Abstract
Aim
To identify predictors of bacteremia in critically ill patients, to evaluate the impact of blood cultures on the outcome, and to define conditions for breakthrough bacteremia despite concurrent antibiotic treatment.
Methods
A descriptive retrospective study was performed over a two-year period (2007-2008) in the medico-surgical Intensive Care Unit (ICU) of the San Giovanni Hospital in Bellinzona, Switzerland.
Results
Forty-five out of 231 patients (19.5%) had positive blood cultures. Predictors of positive blood cultures were elevated procalcitonin levels (>2 µg/L, P < 0.001), higher severity scores (Simplified Acute Physiology Score II>43, P = 0.014; Sequential Organ Failure Assessment >4.0, P < 0.001), and liver failure (P = 0.028). Patients with bacteremia had longer hospital stays (31 vs 21 days, P = 0.058), but their mortality was not different from patients without bacteremia. Fever (t > 38.5°C) only showed a trend toward a higher rate of blood culture positivity (P = 0.053). The rate of positive blood cultures was not affected by concurrent antibiotic therapy.
Conclusions
The prediction of positive blood culture results still remains a very difficult task. In our analysis, blood cultures were positive in 20% of ICU patients whose blood was cultured, and positive findings increased with elevated procalcitonin levels, liver failure, and higher severity scores. Blood cultures drawn >4 days after the start of antibiotic therapy and >5 days after surgery could detect pathogens responsible for a new infection complication.
Sepsis is a common and threatening occurrence in the intensive care unit (ICU), where up to 35% of patients develop such a condition at some point during their stay (1). The associated mortality is 27% but exceeds 50% in cases of septic shock (1,2).
Blood cultures represent an important diagnostic tool, though they detect bacteremia in only about 50% of patients who are clinically suspected of having sepsis (2), with an even lower rate of positivity when drawn in the presence of ongoing antibiotic therapy (3-6). The presence of a blood pathogen represents a negative prognostic factor (7), but the isolation of such pathogen is crucial for verifying the appropriateness of antibiotic therapy, which is known to reduce morbidity and mortality (8,9). Furthermore, cultures of specific sites of suspected infection do not reliably predict the findings of blood cultures (10). Conversely, false-positive results from bacterial contaminants may lead to unnecessary antibiotic therapy, longer hospital stays (11), and selection of resistant microorganisms (12,13).
Most physicians have a low threshold for ordering blood cultures, regardless of concurrent antibiotics, whenever a patient develops a new fever. The same usually occurs in the case of a clinical decline that is potentially caused by infection or of laboratory signs of a worsening inflammatory state, bearing in mind that correlations are lacking between degree of fever, leukocytosis, and bacteremia (14-17).
We conducted a retrospective study in our multidisciplinary ICU in Switzerland to investigate the rate of positive blood cultures drawn from our ICU patients in case of temperature over 38.5°C and/or clinical decline with a concomitant worsening inflammatory state. We aimed to determine the influence of concurrent antibiotics, to identify predictors of bacteremia and conditions for breakthrough bacteremia despite antibiotics, and to compare our findings with those published mainly by US and Canadian university hospitals.
Methods
Setting
This descriptive retrospective study was performed in the ICU of the San Giovanni Hospital in Bellinzona, Switzerland. This is an eight-bed multidisciplinary teaching structure with about 750 adult admissions per year from internal medicine, oncology, general surgery (no cardiac surgery or organ transplantations), urology, orthopedic surgery, ear, nose, and throat, and gynecology. Considering the retrospective, non-interventional design of this quality assurance study, no informed consent was required by the Cantonal Ethics Committee.
Study population, data collection and definitions
Patients aged ≥16 years who had at least one blood culture drawn in the ICU or within 24 hours before admission were retrospectively identified by our microbiology laboratory. Hospital charts were reviewed to collect personal data (age, sex), diagnosis at admission, the Simplified Acute Physiology Score (SAPS) II (at 24 hours from ICU admission) and Simplified Organ Failure Assessment (SOFA, computed on the day of sampling) (18,19), and lengths of stay in the ICU and in the hospital (also considering pre- and post-culture values). We recorded antibiotic status (dividing samples into pre-antibiotic and antibiotic blood culture groups, with the latter drawn with concurrent antibiotics), comorbidities (hepatic failure, active malignancy, diabetes mellitus), immunodeficiency (AIDS, immunosuppressive drugs, chemotherapy, steroids), and conditions on the day of the sampling potentially associated with the outcomes of the exams (body temperature, laboratory tests, invasive mechanical ventilation, indwelling venous/arterial or urinary catheters, surgical wounds). The number and timing of blood cultures, their results, interpretation, and data regarding antibiotic therapy administered before sampling were also obtained.
