Abstract
Context
Associations of major lipids and apolipoproteins with the risk of vascular disease have not been reliably quantified.
Objective
To assess major lipids and apolipoproteins in vascular risk.
Design, Setting, and Participants
Individual records were supplied on 302 430 people without initial vascular disease from 68 long-term prospective studies, mostly in Europe and North America. During 2.79 million person-years of follow-up, there were 8857 nonfatal myocardial infarctions, 3928 coronary heart disease [CHD] deaths, 2534 ischemic strokes, 513 hemorrhagic strokes, and 2536 unclassified strokes.
Main Outcome Measures
Hazard ratios (HRs), adjusted for several conventional factors, were calculated for 1-SD higher values: 0.52 loge triglyceride, 15 mg/dL high-density lipoprotein cholesterol (HDL-C), 43 mg/dL non-HDL-C, 29 mg/dL apolipoprotein AI, 29 mg/dL apolipoprotein B, and 33 mg/dL directly measured low-density lipoprotein cholesterol (LDL-C). Within-study regression analyses were adjusted for within-person variation and combined using meta-analysis.
Results
The rates of CHD per 1000 person-years in the bottom and top thirds of baseline lipid distributions, respectively, were 2.6 and 6.2 with triglyceride, 6.4 and 2.4 with HDL-C, and 2.3 and 6.7 with non-HDL-C. Adjusted HRs for CHD were 0.99 (95% CI, 0.94-1.05) with triglyceride, 0.78 (95% CI, 0.74-0.82) with HDL-C, and 1.50 (95% CI, 1.39-1.61) with non-HDL-C. Hazard ratios were at least as strong in participants who did not fast as in those who did. The HR for CHD was 0.35 (95% CI, 0.30-0.42) with a combination of 80 mg/dL lower non-HDL-C and 15 mg/dL higher HDL-C. For the subset with apolipoproteins or directly measured LDL-C, HRs were 1.50 (95% CI, 1.38-1.62) with the ratio non-HDL-C/HDL-C, 1.49 (95% CI, 1.39-1.60) with the ratio apo B/apo AI, 1.42 (95% CI, 1.06-1.91) with non-HDL-C, and 1.38 (95% CI, 1.09-1.73) with directly measured LDL-C. Hazard ratios for ischemic stroke were 1.02 (95% CI, 0.94-1.11) with triglyceride, 0.93 (95% CI, 0.84-1.02) with HDL-C, and 1.12 (95% CI, 1.04-1.20) with non-HDL-C.
Conclusion
Lipid assessment in vascular disease can be simplified by measurement of either total and HDL cholesterol levels or apolipoproteins without the need to fast and without regard to triglyceride.
INTRODUCTION
Reliable assessment of the separate and joint associations of major blood lipids and apolipoproteins with the risk of vascular disease is important for the development of screening and therapeutic strategies.1,2 Expert opinion is divided about whether assessment of apolipoprotein AI (apo AI) and apolipoprotein B (apo B) should replace assessment of high-density lipoprotein cholesterol (HDL-C) and total cholesterol levels in assessment of vascular risk.3-5
Although there is agreement about the value of reducing low-density lipoprotein cholesterol (LDL-C or, approximately analogously, non-high-density lipoprotein cholesterol [non-HDL-C]), uncertainty persists about the merits of modification or measurement of triglycerides or HDL-C.3 There are strongly positive epidemiological associations of triglyceride concentration with risk of vascular disease,6,7 but it is not clear to what extent these relationships depend on cholesterol levels or vary with fasting state.
Similarly, although previous analyses have generally reported inverse associations of HDL-C with risk of vascular disease, many studies have not investigated the extent to which they depend on triglyceride concentration.8 The failure of torcetrapib has raised questions about the value of raising HDL-C and highlighted the need to characterize more reliably the relationship between HDL-C and vascular risk, particularly at high HDL-C levels.9
Different uncertainties apply in relation to the risk of ischemic stroke and the cholesterol content of proatherogenic lipoproteins. The reduction in ischemic stroke in randomized trials of statins (which principally lower LDL-C) is remarkable in light of the weak epidemiological association reported between circulating LDL-C concentration and ischemic stroke, 10,11 suggesting the need for more powerful and detailed prospective analyses of blood lipids and stroke subtypes.
