Through analytic synthesis of data from observational cohorts of healthcare providers from the 1920s through the 1950s, we estimate that individuals with latent tuberculosis have a 79% lower risk of tuberculosis disease following reinfection compared with initial infection.
Abstract
(See the Editorial Commentary by Vernon and Villarino, on pages 792–3.)
Background. The risk of progression to active tuberculosis is greatest in the several years following initial infection. The extent to which latent tuberculosis infection reduces the risk of progressive disease following reexposure and reinfection is not known. Indirect estimates from population models have been highly variable.
Methods. We reviewed prospective cohort studies of persons exposed to individuals with infectious tuberculosis that were published prior to the widespread treatment of latent tuberculosis to estimate the incidence of tuberculosis among individuals with latent tuberculosis infection (LTBI group) and without latent tuberculosis (uninfected; UI group). We calculated the incidence rate ratio (IRR) of tuberculosis disease following infection between these 2 groups. We then adjusted incidence for expected reactivation, proportion of each group that was infected, and median time of observation following infection during the study.
Results. We identified 18 publications reporting tuberculosis incidence among 23 paired cohorts of individuals with and without latent infection (total N = 19 886). The weighted mean adjusted incidence rate of tuberculosis in the LTBI and UI groups attributable to reinfection was 13.5 per 1000 person-years (95% confidence interval [CI]: 5.0–26.2 per 1000 person-years) and that attributable to primary infection was 60.1 per 1000 person-years (95% CI: 38.6–87.4 per 1000 person-years). The adjusted IRR for tuberculosis in the LTBI group compared with the UI group was 0.21 (95% CI: .14–.30).
Conclusions. Individuals with latent tuberculosis had 79% lower risk of progressive tuberculosis after reinfection than uninfected individuals. The risk reduction estimated in this study is greater than most previous estimates made through population models.
“Is it better to have a positive or negative tuberculin test? Show me the student who has not asked this question. I might add, show me the physician who can give an unequivocal answer.”—Charles Connor, 1940 [1].
Following infection with Mycobacterium tuberculosis, individuals may develop either clinical disease (‘primary progressive tuberculosis’) or latent tuberculosis infection (LTBI), which may later reactivate, resulting in disease (‘reactivation tuberculosis’). The risk of disease in the first 5 years following infection is greater than the risk in subsequent years [2]. Upon reinfection with M. tuberculosis, the extent to which LTBI provides protection against progressive disease is debated [3, 4].
The reduction in tuberculosis incidence following reinfection compared with primary infection has important implications for understanding and projecting the epidemiology of tuberculosis. Models have shown this to be a critical parameter [5, 6]. When drug-resistant tuberculosis strains are introduced into a community with high tuberculosis burden, their spread is limited by the size of the susceptible population, which is largely determined by the degree of susceptibility among those latently infected [7]. If latent tuberculosis provides substantial protection, the susceptible pool for infection is small and limits outbreaks. The extent of protection conferred by latent infection may also predict the efficacy of vaccines that mimic host responses to tuberculosis [8].
The principal challenge in studying M. tuberculosis reinfection is the lack of a test to demonstrate reinfection. Tuberculin skin tests (TSTs) and interferon-γ release assays cannot distinguish reinfection from prior LTBI. Therefore, studies of reinfection rely on the proxy measure of reexposure of latently infected individuals. Today, infection rates outside of high human immunodeficiency virus (HIV)-burden communities are <3% per year, making it challenging to study reinfection. In high HIV-burden communities, the negative impact of HIV on immunity likely outweighs possible protection afforded by latent infection.
Consequently, previous studies attempting to estimate the relative risk of disease following reinfection relied on population models [9–11]. Estimates using these indirect methods have ranged from 41% to 81% risk reduction. More recently, Brooks-Pollock and colleagues modeled transmission and protective immunity from cross-sectional data in households in Lima, yielding an estimate of 35% risk reduction among previously infected individuals [12].
In the 1920s, Norwegian physician Olaf Scheel observed a high incidence of tuberculosis among nursing students in their first year of clinical training [13]. After 1924, he and colleague Johannes Heimbeck performed tuberculin tests on all nursing students upon entry to their training. They noted a high incidence of infection and disease among nursing students with negative tuberculin tests at entry. This work was replicated in hospitals across the United States and Europe over the next 2 decades. The high rates of infection and lack of drugs for treatment of LTBI allowed researchers to conduct observational studies that could not be performed today.