A blood culture was considered positive when it yielded Staphylococcus aureus, non-viridans group Streptococci (including group A or group B Streptococci), Enterococci, enteric gram-negative bacilli, Pseudomonas spp, Bacteroides spp, or fungi. Bacteria like viridans group Streptococci, coagulase-negative Staphylococci, Propionibacterium spp, Corynebacterium spp or Bacillus spp were considered pathogens (20) only if two separate blood cultures were positive and at least two SIRS criteria (21) were fulfilled. Otherwise, they were considered contaminants and blood cultures were not included in the bacteremic group.
Specimen collection and processing
Blood samples were collected by nurses following a sterile procedure in accordance with the local protocol: 20 mL of blood was obtained for each blood culture, with 10 mL inoculated into each aerobic and anaerobic bottle. At least one blood culture (one bottle for aerobic growth and one for anaerobic growth) was drawn. Samples were processed by the Institute of Microbiology (Bellinzona) in a Bact/Alert 3D (bioMérieux SA, Marcy L’Etoile, France) automatic system at 35°C for at least seven days. Microbial growth was detected by continuously monitoring the CO2 production in the bottles. Identification of microorganisms and antibiotic susceptibility testing were performed according to standard laboratory operating procedures.
Statistical analysis
Continuous values are shown as means ± standard deviations, and medians and ranges whenever the distribution was not normal. Categorical values were expressed as counts and percentages. To compare continuous values we used t test and Wilcoxon rank-sum test if the hypothesis of normality was rejected and for categorical values we used χ2 tests. We also considered analyses for every unit of observation (series of blood cultures) to neutralize discrepancies between patients with different number of examined septic episodes and different number of blood cultures per septic episode. A series of blood cultures was defined as all blood cultures obtained from one patient on the same day. This served to identify potential predictors of bacteremia. If at least one blood culture was positive (according to the previously mentioned criteria), the series was considered positive for bacteremia. The link between bacteremia and different factors was investigated with a logistic regression model, where age and presence of antibiotic therapy were systematically incorporated into the model to reduce the effect of potential biases. All analyses were performed with Spotfire S+® 8.1 for Windows (TIBCO Software Inc. Palo Alto, CA, USA).
Results
Study population
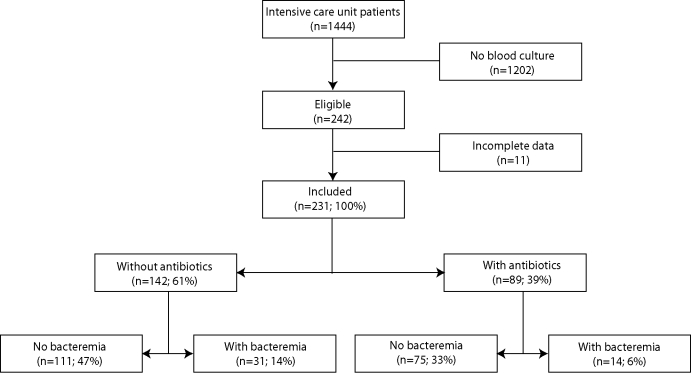
From 1444 patients admitted to the ICU from January 2007 to December 2008, 231 were eligible for the study (Figure 1), with 616 blood cultures and 310 series of blood cultures. There were 89 patients (39%) with 269 antibiotic blood cultures (44%) and 138 series of antibiotic blood cultures (45%). The blood of most patients (79%) was cultured on one day.
Figure 1.
Study population and rates of negative and positive blood cultures
Negative blood cultures were found in 186 patients (81%), with 23 contaminations, 107 of whom were not given concurrent antibiotic therapy and 79 were. Forty-five patients had at least one positive blood culture (31 patients without and 14 patients with concurrent antibiotics), resulting in a true positive rate of 19.5% and an incidence of bacteremia of 3.1 per 100 ICU admissions.
The diagnosis at admission most frequently associated with positive results (69%) was sepsis/septic shock (Table 1; about 50% of patients with sepsis/septic shock had positive blood cultures). While the length of the ICU stay was unaffected by blood culture results, there was a trend toward longer hospital stays in bacteremic patients (Table 2). ICU and hospital mortality rates were 10% and 15%, respectively, and were not affected by positive blood culture results.