The objective of this report is to produce reliable estimates of the associations of major lipids and apolipoproteins in relation to coronary heart disease (CHD) and ischemic stroke, incorporating adjustment for confounding caused by other risk factors and correction for regression dilution.
METHODS
Study Design
Details of study selection, data collection, and harmonization procedures of the Emerging Risk Factors Collaboration (ERFC) have been described previously.13
One hundred twelve prospective studies of cardiovascular risk factors, involving a total of 1.2 million participants, have shared individual records in the ERFC (eFigure 1, available at http://www.jama.com). These studies were approximately population-based (ie, did not select participants on the basis of having previous cardiovascular disease); recorded cause-specific mortality or vascular morbidity using accepted criteria; and had accrued more than 1 year of follow-up. eTable 1 lists details of the 68 studies—involving a total of 302 430 participants without any known history of CHD (ie, myocardial infarction [MI], angina, or stroke, which were defined in each study) at the initial (“baseline”) examination—that had complete information at baseline on total cholesterol, HDL-C, and triglyceride levels and several conventional risk factors (ie, age, sex, smoking status, history of diabetes mellitus, systolic blood pressure, body mass index). References for studies in eTable 1 are in eAppendix 1 and in a previously published reference list.13 Twenty-two studies with 91 307 participants had information on the preceding variables plus apo B and apo AI, and 8 studies with 44 234 participants had directly measured LDL-C values. The AMORIS study provided data for the ERFC, but it could not be incorporated into the current analyses because AMORIS did not measure baseline levels of HDL-C, blood pressure, smoking, body mass index, or diabetes.14
All but 1 study used enzymatic methods to assay triglyceride, and all but 2 studies used precipitation methods to assay HDL-C (eTable 2). For assay of apolipoproteins, 16 studies used immunoturbidimetry or nephelometry, 4 used immunoradiometric assays, 1 used immunoelectrophoresis, and 1 involved immunochemical methods. For assay of LDL-C, 4 studies used ultracentrifugation, 2 used direct homogeneous methods, 1 used chemical precipitation, and 1 used electrophoresis. In registering fatal outcomes, all contributing studies used coding from the International Classification of Diseases to at least 3 digits and ascertainment was based on death certificates. Fifty-two of 68 contributing studies also involved medical records, autopsy findings, and other supplementary sources to help classify deaths. Sixty-two studies used standard definitions of MI based on World Health Organization criteria. Fifty-six studies reported diagnosis of strokes on the basis of typical clinical features and characteristic changes on brain imaging, and all at tempted to provide attribution of stroke subtype.
Statistical Analyses
Non-HDL-C (calculated by subtraction of HDL-C from total cholesterol, yielding a measure that encompasses low-, intermediate-, and very-low-density lipoprotein cholesterol) was used as the principal marker of cholesterol content in proatherogenic lipoproteins in order to avoid the biases that may arise when using LDL-C values estimated by the Friedewald formula15 (eAppendix 2). Triglyceride was loge transformed to improve its normality. Details of the statistical methods have been described previously.16 The primary outcome was CHD (ie, first-ever MI or fatal CHD). Analyses involved a 2-stage approach with estimates of association calculated separately within each study before pooling across studies by random-effects meta-analysis.
For the 64 studies analyzed as prospective cohort studies, hazard ratios (HRs) were calculated using Cox proportional hazard regression models stratified by sex (and, where appropriate, by trial group). The proportional hazards assumption was satisfied for each lipid in each of the studies. Participants contributed only their first nonfatal outcome or death recorded at age 40 years or older (ie, deaths proceeded by nonfatal CHD or stroke were not included in the main analyses). For the 4 contributing individually matched nested case-control studies within prospective cohorts, odds ratios were calculated using conditional logistic regression models. Odds ratios approximated HRs because these studies selected cases and controls con-currently and matched for age and sex.