Collectively, these studies provide direct evidence of risk of tuberculosis following reexposure among latently infected individuals. We reviewed and summarized the results of these studies; by proposing simple adjustments to account for endogenous reactivation, we estimated the risk reduction in active disease associated with prior latent infection.
METHODS
Search Strategy
We searched for English language publications that reported tuberculosis incidence among longitudinal cohorts with and without LTBI within the same study. The majority of studies was published prior to 1950 and not reliably indexed on MEDLINE or other major databases. The search strategy therefore consisted of cross-referencing publications cited by key studies in the fields of tuberculosis reinfection and infection control [14]. Cross-referencing was performed iteratively until no additional relevant publications were identified.
Study Selection
We narrowed the list of publications, including studies only if they reported the following information: (1) tuberculosis cases among latently infected (LTBI) and uninfected (UI) populations, with cases reported according to latency status; (2) annual risk of tuberculosis infection (or proportion of subjects with new tuberculosis infections during the study); (3) duration of exposure; and (4) duration of follow-up.
We excluded cohorts in which any individuals received isoniazid or Bacille Calmette-Guérin (BCG) vaccination. To focus on populations with intensive exposure, thus limiting the impact of uncertainty around latent tuberculosis reactivation, we excluded studies with annual risk of tuberculosis infection <5%.
Data Abstraction
We defined a case of active tuberculosis as any case reported as tuberculosis disease by the authors of the original papers. Diagnostic methods differed between studies; most included radiography, sputum microscopy, and clinical evaluation. Individuals with a known history of active tuberculosis were excluded. We defined cases of latent tuberculosis as subjects with a positive TST reaction in whom active tuberculosis was excluded through radiograph and clinical evaluation. Various forms of TST (purified protein derivative, old tuberculin, Pirquet test) were used, and positivity criteria (eg, size of induration) were unspecified in many studies. We accepted authors’ definitions for a “positive TST reaction.” In studies in which multiple TST doses were reported, we used findings associated with a dose of 1.0 mg old tuberculin or equivalent (0.005 mg purified protein derivative [PPD]) [15].
Annual risk of infection (ARI) with tuberculosis was abstracted if reported. In studies in which ARI was not reported, the ARI was calculated from the chain-binomial formula: ARI = 1−(1−P)1/Texp, where P is the proportion of subjects without LTBI at baseline who converted their TST, and Texp is the time period of exposure.
Two authors (J. R. A. and R. C.) independently reviewed and abstracted data from all papers; a third author (C. R. H.) reviewed and adjudicated all discrepancies.
Quantitative Data Synthesis
Incidence rate of tuberculosis was calculated by dividing the number of cases by the number of person-years of observation. The incidence rate ratio (IRR) of tuberculosis among latently infected individuals was calculated using uninfected individuals as the referent.
To estimate the incidence rates of tuberculosis in the LTBI and UI groups attributable to infection or reinfection, we made the following adjustments:
Adjusting for Proportion of Population Infected
Not all individuals in the study were infected during the study period, particularly in those studies with lower ARI. To determine the incidence of disease following infection, we divided the observed incidence by the proportion of the study population infected during the study. Most of the studies listed the proportion of tuberculin converters; in studies in which this was not reported, it was estimated through the ARI and exposure period: P = 1–(1–ARI)Texp.
Adjusting for Timing of Infection
To estimate the incidence of disease following infection, we first calculated the median time to infection (T50) by the chain-binomial formula relating prevalence and ARI. We subtracted this from the observation period to estimate the infected period: Tinf = Tobs−T50.
Adjustment for Reactivation Among Latently Infected Individuals
We subtracted the incidence rate of reactivation (Rreact) from the incidence rate of tuberculosis in the LTBI group. We used a reactivation rate of 1.5 per 1000 person-years [2].