Table 1.
Characteristics of patients with negative and positive blood cultures*
| No. (%) of patients |
||||
|---|---|---|---|---|
| all (n = 231) | negative (n = 186) | positive (n = 45) | P | |
| Age, mean ±SD (years) |
65 ± 16 |
64 ± 16 |
67 ± 12 |
0.583 |
| Sex: |
||||
| male |
153
(66) |
121
(65) |
32
(71) |
0.552 |
| female |
78
(34) |
65
(35) |
13
(29) |
|
| SAPS II, mean ±SD |
44.29 ± 19.78 |
42.44 ± 18.73 |
51.93 ± 21.75 |
0.007 |
| Blood cultures performed, median (range) |
2 (1-11) |
2 (1-8) |
2 (1-11) |
<0.001 |
| Diagnosis: |
||||
| sepsis/septic shock |
63
(27) |
32
(17) |
31
(69) |
<0.001 |
| non-septic shock |
10
(4) |
9
(5) |
1
(2) |
0.439 |
| cardiovascular disease |
35
(15) |
30
(16) |
5
(11) |
0.400 |
| cardiac arrest |
5
(2) |
5
(3) |
0
(0) |
0.266 |
| respiratory disease |
45
(19) |
42
(23) |
3
(7) |
0.016 |
| gastrointestinal disease |
6
(3) |
6
(3) |
0
(0) |
0.222 |
| neurological disease |
18
(8) |
15
(8) |
3
(8) |
0.754 |
| trauma |
8
(3) |
8
(4) |
0
(0) |
0.157 |
| surgery |
25
(11) |
23
(12) |
2
(4) |
0.205 |
| psychiatric disease |
3
(1) |
3
(2) |
0
(0) |
0.391 |
| others | 13 (6) | 13 (7) | 0 (0) | 0.070 |
*Abbreviations: SD – standard deviation; SAPS II – Simplified Acute Physiology Score II (18).
Table 2.
Outcomes of patients with negative and positive blood cultures*
| Patients |
||||
|---|---|---|---|---|
| all (n = 231) | negative (n = 186) | positive (n = 45) | P | |
| Length of stay in days, median (range): |
||||
| intensive care unit |
5 (1-125) |
5 (1-125) |
6 (2-46) |
0.221 |
| hospital |
17 (2-194) |
16 (2-136) |
19 (2-194) |
0.058 |
| hospital before blood culture |
1 (0-64) |
1 (0-26) |
0 (0-64) |
0.445 |
| hospital after blood culture |
14 (1-192) |
13 (1-135) |
17 (1-192) |
0.117 |
| Mortality, n (%) |
||||
| intensive care unit |
22
(10) |
18
(10) |
4
(9) |
0.872 |
| hospital | 35 (15) | 28 (15) | 7 (16) | 0.933 |
Link between bacteremia and other factors
Positive blood cultures were associated with higher severity scores (SAPS II>43 at admission and SOFA score >4 the day of sampling; cut-off levels were set at the median of the observations) and hepatic failure (Table 3). A predictive factor were also procalcitonin levels above 2 µg/L, while fever showed only a trend toward a positive correlation. Fever higher than 38.5°C had a low positive predictive value (0.23; 95% confidence interval [CI], 0.14-0.33), while absence of fever had a higher predictive value (negative predictive value, 0.87; 95% CI, 0.81-0.91) without being discriminating. Actually, afebrile bacteremia (Tmax ≤38.5°C) was identified in 13% of units of observation.
Table 3.