To help characterize shapes of associations, study-specific HRs calculated within overall quantiles of baseline lipid levels were pooled on the log scale by multivariate random-effects meta-analysis and plotted against pooled mean usual levels of the relevant lipid marker within each quantile. Ninety-five percent confidence intervals (CIs) were estimated from the variances that reflect the amount of information underlying each group (including the reference group). As associations were approximately log-linear, regression coefficients were calculated to estimate the HRs associated with 1-SD higher baseline values of each lipid: 0.52 loge triglyceride, 15 mg/dL HDL-C, 43 mg/dL non-HDL-C (and, in subsets, 29 mg/dL apo AI, 29 mg/ dL apo B, and 33 mg/dL for directly measured LDL-C). (To convert HDL-C and LDL-C to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113.)
Hazard ratios were adjusted progressively for age, sex, systolic blood pressure, smoking status, history of diabetes, body mass index, and lipid measures, with the evidence of association indicated by the Wald χ2 statistic and heterogeneity between studies assessed by the I2 statistic.17 Investigation of effect modification was quantified by formal tests of interaction. Diversity at the study level was investigated by grouping studies by recorded characteristics and by meta-regression.
We corrected for bias caused by variability in levels of both lipids and potential confounding factors. Regression dilution ratios were obtained by regressing serial measurements of risk factors, taken from up to 89 073 participants (mean interval, 4.9 years), on baseline levels of the relevant characteristic and duration of follow-up. Correction for within-person variation in risk factors was achieved by use of conditional expectations of long-term average levels (“usual levels”) of these risk factors, which were predicted from these regression calibration models and used in the estimation of HRs, as described previously.12,18
Analyses involved Stata software, release 10 (StataCorp, College Station, Texas); 2-sided P values and 95% CIs are presented. This study was approved by the Cambridgeshire ethics review committee and was conducted and anlyzed independently from its funders.
RESULTS
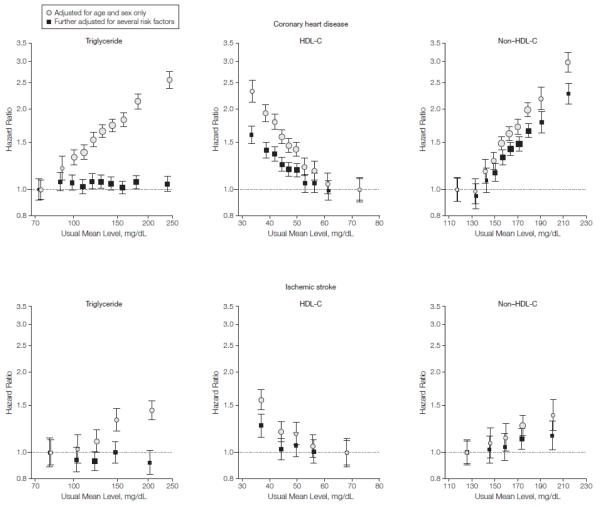
Mean (SD) age at entry of participants was 59 (8) years, 43% were women, 60% were in western Europe, and 32% in North America (eTable 3). During 2.79 million person-years at risk (median, 6.1 years to first outcome), there were, counting only first-ever outcomes, 8857 nonfatal MIs, 3928 CHD deaths, 2534 ischemic strokes, 513 hemorrhagic strokes, and 2536 unclassified strokes (eTable 4). Mean (SD) levels of loge triglyceride, HDL-C, and non-HDL-C were each broadly similar across studies (eTable 1). Loge triglyceride, HDL-C, and non-HDL-C were correlated with one another, with particularly strong correlations of non-HDL-C with apo B and directly measured LDL-C, and of HDL-C with apo AI (eTable 3). Serial measurements yielded age- and sex-adjusted regression dilution ratios of 0.63 (95% CI, 0.60-0.67) for loge triglyceride, 0.69 (95% CI, 0.64-0.74) for HDL-C, 0.60 (95% CI, 0.54-0.65) for non-HDL-C, 0.57 (95% CI, 0.46-0.69) for apo AI, 0.61 (95% CI, 0.47-0.75) for apo B, and 0.64 (95% CI, 0.57-0.71) for directly measured LDL-C. The rates of CHD per 1000 person-years in the bottom and top thirds of baseline lipid distributions, respectively, were 2.6 and 6.2 with triglyceride, 6.4 and 2.4 with HDL-C, and 2.3 and 6.7 with non-HDL-C. In analyses adjusted for age and sex only, each lipid studied was approximately log-linearly associated with CHD risk, with possible attenuation at very high HDL-C and at low non-HDL-C concentration (FIGURE 1).