Combining these adjustments, the adjusted incidence rate of tuberculosis among latently infected individuals (Radj,+) is:
The adjusted incidence rate among those without latent tuberculosis is (Radj,-):
where Cobs,+ and Cobs,- are observed cases among LTBI (N+) and UI (N−) subjects. As we assumed that the proportion of the population infected and the timing of infection were the same for both the LTBI and UI groups, only the adjustment for reactivation impacted the relative rates of active tuberculosis disease and therefore the IRR.
To evaluate uncertainty around estimated adjusted incidence, we performed a simulation study by drawing from parameter distributions to derive empirical distributions of incidence and their credible intervals. The observed incidence and proportion of individuals infected during the study were drawn from β distributions, whereas the reactivation rate was drawn from a binomial distribution [2]. We performed 100 000 simulations and report the median and 95% credible intervals for adjusted incidence in the LTBI and UI groups. Finally, to determine the proportion of cases attributable to reinfection among the LTBI group, we divided the adjusted incidence attributable to reinfection (Radj,+) by the adjusted incidence including reactivation.
Meta-analysis and Meta-Regression
We used a random effects model to determine the pooled adjusted IRR weighted inversely to each study variance [16]. A log transformation of the IRR was used to normalize its distribution; a small constant was added to the case estimate to avoid taking a log of 0.
We then built a mixed-effects meta-regression to assess the relationship between study characteristics and the IRR. Study characteristics examined included midpoint study year, sex of the study population, baseline prevalence of LTBI, ARI, and study population (medical students, nursing students, etc). We evaluated study heterogeneity by calculation of τ2.
Analyses were performed in R software [17]. Meta-analysis and meta-regression were performed using the metafor package in R [16].
Sensitivity Analysis
We performed sensitivity analysis on the reactivation rate by varying this parameter across the 95% confidence interval of estimates from a published meta-analysis [2]. We assessed the impact of varying the reactivation rate on IRR and proportion of cases attributable to reinfection.
RESULTS
Study Characteristics
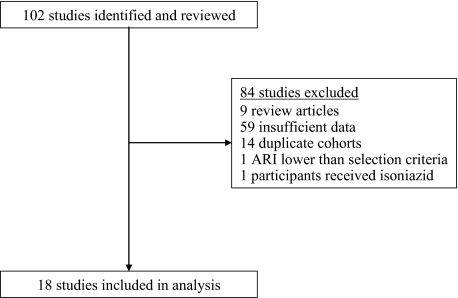
We identified and reviewed the full text of 102 studies for potential inclusion in this analysis (Figure 1). Nine review articles were excluded. Fifty-nine studies did not contain the required data elements and were excluded. Fourteen manuscripts described duplicate cohorts and were excluded. Two papers described populations with annual risk of infection below the predefined threshold and were excluded.
Figure 1.
Selection of studies for analysis. ARI, annual risk of infection.
We therefore identified 18 publications describing 23 paired observational cohorts, all published prior to 1960, of individuals with and without latent tuberculosis infection. The combined paired cohort size ranged from 112 to 2572 (median, 637; Table 1). The total number of individuals in all cohorts was 19 886, which included 10 959 TST-positive (LTBI) and 8927 TST-negative (UI) individuals.
Table 1.