Potential predictors of bacteremia
| Predictor Total | No. (%) of series of BCs (310) | No. (%) of series of BCs with bacteremia | Odds ratio (95% confidence interval)* | P* |
|---|---|---|---|---|
| Antibiotic therapy: |
||||
| yes |
138
(45) |
15
(11) |
0.53
(0.27-1.04) |
0.058 |
| no |
172
(55) |
34
(20) |
1 |
|
| Age (years): |
||||
| up to 55 |
87
(28) |
8
(9) |
1 |
0.063 |
| 56 to 65 |
64
(21) |
17
(27) |
3.33
(1.32-8.40) |
|
| 66 to 75 |
87
(28) |
12
(14) |
1.55
(0.60-1.55) |
|
| ≥76 |
72
(23) |
12
(17) |
1.77
(0.67-4.66) |
|
| Sequential Organ Failure Assessment (19): |
||||
| >4 |
122
(39) |
32
(26) |
3.81
(1.95-7.44) |
<0.001 |
| ≤4 |
188
(61) |
17
(9) |
1 |
|
| Simplified Acute Physiology Score II (18): |
||||
| >43 |
150
(48) |
30
(20) |
2.25
(1.16-4.38) |
0.014 |
| ≤43 |
160
(52) |
21
(12) |
1 |
|
| Hepatic failure |
32
(10) |
10
(31) |
2.81
(1.15-6.86) |
0.028 |
| Temperature (°C): |
||||
| >38.5 |
80
(26) |
18
(22) |
1.98
(1.00-3.94) |
0.053 |
| ≤38.5 |
230
(74) |
30
(13) |
1 |
|
| White blood cells (G/L) |
||||
| >12 |
160
(52) |
22
(14) |
0.77
(0.40-1.45) |
0.411 |
| ≤12 |
150
(48) |
26
(17) |
1 |
|
| C-reactive protein (mg/L):† |
||||
| >100 |
173
(57) |
31
(18) |
1.45
(0.76-2.80) |
0.256 |
| ≤100 |
130
(43) |
18
(14) |
1 |
|
| Procalcitonin (μg/L):‡ |
||||
| >2 |
53
(38) |
15
(28) |
9.68
(1.81-51.93) |
<0.001 |
| 0.5-2 |
40
(28) |
2
(5) |
1 |
|
| <0.5 | 48 (34) | 4 (8) | 1.45 (0.23-9.35) |
*Adjusted by age and presence or absence of antibiotic therapy. BC – blood cultures.
†7 (2%) missing values.
‡169 (55%) missing values.
Factors that were not predictive of positive blood cultures were age (P = 0.063), major comorbidities (endocarditis [P = 0.379], pneumonia [P = 0.771], malignancy [P = 0.483], diabetes [P = 0.256]), immunodepression (chemotherapy [P = 0.599], immunosuppressive drugs [P = 0.139], steroids [P = 0.232]), and medical devices (central venous catheter [P = 0.508], urinary catheter [P = 0.139], drains [P = 0.829]). Indwelling arterial catheters and mechanical ventilation were actually correlated with negative blood cultures (odds ratio, 0.45; P = 0.020, and odds ratio, 0.46; P = 0.045, respectively). Seventy-three patients received invasive mechanical ventilation (36 of whom had major infections at admittance), with 242 blood cultures (176 antibiotic blood cultures, 73%). Seven of these patients (10% of patients and 9% of series of blood cultures) had positive blood cultures, independent of the length of mechanical ventilation (≤6 days vs >6 days, P = 0.101).
Positive blood cultures were not correlated with surgical wounds (P = 0.286). There were 36 surgical patients with 38 pre-antibiotic (31.7%) and 82 antibiotic blood cultures (68.3%). All 20 patients who were sampled within the first three postoperative days had negative blood cultures. All 4 patients with positive results had blood cultures drawn more than six days after surgery.
Bacteremia showed only a trend toward correlation with ongoing antibiotic therapy (P = 0.058), with no significant impact of its length: patients with negative blood cultures were treated for a mean of 6.2 days and patients with positive blood cultures were treated for a mean of 5.3 days. Nevertheless, 96.1% of blood cultures drawn within the first 72 hours were negative, and 83.3% of positive blood cultures were drawn more than 4 days after the start of antibiotic treatment. Bacteremic patients had a greater number of obtained blood cultures (Table 1) but also a greater number of series of blood cultures than non-bacteremic patients (1.7 for bacteremic patients and 1.2 for non-bacteremic patients, P = 0.009).
Microbiology analysis
Pathogens were isolated from antibiotic blood cultures in 14 of 45 patients with positive blood cultures (Table 4). Gram-positive and Gram-negative bacteria were identified in the same proportions, and fungi were identified in three singular cases. Staphylococcus aureus was the only organism that grew in antibiotic blood cultures (two patients), despite normal immune status and adequate therapy (as assessed by in vitro testing) over four and five days, respectively. In four cases, while pre-antibiotic blood cultures remained sterile, we identified pathogens in antibiotic blood cultures: coagulase-negative Staphylococcus determining two catheter-related bloodstream infections (one from a peripheral venous line and one from a portacath); coagulase-negative Staphylococcus in a patient with superinfection of an aortic graft; and Enterococcus faecium and Enterobacter aerogenes in a patient with tertiary peritonitis. Two patients had different pathogens isolated from their pre-antibiotic and antibiotic blood cultures: Enterococcus faecium after a 7-day therapy for urosepsis (previous strain: Escherichia coli) and Enterococcus faecium and Aerococcus viridans after a 3-day therapy for acute cholangitis (previous strains: Klebsiella oxytoca and Escherichia coli).