Figure 1.
Hazard Ratios for Coronary Heart Disease or Ischemic Stroke Across Quantiles of Usual Triglyceride, HDL-C, and Non-HDL-C Levels.
Analyses for coronary heart disease were based on 302 430 participants (involving 12 785 cases) from 68 studies. Analyses for ischemic stroke were based on 173 312 participants (involving 2534 cases) from 32 studies. Regression analyses were stratified, where appropriate, by sex and trial group. Values with further adjustments were adjusted for age, systolic blood pressure, smoking status, history of diabetes mellitus, and body mass index; furthermore, analyses of loge triglyceride were ad-justed for high-density lipoprotein cholesterol (HDL-C) and non-HDL-C levels, analyses of HDL-C were adjusted for non-HDL-C and loge triglyceride levels, and analyses of non-HDL-C were adjusted for HDL-C and loge triglyceride levels. Studies with fewer than 10 cases were excluded from analysis. Sizes of data markers are proportional to the inverse of the variance of the hazard ratios. The y-axes are shown on a log scale. The x-axes for triglyceride are shown on a log scale. Referent groups are lowest quantiles for triglyceride and non-HDL-C and highest quantiles for HDL-C. Error bars indicate 95% confidence intervals.
Triglyceride
The HR for CHD with triglyceride was 1.37 (95% CI, 1.31-1.42) after adjustment for nonlipid risk factors, but it was reduced to 0.99 (95% CI, 0.94-1.05) after further adjustment for HDL-C and non-HDL-C (the Wald χ2 reduced from 214 to 0) (eTable 5 and Figure 1). There was modest heterogeneity among the contributing studies (I2 = 35%; 95% CI, 12%-52%). Adjusted HRs for CHD were essentially null under a range of circumstances, including by sex (HRs of 0.97 [95% CI, 0.91-1.03] in men and 1.06 [95% CI, 0.96-1.16] in women) and by fasting status (HRs of 1.02 [95% CI, 0.95-1.09] in people who fasted and 0.92 [95% CI, 0.82-1.03] in people who did not fast) (eFigure 2). There was, however, an apparently positive association at lower systolic blood pressure. The adjusted HR was 1.02 (95% CI, 0.94-1.11) for ischemic stroke (eTable 5 and Figure 1), 1.04 (95% CI, 0.82-1.32) for hemorrhagic stroke, and 1.03 (95% CI, 0.94-1.13) for unclassified stroke.