Characteristics of Studies, Participants, and Tuberculosis Cases by Baseline Tuberculin Test Result
| Author, Year | Study Years | Population | No. | TST+ (n) | TST− (n) | LTBI Prev. (%) | ARI (%) | Prop. Converted | Observation Time (years) | Cases LTBI | Cases UI |
| Geer 1934 [18] | 1928–1932 | Nursing students | 112 | 42 | 70 | 37.1 | 88.2 | 1.00 | 2.7 | 1 | 5 |
| Geer 1934 [18] | 1930–1932 | Nursing students | 181 | 55 | 126 | 30.4 | 75.0 | 0.88 | 1.5 | 0 | 3 |
| Heimbeck 1938 [19] | 1924–1936 | Young women | 1458 | 467 | 991 | 32.0 | 5.5 | 0.23 | 4.7 | 14 | 52 |
| Heimbeck 1938 [19] | 1924–1937 | Nurses | 682 | 403 | 279 | 59.1 | 8.8 | 0.26 | 3.3 | 6 | 20 |
| Heimbeck 1938 [19] | 1924–1938 | Nursing students | 952 | 668 | 284 | 70.2 | 100.0 | 1.00 | 2.6 | 22 | 97 |
| Myers 1940 [20] | 1929–1938 | Nursing students | 1100 | 281 | 819 | 25.5 | 21.2 | 0.51 | 3.0 | 7 | 33 |
| Myers 1941 [21] | 1929–1936 | Medical students | 449 | 160 | 289 | 35.6 | 16.0 | 0.50 | 4.0 | 2 | 11 |
| Hastings 1941 [22] | 1929–1938 | Nursing students | 504 | 142 | 362 | 28.2 | 17.1 | 0.43 | 4.0 | 1 | 9 |
| Brahdy,1941 [23] | 1935–1940 | Nursing students | 2230 | 1320 | 910 | 59.2 | 46.9 | 0.85 | 3.0 | 6 | 34 |
| Israel 1941 [24] | 1935–1941 | Nursing students | 637 | 177 | 460 | 27.8 | 78.1 | 0.95 | 2.2 | 11 | 56 |
| Wright 1941 [25] | 1936–1940 | Nursing students | 141 | 36 | 105 | 25.5 | 34.1 | 0.72 | 3.0 | 0 | 10 |
| Schwartz 1942 [26] | 1936–1941 | Nursing students | 228 | 114 | 114 | 59.4 | 80.1 | 0.24 | 3.0 | 1 | 5 |
| Daniels1944 [27] | 1934–1943 | Nurses | 2572 | 2120 | 452 | 82.4 | 56.9 | 0.92 | 2.8 | 30 | 34 |
| Lim-Yuen 1946 [28] | 1938–1943 | Sanitorium workers | 500 | 268 | 232 | 53.6 | 33.6 | 0.53 | 1.3 | 2 | 8 |
| Madsen1942 [29] | 1934–1940 | Medical students | 1258 | 936 | 322 | 74.4 | 18.0 | 0.47 | 3.3 | 12 | 38 |
| Madsen 1942 [29] | 1934–1940 | Students | 1676 | 1135 | 541 | 67.7 | 9.0 | 0.17 | 2.0 | 5 | 14 |
| Holm 1946 [30] | 1942–1945 | Adolescent students | 199 | 105 | 94 | 52.8 | 99.6 | 0.74 | 3.0 | 4 | 7 |
| Thompson 1949 [31] | 1942–1948 | Nursing students | 503 | 249 | 254 | 49.5 | 8.0 | 0.28 | 4.0 | 7 | 17 |
| Badger 1949 [32] | 1932–1948 | Nursing students | 736 | 374 | 362 | 50.8 | 46.4 | 0.85 | 5.0 | 22 | 36 |
| Dickie 1950 [33] | 1934–1947 | Medical students | 373 | 141 | 232 | 37.8 | 28.7 | 0.74 | 5.0 | 4 | 25 |
| Dickie 1950 [33] | 1934–1944 | Nursing students | 122 | 33 | 89 | 27.0 | 30.4 | 0.66 | 4.0 | 1 | 8 |
| Poole 1954 [34] | 1939–1952 | Nursing students | 1653 | 986 | 667 | 59.6 | 92.4 | 0.73 | 4.0 | 15 | 25 |
| Karns 1959 [35] | 1934–1954 | Medical students | 1620 | 747 | 873 | 46.1 | 12.4 | 0.41 | 4.0 | 5 | 11 |
| Median | 637 | 268 | 289 | 49.5 | 33.6 | 0.66 | 3.0 | 5.0 | 17.0 | ||
| Range | (112–2572) | (33–-2120) | (70 991) | (25.5–82.4) | (5.5–100.0) | (0.17–1.00) | (1.3–5.0) | (0–30) | (3–97) |
Abbreviations: ARI, annual risk of infection; LTBI, latent tuberculosis infection; TST, tuberculosis skin test; UI, uninfected.
Nursing students and medical students comprised the majority of cohorts (56.5% and 13.0%, respectively). The prevalence of latent tuberculosis at baseline ranged from 25.5% to 82.4%, with a median of 49.5%. The mean duration of exposure and follow-up were 2.8 and 3.3 years, respectively. The mean annual risk of infection was 43.8% (range, 5.5%–100.0%).
