M. S., Lunden I., Cseh G., Anggard E. 1980. Prostaglandin profiles in nervous tissue and blood vessels of the brain of various animals. Prostaglandins. 19: 249–258. [DOI] [PubMed] [Google Scholar]
- 47.Peters O., Back T., Lindauer U., Busch C., Megow D., Dreier J., Dirnagl U. 1998. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 18: 196–205. [DOI] [PubMed] [Google Scholar]
- 48.Marin J. G., Cornet S., Spinnewyn B., Demerlé-Pallardy C., Auguet M., Chabrier P. E. 2000. BN 80933 inhibits F2α isoprostane elevation in focal cerebral ischaemia and hypoxic neuronal cultures. Neuroreport. 11: 1357–1360. [DOI] [PubMed] [Google Scholar]
- 49.Sun G. Y., MacQuarrie R. A. 1989. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann. N. Y. Acad. Sci. 559: 37–55. [DOI] [PubMed] [Google Scholar]
- 50.Rapoport S. I. 2003. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J. Pediatr. 143: S26–S34. [DOI] [PubMed] [Google Scholar]