High-Density Lipoprotein Cholesterol
The HR for CHD with HDL-C was 0.71 (95% CI, 0.68-0.75) after adjustment for nonlipid risk factors, and it was 0.78 (95% CI, 0.74-0.82) after further adjustment for non-HDL-C and loge triglyceride (the Wald χ2 reduced from 149 to 84) (eTable 5 and Figure 1). There was modest heterogeneity among the contributing studies (I2 = 40%; 95% CI, 20%-55%). The HR for CHD was stronger at younger ages and at lower systolic blood pressure, but it did not vary importantly by sex, other lipid fractions, diabetes, or body mass index (eFigure 2). Findings did not vary materially in sub-analyses that additionally adjusted for C-reactive protein or fibrinogen concentration (eTable 6) or alcohol consumption (or that excluded alcohol abstainers). Hazard ratios were 0.79 (95% CI, 0.74-0.84) in participants who fasted and 0.75 (95% CI, 0.68-0.83) in participants who did not fast. The adjusted HR for ischemic stroke was 0.93 (95% CI, 0.84-1.02) (eTable 5 and Figure 1), with modest heterogeneity among the contributing studies (I2 = 27%; 95% CI, 0%-53%) (eTable 5). Adjusted HRs were 1.09 (95% CI, 0.92-1.29) for hemorrhagic stroke and 0.87 (95% CI, 0.80-0.94) for unclassified stroke.
Non-High-Density Lipoprotein Cholesterol
The HR for CHD with non-HDL-C was 1.56 (95% CI, 1.47-1.66) after adjustment for nonlipid risk factors, and it was 1.50 (95% CI, 1.39-1.61) after further adjustment for HDL-C and loge triglyceride (the Wald χ2 reduced from 229 to 122) (eTable 5 and Figure 1). There was considerable heterogeneity among the contributing studies (I2 = 73%; 95% CI, 66%-79%), partly explained by more extreme HRs in participants who did not fast vs those who fasted (1.72 [95% CI, 1.51-1.95] vs 1.41 [95% CI, 1.30-1.53]; P = .01) and in studies with serum than those with other types of blood samples (1.60 [95% CI, 1.47-1.74] vs 1.31 [95% CI, 1.17-1.47]; P = .008) (eFigure 2). The HR for CHD was slightly stronger at younger ages (although it remained strong even at older ages) and more extreme at lower systolic blood pressure. Hazard ratios for CHD did not vary importantly by sex, levels of other lipid fractions, diabetes, or body mass index (eFigure 2). In the subset of participants with available measurements, the adjusted HRs for CHD were 1.38 (95% CI, 1.09-1.73) with directly measured LDL-C and 1.42 (95% CI, 1.06-1.91) with non-HDL-C (eTable 7). The adjusted HR for ischemic stroke was 1.12 (95% CI, 1.04-1.20) with non-HDL-C, about 4 times weaker than that for CHD (eTable 5 and Figure 1). Adjusted HRs were 0.98 (95% CI, 0.82-1.17) for hemorrhagic stroke and 1.01 (95% CI, 0.93-1.09) for unclassified stroke.
Combined Lipid Analyses and Apolipoproteins
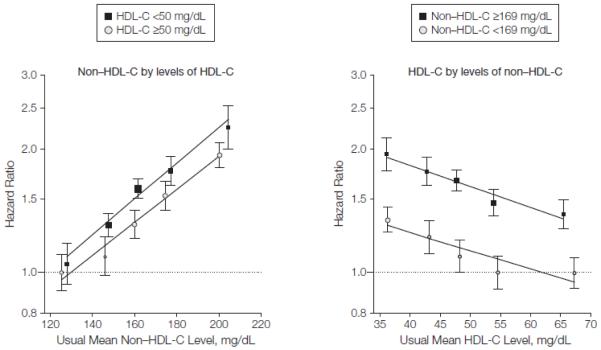
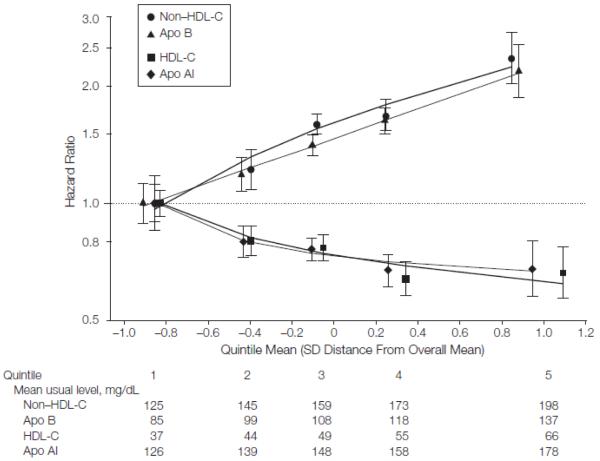