Risk of Tuberculosis Infection
Among the LTBI cohorts, a mean of 1.6% (range, 0–6.2%) developed tuberculosis during the study periods. Among the UI cohort, a mean of 6.3% (range, Texp 1.2%–34.2%) developed tuberculosis. The incidence rates of active tuberculosis in the LTBI and UI groups were 5.1 per 1000 person-years (95% CI: 4.4–5.9 per 1000 person-years) and 18.2 per 1000 person-years (95% CI: 16.8–19.8 per 1000 person-years), respectively. The unadjusted incidence rate ratio was 0.28 (95% CI: .21–.39).
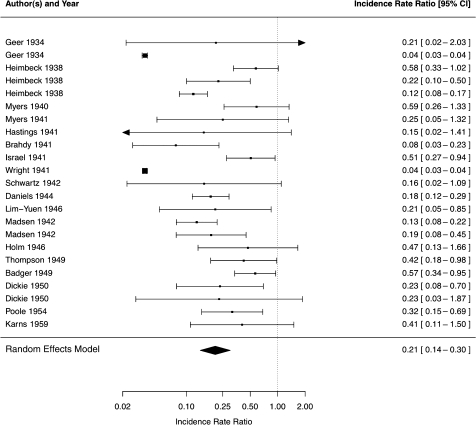
After adjusting for reactivation, the proportion of subjects infected during the study, and the average timing of infection, the adjusted incidence rates of tuberculosis in the LTBI and UI groups were 13.5 per 1000 person-years (95% CI: 5.0–26.2 per 1000 person-years) and 60.1 per 1000 person-years (95% CI: 38.6–87.4 per 1000 person-years), respectively. The weighted adjusted risk ratio was 0.21 (95% CI: .14–.30) (Figure 2). There was nontrivial heterogeneity (τ2 = 0.70) among studies.
Figure 2.
Incidence rate ratio of tuberculosis in the latent tuberculosis infection group compared with uninfected group by study, with weighted result in random effects model.
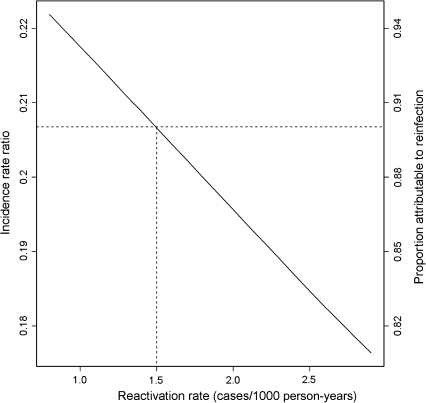
The majority (90%) of tuberculosis cases in the LTBI group were attributable to reinfection. In sensitivity analysis, varying the reactivation rate from 0.8 to 2.9 per 1000 person-years changed the IRR from 0.22 to 0.18 and the proportion of cases attributable to reinfection from 0.94 to 0.82 (Figure 3).
Figure 3.
Incidence rate ratio estimate and proportion of cases attributable to reinfection in the latent tuberculosis infection group according to reactivation rate used in the model (dotted line denotes base case).
Study Factors Associated With Incidence Rate Ratio
In a model incorporating study year, sex, baseline prevalence of LTBI, ARI, and study population, no study factors were associated with IRR.
DISCUSSION
We identified 23 observational cohorts that provide direct estimates of the incidence of active tuberculosis among subjects with and without LTBI following intensive exposure. Adjusting for endogenous reactivation, we estimated the IRR of tuberculosis among latently infected subjects to be 0.21, corresponding to a risk reduction of 79%. With the most conservative estimate that no cases of reactivation occurred in the LTBI group, the risk reduction would be 72%.
Population models have long formed the basis for estimates of tuberculosis incidence following reinfection and, accordingly, the risk reduction associated with latent tuberculosis. In 1982, Sutherland and colleagues [9] published a landmark model on tuberculosis in the Netherlands from 1951 to 1970. Using historical data on latent tuberculosis prevalence, they estimated the proportion of individuals in various age groups that fell into 3 groups: latently infected, latently infected with recent reinfection, and recently infected. They then fit these estimates to notification data to estimate the proportion of cases attributable to each group. Their estimates for tuberculosis incidence following primary infection and reinfection were 5.06% and 1.91% for men and 5.85% and 1.10% for women, corresponding to a risk reduction conferred by latent tuberculosis of 63% for men and 81% for women.