Table 4.
Pathogens isolated from blood
| Organism | Patients with positive pre-antibiotic BCs* | Patients with positive antibiotic BCs* | Concurrent therapy | Duration of therapy (days) | Susceptibility to concurrent antibiotic therapy |
|---|---|---|---|---|---|
|
Gram-positive bacteria (n = 25): |
|||||
| Staphylococcus aureus |
4 |
3 |
amoxicillin/clavulanate
imipenem/cilastatin, vancomycin‡
amoxicillin/clavulanate, gentamycin |
4
0¶
5,2 |
susceptible†
susceptible
susceptible† |
| Coagulase-negative Staphylococcus |
3 |
4 |
amoxicillin/clavulanate
amoxicillin/clavulanate
ceftriaxone
ceftriaxone, metronidazole |
9
3
2
6,2 |
resistant
resistant
resistant
resistant |
| Streptococcus pneumoniae |
3 |
0 |
|||
| Streptococcus bovis |
1 |
0 |
|||
| Enterococcus spp |
2 |
3 |
ceftriaxone, metronidazole§
amoxicillin/clavulanate
imipenem/cilastatin║ |
3
7
6 |
resistant
resistant
resistant |
| Aerococcus viridans |
0 |
1 |
ceftriaxone, metronidazole§ |
3 |
susceptible |
| Gram-positive anaerobic cocci |
1 |
0 |
|||
|
Gram-negative bacteria (n = 22): |
|||||
| Escherichia coli |
12 |
1 |
amoxicillin/clavulanate, ciprofloxacin |
0 |
susceptible |
| Enterobacter spp |
1 |
1 |
imipenem/cilastatin║ |
6 |
susceptible |
| Klebsiella spp |
3 |
1 |
amoxicillin/clavulanate |
9 |
resistant |
| Citrobacter spp |
2 |
0 |
|||
| Salmonella group E |
1 |
0 |
|||
|
Fungi (n = 3): |
|||||
| Candida glabrata |
0 |
2 |
tazobactam/piperacillin, vancomycin, fluconazole
imipenem/cilastatin, fluconazole |
11
11, 5 |
resistant
resistant |
| Candida pelliculosa |
0 |
1 |
imipenem/cilastatin, vancomycin‡ |
0 |
resistant |
| Total | 33 | 17 |
*Pre-antibiotic denotes blood cultures (BC) drawn without concurrent antibiotic therapy. Antibiotic denotes BCs drawn with concurrent antibiotic therapy. For microorganisms identified in the antibiotic BC type, duration and susceptibility to concurrent therapy are reported.
†In two cases, we observed persistent Staphylococcus aureus infection despite adequate antibiotic therapy.
‡Staphylococcus aureus and Candida pelliculosa were isolated in the same BC.
§Enterococcus spp. and Aerococcus viridians were isolated in the same BC.
║Enterococcus spp. and Enterobacter spp. were isolated in the same BC.
¶0 indicates that antibiotics were started on the same day but prior to sampling.
Discussion
Our results showed that blood cultures obtained as part of an “extended” infectious work-up in a general ICU population had a limited chance of identifying pathogens. This confirms previous data reported in large US/Canadian tertiary hospitals (10,17,22,23). In our antibiotic-free population, 20% of the series of blood cultures was positive, and 11% was positive if obtained in the presence of a concurrent antibiotic therapy. Thus, only a trend toward difference was detected based on the antibiotic status.
The overall incidence of bacteremia was 3.1 per 100 ICU admissions, a rate comparable with that reported in an adult ICU in a 25-year observation period (24). Our results support the common notion that bacteremia in ICU patients is difficult to predict. We confirmed the results of other studies (3,25) that showed that fever alone cannot be considered a solid predictor of bacteremia, as it could also be an expression of non-infectious inflammatory reactions. Conversely, an absence of fever was associated with a low rate of blood culture positivity but was not discriminating, as 13% of series of blood cultures revealed microbiological growth.