Hazard ratios for CHD with non HDL-C were generally similar at different HDL-C levels and vice versa (FIGURE 2). The HR for CHD was 0.35 (95% CI, 0.30-0.42) with a combination of 15 mg/dL higher HDL-C and 80 mg/dL lower non-HDL-C, alterations that are attainable with available lipid lowering agents.19,20 The HR was not materially changed by addition of information on triglyceride. Non-HDL-C and apo B each had very similar shape and magnitude of associations with CHD, as did HDL-C and apo AI (FIGURE 3 and eTable 8). Hazard ratios for CHD were 1.50 (95% CI, 1.381.62) with the ratio of non-HDL-C/ HDL-C (which is statistically equivalent to the ratio of total cholesterol to HDL-C) and 1.49 (95% CI, 1.39-1.60) with the ratio of apo B/apo AI (eTable 8). For ischemic stroke, there were also similar findings with cholesterol levels and apolipoproteins.
Figure 2.
Hazard Ratios for Coronary Heart Disease Across Fifths of Non-HDL-C by Levels of HDL-C and Fifths of HDL-C by Levels of Non-HDL-C
Analyses were based on 302 430 participants (involving 12 785 cases) from 68 studies. Median values in the Emerging Risk Factors Collaboration were 50 mg/dL for high-density lipoprotein cholesterol (HDL-C) and 169 mg/dL for non-HDL-C. Regression analyses were stratified, where appropriate, by sex and trial group and adjusted for age, systolic blood pressure, smoking status, history of diabetes mellitus, body mass index, and loge triglyceride levels. Studies with fewer than 10 cases were excluded from analysis. Sizes of data markers are proportional to the inverse of the variance of the hazard ratios. The y-axes are shown on a log scale. Referent groups are lowest fifth of non-HDL-C in the higher level of HDL-C and highest fifth of HDL-C in the lower level of non-HDL-C. Lines are fitted by log-linear regression of log hazard ratios on mean levels. Error bars indicate 95% confidence intervals.
Figure 3.
Hazard Ratios for Coronary Heart Disease Across Fifths of Usual Lipids or Apolipoproteins
Analyses were based on 91 307 participants (involving 4499 cases) from 22 studies. Regression analyses were stratified, where appropriate, by sex and trial group and adjusted for age, systolic blood pressure, smoking status, history of diabetes mellitus, and body mass index; furthermore, analyses of non-HDL-C were adjusted for HDL-C and loge triglyceride, analyses of apolipoprotein B (apo B) were adjusted for apolipoprotein AI (apo AI) and loge triglyceride, analyses of HDL-C were adjusted for non-HDL-C and loge triglyceride, and analyses of apo AI were adjusted for apo B and loge triglyceride. Studies with fewer than 10 cases were excluded from analysis. Sizes of data markers are proportional to the inverse of the variance of the hazard ratios. Referent groups are lowest fifths. Lines are fitted by first-degree fractional polynomial regression of log hazard ratios on mean SD score. Error bars indicate 95% confidence intervals. The y-axis is shown on a log scale. The x-axis is shown on a Z-transformed scale.
Qualitatively similar results to those reported here were observed in analyses that used fixed-effect models (eFigure 3), compared larger vs smaller studies, ignored regression dilution, replaced non-HDL-C with total cholesterol, included fatal outcomes with-out censoring previous nonfatal outcomes, and omitted the 44 108 participants from clinical trials (eFigure 2).