Clark and Vynnycky [10] extended this work by using maximum likelihood methods to fit data among First Nations people in British Columbia from 1926 to 2000; they arrived at an estimate of 73% reduced risk. Vynnycky and Fine [11] used an age-structured deterministic model, fit to data from England and Wales from 1950 through the 1980s, and estimated a 41% risk reduction. Most recently, Brooks-Pollock and colleagues [12] used cross-sectional data on cases within households to estimate disease probabilities, inferring that the reduced secondary attack rate in households with multiple tuberculosis episodes represented protective immunity. They estimated a risk reduction of 35%.
Our estimates for the tuberculosis risk reduction among individuals with LTBI upon reinfection are higher than most of these estimates. However, by observing infections in the uninfected group and observing cases of tuberculosis directly, our estimates rely on fewer assumptions and thus provide a more direct estimate.
In our study, LTBI was associated with a significantly reduced risk of disease upon reinfection; nevertheless, the majority of new infections (90%) among LTBI subjects were attributable to reinfection, a consequence of the high annual risk of infection. These findings are consistent with epidemiologic models, which estimate that the majority of new cases of tuberculosis in high transmission settings occur as a result of new infections rather than reactivation [11, 36]. Preventing tuberculosis transmission may therefore be more important than treating latent infection in high-burden settings.
These data must be interpreted within the context of the quality of the studies assessed. There was substantial between-study heterogeneity in the IRR; however, the range of estimates was 0.04–0.58, all signifying a marked risk reduction in tuberculosis following reinfection among individuals with LTBI. Tuberculosis cases were not uniformly defined, making consistency across studies difficult to ensure. The incidence of tuberculosis following infection was in accordance with Sutherland’s and Clark’s findings but higher than that estimated in other studies [2], suggesting the definition of tuberculosis may have been too lenient. Nevertheless, the World Health Organization recognizes the cases judged by a clinician to represent tuberculosis, and we took this flexible definition as most suitable. Clinicians in the studies evaluated were not blinded to the results of the tuberculin tests; however, unless the definition was applied differently in LTBI and UI individuals within the same study, the rate ratio we estimated would not be biased.
These cohorts consisted of young, presumably healthy individuals and do not shed light on the important question of risk reduction in HIV-infected and other immunocompromised individuals. Several studies have shown that HIV increases the risk of disease due to reinfection following active tuberculosis [37, 38], but there has been no direct assessment of the risk of disease following reinfection among individuals with latent infection. A recent study found that among HIV-infected individuals, the proportion of cases clustered did not differ among those with and without a history of LTBI, indirectly suggesting that latent infection did not provide protection from reinfection [39]. Finally, most of the individuals with LTBI in our study were young and thus had relatively recent infection. Like vaccine-induced immunity, protection conferred by LTBI may wane with time, and it is not known whether latent infection provides similar protection decades after initial infection.
We assumed that exposure was balanced between the LTBI and UI groups; it is conceivable that the LTBI group had higher risk characteristics that led to initial infection. As the elevated risk of tuberculosis among UI individuals was well known by the 1930s, it is conceivable that after this time, UI individuals were more cautious to avoid infection. Such biases would mean that the higher rates of active tuberculosis in the UI group were in the context of lesser exposure, rendering our estimate conservative. It also is conceivable that some individuals were exposed outside the hospital, but this would be unlikely to only affect the UI group. Moreover, the average ARI of 43.8% greatly exceeded the community ARI from this time period.
The reduction in tuberculosis incidence in the LTBI group may not be fully explained by immunity established as a result of prior infection. Individuals who established LTBI without developing primary disease upon first infection may have innate immune characteristics associated with a reduced tuberculosis risk. The immunity conferred by LTBI may be but one component in the observed risk reduction.
The higher degree of protection afforded by LTBI in these studies, compared with population models, could be related to the very high ARI in this population of healthcare workers in this earlier era. However, we did not find an association between ARI and IRR in our analysis.