C-reactive protein and elevated white blood cells had low predictive values for bacteremia, while elevated procalcitonin levels were correlated with positive blood culture findings, confirming its diagnostic value in general ICU settings (26) and in particular clinical scenarios (27,28). However, we also identified some cases of bacteremia with procalcitonin levels below 0.5 μg/L – a level only slightly above the cut-off value for ICU patients of 0.38 μg/L (26), which further strengthens the importance of a cautious clinical approach. The definite diagnosis of sepsis might not rely on a single measurement of procalcitonin but on a complete clinical and laboratory evaluation of the patient, with procalcitonin playing a considerable role.
Among the analyzed comorbid conditions, a predictor of positive blood culture results was liver failure. Acute and chronic liver diseases are known to be associated with an increased risk of bacteremia (29-31) and have been shown to be independent risk factors for the development of bacteremia in patients with community-acquired pneumonia (32). This feature has been correlated with impaired function of the hepatic reticuloendothelial system (33), and complement (34) and polymorphonuclear cells.
We identified no associations between bacteremia and immunodeficiency or diabetes, confirming the results of Grace et al (3). Interestingly, this is in contrast with the results reported by Stoeckle et al (35), who found a relative frequency of bloodstream infections to be more than 4 times higher in diabetic than in non-diabetic general inpatients.
Bacteremia was not associated with the presence of a surgical wound or mechanical ventilation. All blood cultures obtained within 72 hours after a surgical procedure remained sterile, which is in accordance with the finding that most early postoperative febrile episodes resolved spontaneously without confirmation of infection (36). Late blood cultures (6, 7, and 15 days after surgery) disclosed infective complications in only three cases. Blood cultures obtained from patients with mechanical ventilation had very low yields. This can be explained by patient selection (about 50% were ventilated for reasons other than infection) and the known low sensitivity of blood cultures for disclosing pathogenic microorganisms in ventilator-associated pneumonia (37).
We aimed to evaluate the utility of obtaining repeat blood cultures in patients receiving antibiotic therapy with new suspected septic episodes. Antibiotic blood cultures were very often negative, particularly if the pre-antibiotic blood culture had been negative; otherwise, the same pathogen was isolated as in the pre-antibiotic cultures. Nevertheless, in our analysis, which was not limited to the first three days of antibiotic therapy but included the entire ICU stay, we identified microorganisms not found in the pre-antibiotic samples in 6.5% of patients, demonstrating that this practice is not always useless and may even be very important for detecting either polymicrobial infections or new infection complications.
Bacteremic patients were investigated more often (ie, series of blood cultures) and with more blood cultures than non-bacteremic patients. However, the number of blood cultures drawn for each suspected septic episode was the same. Thus, our data do not challenge the current guidelines (20), which propose to limit the number of blood cultures drawn per suspected septic episode to two or three. A study in an ICU (38) demonstrated that limitation of blood cultures to up to three sets reduced the number of blood cultures ordered for suspected septic episodes from 3.0 to 2.2 with no untoward effects on patient care.
Patients with nosocomial bloodstream infections were shown in one study to have a worse outcome with a longer hospital stay and an attributable mortality rate (7). Other studies (23,39-41) did not find the association of bacteremia with excess mortality but did with longer hospital stays. Our data tend to confirm the latter, as we observed only a trend toward longer hospital stays, while the length of ICU stay and mortality rates were not different between the bacteremic and non-bacteremic patients. Eventually, we cannot exclude the possibility that our study population was too small for a meaningful assessment, as demonstrated by the inconsistence of mortality, illness severity, and prevalence of bacteremia. SOFA and SAPS II scores differed between bacteremic and non-bacteremic patients, but their discriminatory capacities should be checked prospectively (eg, by calculating receiver operating characteristic curves on a larger study population).
Our study has several limitations. The retrospective observational design implies selection biases and some missing values (eg, for procalcitonin). Nevertheless, we attempted to neutralize the effects of a different number of examined septic episodes and blood cultures per septic episode by opting for a unit of observation analysis (series of blood cultures) that considered all blood cultures drawn on a single day from one patient, which allowed us to adjust the impact of clinical characteristics of a given patient compared to another patient subjected to a different number of blood cultures. Also, data collection spanned over two years and the number of patients (ie, blood cultures) was modest, thus limiting the power of the study. Although blood cultures were collected following a local standard operating procedure, we cannot rule out some technical differences. This is a critical remark, as the yields of blood cultures are known to increase when appropriate measures are applied (eg, blood volume) (42). Triggers for blood culture collection were generally mentioned in clinical records, but we cannot exclude the possibility that there were more unlisted triggers. Similarly, we cannot verify how often blood cultures were not performed despite the presence of triggers for blood culture collection.