Finally, as this study makes inferences about exposure among LTBI individuals from observed infection rates among UI individuals, these estimates do not allow us to distinguish between reduction in risk of active disease versus reduction in risk of reinfection (ie, the establishment of a second infection). Nevertheless, these results provide insight into the composite reduction in tuberculosis disease following reexposure, which is a critical parameter in the epidemiologic modeling of tuberculosis.
The findings from this analysis demonstrate that latent tuberculosis infection is associated with a reduced risk of tuberculosis following reexposure. Clinicians and public health practitioners should consider individuals with LTBI at lower risk of disease upon reexposure, which may be important in evaluating household contacts or tuberculosis control strategies in high transmission settings. Vaccines that replicate this level of risk reduction would likewise be important in controlling tuberculosis. However, the extent to which HIV coinfection, age, other host factors and tuberculosis strain affect this risk reduction remain important areas for further investigation.
Notes
Acknowledgments.
We thank Corina Rusu for her assistance in preparing the tables and figures.
J. R. A. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which played no role in the study design, methods, interpretation of results, the content of this manuscript, or the decision to submit it for publication.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases [grants R01 AI058736 and T32 AI007433-20]; the AIDS Clinical Trials Group [grant U01 AI068636]; the Doris Duke Charitable Foundation [Clinical Scientist Development Award]; and the National Institute of General Medical Sciences [grant U54 GM088558]
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Connor C. Tuberculosis among medical students. The Diplomate. 1940;12:241–6. [Google Scholar]
- 2.Horsburgh CR. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060–7. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 3.Stead WW. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95:729–45. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C-Y, Riley LW. Exogenous reinfection in tuberculosis. Lancet Infect Dis. 2005;5:629–36. doi: 10.1016/S1473-3099(05)70240-1. [DOI] [PubMed] [Google Scholar]
- 5.Colijn C, Cohen T, Murray M. Mathematical models of tuberculosis: accomplishments and future challenges. International Symposium on Mathematical and Computational Biology. BIOMAT 2006: International Symposium on Mathematical and Computational Biology. Singapore, World Scientific. 2006 [Google Scholar]
- 6.Cohen T, Colijn C, Finklea B, Murray M. Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J R Soc Interface. 2007;4:523–31. doi: 10.1098/rsif.2006.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu S, Orenstein E, Galvani AP. The theoretical influence of immunity between strain groups on the progression of drug-resistant tuberculosis epidemics. J Infect Dis. 2008;198:1502–13. doi: 10.1086/592508. [DOI] [PubMed] [Google Scholar]
- 8.von Reyn CF, Horsburgh CR. Reinfection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;173:133–4. doi: 10.1164/ajrccm.173.1.133a. author reply 134–135. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland I, Svandová E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63:255–68. doi: 10.1016/s0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 10.Clark M, Vynnycky E. The use of maximum likelihood methods to estimate the risk of tuberculous infection and disease in a Canadian First Nations population. Int J Epidemiol. 2004;33:477–84. doi: 10.1093/ije/dyh001. [DOI] [PubMed] [Google Scholar]
- 11.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks-Pollock E, Becerra MC, Goldstein E, Cohen T, Murray MB. Epidemiologic inference from the distribution of tuberculosis cases in households in Lima. Peru J Infect Dis. 2011;203:1582–9. doi: 10.1093/infdis/jir162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjartveit K. Olaf Scheel and Johannes Heimbeck: their contribution to understanding the pathogenesis and prevention of tuberculosis. Int J Tuberc Lung Dis. 2003;7:306–11. [PubMed] [Google Scholar]