In conclusion, blood cultures in a general ICU represent an important diagnostic tool to identify bacteremia and to guide diagnostic and therapeutic choices. According to our analysis, their rate of positivity increases with illness severity (SAPS II and SOFA scores), elevated procalcitonin levels, and the presence of hepatic failure, but does not seem to be clearly influenced by the presence of ongoing antibiotic therapy. Early blood cultures drawn in the presence of ongoing antibiotics rarely identified pathogens not found in the pre-antibiotic blood cultures. Therefore, the decision to perform a repeat blood culture should rely on careful clinical judgment, bearing in mind that blood cultures drawn 4 days after an initially appropriate antibiotic therapy and 6 days after surgery can detect new pathogens, which mainly reveal new infection complications.
Acknowledgments
Funding None.
Ethical approval Not required.
Declaration of authorship MP and MG contributed the same extent to the study conception and design and to data acquisition, analysis, interpretation, article drafting and revision for important intellectual content. They also gave the final approval. BC participated in data interpretation, data acquisition, and gave the final approval. MD participated in data interpretation, revision of the article, drafting of the article, and gave the final approval. AP contributed to study conception, revision and drafting of the article, and gave the final approval.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 2.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–74. doi: 10.1001/jama.1995.03530120060042. [DOI] [PubMed] [Google Scholar]
- 3.Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis. 2001;32:1651–5. doi: 10.1086/320527. [DOI] [PubMed] [Google Scholar]
- 4.Schermer CR, Sanchez DP, Qualls CR, Demarest GB, Albrecht RM, Fry DE. Blood culturing practices in a trauma intensive care unit: does concurrent antibiotic use make a difference? J Trauma. 2002;52:463–8. doi: 10.1097/00005373-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie R, Reimer LG. Effect of antimicrobials on blood cultures in endocarditis. Diagn Microbiol Infect Dis. 1987;8:165–72. doi: 10.1016/0732-8893(87)90167-2. [DOI] [PubMed] [Google Scholar]
- 6.Pazin GJ, Saul S, Thompson ME. Blood culture positivity: suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med. 1982;142:263–8. doi: 10.1001/archinte.1982.00340150063012. [DOI] [PubMed] [Google Scholar]
- 7.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–601. doi: 10.1001/jama.1994.03510440058033. [DOI] [PubMed] [Google Scholar]
- 8.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379–86. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 10.Schwenzer KJ, Gist A, Durbin CG. Can bacteremia be predicted in surgical intensive care unit patients? Intensive Care Med. 1994;20:425–30. doi: 10.1007/BF01710653. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365–9. doi: 10.1001/jama.1991.03460030071031. [DOI] [PubMed] [Google Scholar]
- 12.Ortega M, Marco F, Soriano A, Almela M, Martínez JA, Munoz A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63:568–74. doi: 10.1093/jac/dkn514. [DOI] [PubMed] [Google Scholar]
- 13.Zarrilli R, Crispino M, Bagattini M, Barretta E, Di Popolo A, Triassi M, et al. Molecular epidemiology of sequential outbreaks of Acinetobacter baumannii in an intensive care unit shows the emergence of carbapenem resistance. J Clin Microbiol. 2004;42:946–53. doi: 10.1128/JCM.42.3.946-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galicier C, Richet H. A prospective study of postoperative fever in a general surgery department. Infect Control. 1985;6:487–90. doi: 10.1017/s0195941700063608. [DOI] [PubMed] [Google Scholar]
- 15.Bell DM, Goldmann DA, Hopkins CC, Karchmer AW, Moellering RC., Jr Unreliability of fever and leukocytosis in the diagnosis of infection after cardiac valve surgery. J Thorac Cardiovasc Surg. 1978;75:87–90. [PubMed] [Google Scholar]