- 14.Sepkowitz KA. Tuberculosis and the health care worker: a historical perspective. Ann Intern Med. 1994;120:71–9. doi: 10.7326/0003-4819-120-1-199401010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Smith DT. Diagnostic and prognostic significance of the quantitative tuberculin tests: the influence of subclinical infections with atypical mycobacteria. Ann Intern Med. 1967;67:919–46. doi: 10.7326/0003-4819-67-5-919. [DOI] [PubMed] [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 17.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. Available at: http://www.R-project.org. [Google Scholar]
- 18.Geer E. Primary tuberculosis among nurses. Am Rev Tuberculosis. 1934;29:88–97. [Google Scholar]
- 19.Heimbeck J. Incidence of tuberculosis in young adult women with special reference to employment. Br J Tuberculosis. 1938;32:154–66. [Google Scholar]
- 20.Myers J, Boynton R, Diehl H, Streukens T, Ch’iu P. Tuberculosis among students and graduates in nursing. Ann Intern Med. 1940;14:873–97. [Google Scholar]
- 21.Myers J, Diehl H, Boynton R, Ch’iu P, Streukens T, Trach B. Tuberculosis among students and graduates of medicine. Ann Intern Med. 1941;14:1575–94. [Google Scholar]
- 22.Hastings D, Behn B. Tuberculosis among nurses: a study of the effect of tuberculosis service on the incidence of tuberculosis infection and disease among student nurses. Am Rev Tuberculosis. 1941;44:681–94. [Google Scholar]
- 23.Brahdy L. Immunity and positive tuberculin reaction. Am J Public Health Nations Health. 1941;31:1040–3. doi: 10.2105/ajph.31.10.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israel HL, Hetherington MD, Ord JG. A study of tuberculosis among students of nursing. J Am Med Assoc. 1941;117:839–44. [Google Scholar]
- 25.Wright HP. The comparative value of various tuberculin tests in children, medical students, and nurses-in-training. Can Med Assoc J. 1941;44:44–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz S. Tuberculin sensitivity and the development of tuberculosis among nurses. Am Rev Tuberculosis. 1942;47:19–25. [Google Scholar]
- 27.Daniels M. Primary tuberculosis infection in nurses: manifestations and prognosis. Lancet. 1944;244:165–70. [Google Scholar]
- 28.Lim-Yuen DM. Tuberculosis in sanatorium personnel; a tuberculin survey and incidence of active tuberculosis. Am Rev Tuberc. 1946;54:261–71. doi: 10.1164/art.1946.54.3.261. [DOI] [PubMed] [Google Scholar]
- 29.Madsen T, Holm J, Jensen KA. Studies on the epidemiology of tuberculosis in Denmark. Acta Tuberculosis Scandinavia. 1942;(Supp 6):1–172. [Google Scholar]
- 30.Holm J. BCG vaccination in Denmark. Public Health Rep. 1946;61:1298–315. [PubMed] [Google Scholar]
- 31.Thompson BC. Tuberculosis in nurses in a New Zealand hospital. Tubercle. 1949;30:155–61. doi: 10.1016/s0041-3879(49)80081-x. [DOI] [PubMed] [Google Scholar]
- 32.Badger TL, Ayvazian LF. Tuberculosis in nurses; clinical observations on its pathogenesis as seen in a 15 year follow-up of 745 nurses. Am Rev Tuberc. 1949;60:305–31. doi: 10.1164/art.1949.60.3.305. [DOI] [PubMed] [Google Scholar]
- 33.Dickie HA. Tuberculosis in student nurses and medical students at the University of Wisconsin. Ann Intern Med. 1950;33:941–59. doi: 10.7326/0003-4819-33-4-941. [DOI] [PubMed] [Google Scholar]
- 34.Poole G. Tuberculin sensitivity and tuberculosis in 1,779 nurses. Br J Tuberc Dis Chest. 1954;48:230–7. doi: 10.1016/s0366-0869(54)80113-4. [DOI] [PubMed] [Google Scholar]
- 35.Karns JR. Tuberculosis in medical students at the University of Maryland. Am Rev Tuberc. 1959;79:746–55. doi: 10.1164/artpd.1959.79.6.746. [DOI] [PubMed] [Google Scholar]
- 36.Cohen T, Murray M. Incident tuberculosis among recent US immigrants and exogenous reinfection. Emerg Infect Dis. 2005;11:725–8. doi: 10.3201/eid1105.041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–93. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 38.Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis. 2003;3:282–7. doi: 10.1016/s1473-3099(03)00607-8. [DOI] [PubMed] [Google Scholar]
- 39.Houben RM, Glynn JR, Mallard K, et al. Human immunodeficiency virus increases the risk of tuberculosis due to recent re-infection in individuals with latent infection. Int J Tuberc Lung Dis. 2010;14:909–15. [PMC free article] [PubMed] [Google Scholar]