- 16.Torras-Comamala M, Aceituno-Ruiz R. Influence of axillary temperature on blood culture yield in the emergency department. Enferm Clin. 2007;17:10–6. doi: 10.1016/s1130-8621(07)71759-x. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 17.Claridge JA, Golob JF, Jr, Fadlalla AM, Malangoni MA, Blatnik J, Yowler CJ. Fever and leukocytosis in critically ill trauma patients: it is not the blood. Am Surg. 2009;75:405–10. [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Shafazand S, Weinacker AB. Blood cultures in the critical care unit: improving utilization and yield. Chest. 2002;122:1727–36. doi: 10.1378/chest.122.5.1727. [DOI] [PubMed] [Google Scholar]
- 21.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Tabriz MS, Riederer K, Baran J, Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect. 2004;10:624–7. doi: 10.1111/j.1469-0691.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- 23.Laupland KB, Davies HD, Church DL, Louie TJ, Dool JS, Zygun DA, et al. Bloodstream infection-associated sepsis and septic shock in critically ill adults: a population-based study. Infection. 2004;32:59–64. doi: 10.1007/s15010-004-3064-6. [DOI] [PubMed] [Google Scholar]
- 24.Edgeworth JD, Treacher DF, Eykyn SJ. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit Care Med. 1999;27:1421–8. doi: 10.1097/00003246-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Elekes S, Weinstein MP. Blood cultures. Infect Dis Clin North Am. 1993;7:221–34. [PubMed] [Google Scholar]
- 26.Nakamura A, Wada H, Ikejiri M, Hatada T, Sakurai H, Matsushima Y, et al. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock. 2009;31:586–91. doi: 10.1097/SHK.0b013e31819716fa. [DOI] [PubMed] [Google Scholar]
- 27.Muller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138:121–9. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 28.Koivula I, Hamalainen S, Jantunen E, Pulkki K, Kuittinen T, Nousiainen T, et al. Elevated procalcitonin predicts Gram-negative sepsis in haematological patients with febrile neutropenia. Scand J Infect Dis. 2011;43:471–8. doi: 10.3109/00365548.2011.554855. [DOI] [PubMed] [Google Scholar]
- 29.Graudal N, Milman N, Kirkegaard E, Korner B, Thomsen AC. Bacteremia in cirrhosis of the liver. Liver. 1986;6:297–301. doi: 10.1111/j.1600-0676.1986.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 30.Graudal N, Hubeck B, Bonde J, Thomsen AC. The prognostic significance of bacteremia in hepatic cirrhosis. Liver. 1987;7:138–41. doi: 10.1111/j.1600-0676.1987.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 31.Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
- 32.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–7. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 33.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg. 2000;7:40–8. doi: 10.1007/s005340050152. [DOI] [PubMed] [Google Scholar]
- 34.Unsworth DJ. Complement deficiency and disease. J Clin Pathol. 2008;61:1013–7. doi: 10.1136/jcp.2008.056317. [DOI] [PubMed] [Google Scholar]
- 35.Stoeckle M, Kaech C, Trampus A, Zimmerli W. The role of diabetes mellitus in patients with blodstream infections. Swiss Med Wkly. 2008;138:512–9. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 36.Garibaldi RA, Brodine S, Matsumiya S, Coleman M. Evidence for the non-infectious etiology of early postoperative fever. Infect Control. 1985;6:273–7. doi: 10.1017/s0195941700061749. [DOI] [PubMed] [Google Scholar]
- 37.Luna CM, Videla A, Mattera J, Vay C, Famiglietti A, Vujacich P, et al. Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator-associated pneumonia. Chest. 1999;116:1075–84. doi: 10.1378/chest.116.4.1075. [DOI] [PubMed] [Google Scholar]
- 38.Gross PA, Van Antwerpen CL, Hess WA, Reilly KA. Use and abuse of blood cultures: program to limit use. Am J Infect Control. 1988;16:114–7. doi: 10.1016/0196-6553(88)90048-X. [DOI] [PubMed] [Google Scholar]
- 39.Raymond DP, Pelletier SJ, Crabtree TD, Gleason TG, Pruett TL, Sawyer RG. Impact of bloodstream infection on outcomes among infected surgical inpatients. Ann Surg. 2001;233:549–55. doi: 10.1097/00000658-200104000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 41.Digiovine B, Chenoweth C, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med. 1999;160:976–81. doi: 10.1164/ajrccm.160.3.9808145. [DOI] [PubMed] [Google Scholar]
- 42.Cockerill FR, III, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, et al. Optimal testing parameters for blood cultures. Clin Infect Dis. 2004;38:1724–30. doi: 10.1086/421087. [DOI] [PubMed] [Google Scholar]