COMMENT
The current analysis of more than 300 000 people has demonstrated that lipid assessment in vascular disease can be simplified by measurement of either cholesterol levels or apolipoproteins without the need to fast and without regard to triglyceride. This conclusion derives from several findings. First, HRs with non-HDL-C and HDL-C were nearly identical to those seen with apo B and apo AI. This finding suggests that current discussions about whether to measure cholesterol levels or apolipoproteins in vascular risk assessment should hinge more on practical considerations (eg, cost, availability, and standardization of assays) than on major differences in strength of epidemiological associations. Second, HRs for vascular disease with lipid levels were at least as strong in participants who did not fast as in those who fasted. Third, HRs were similar with non-HDL-C as with directly measured LDL-C. Finally, in contrast with previous findings based on much less data, triglyceride concentration was not independently related with CHD risk after controlling for HDL-C, non-HDL-C, and other standard risk factors, including null findings in women and under nonfasting conditions.21,22 Hence, for population-wide assessment of vascular risk, triglyceride measurement provides no additional information about vascular risk given knowledge of HDL-C and total cholesterol levels, although there may be separate reasons to measure triglyceride concentration (eg, prevention of pancreatitis).
Concentrations of HDL-C and non-HDL-C were each strongly associated— in opposite directions—with CHD risk in an approximately log-linear manner. In contrast with the null triglyceride findings after adjustment, HDL-C and non-HDL-C were largely independent from each other on a multiplicative scale, as well as from triglyceride concentration and other risk factors. Hence, whereas prevailing therapeutic strategies focus on lowering of LDL-C (or, approximately analogously, non-HDL-C), the current findings suggest that therapy directed at HDL-C as well as non-HDL-C may generate substantial additional benefit. For example, CHD risk is approximately two-thirds lower in people with 15 mg/dL higher HDL-C and 80 mg/dL lower non-HDL-C, which are alterations that are attainable with, say, extended-release niacin plus a potent statin.19,20 Long-term randomized trials of such lipid-modifying regimens are therefore needed to test this epidemiologically expected risk reduction.23-25
Because associations of higher non-HDL-C concentration with CHD are similar at both higher and lower HDL-C concentrations, the absolute benefits of lowering LDL-C are likely to be greater if HDL-C concentration is low (or when absolute risk is high for some other reason). While the current findings can-not confirm or refute causality for either triglyceride or HDL-C concentration, they encourage large CHD studies of therapies and genotypes that specifically affect each of these lipid measures to help judge etiological relevance.23-29 Observational analyses focused on etiology should ideally allow for the possibility of disparate associations of different non-HDL-C components with vascular risk, which requires information on each type of low-, intermediate-, and very-low-density lipoprotein cholesterol (such information was not available in most studies contributing to the current analysis).
Hemorrhagic stroke was unrelated to any of the lipids studied here. Only proatherogenic lipids appeared to be associated with risk of ischemic stroke, albeit modestly. Indeed, the current study found an HR for CHD with non-HDL-C about 4 times greater than that for ischemic stroke. Because statin medications reduce risk of both CHD and ischemic stroke to a similar extent,10 the quantitative discrepancy observed between epidemiological associations of non-HDL-C with CHD and ischemic stroke is striking.30 To characterize this risk in more detail, stud ies are needed that can subtype the diverse etiologies for ischemic stroke.31 Given the essentially null associations observed between HDL-C concentration and stroke risk, considerable loss of statistical power may result from inclusion of stroke in primary outcomes of HDL-C-raising trials 23,25,29 (unless similar effects are observed to those in the previous trials of statin).
There was some heterogeneity in the findings, but the broad consistency of results across 68 studies in 21 countries supports their generalizability. Confounding was minimized by adjustment of HRs for long-term average levels of risk factors based on more than 89 000 serial measurements. As the logarithm of triglyceride concentration had a regression dilution ratio comparable with those of other lipid measures, such variability cannot account for the different HRs for CHD that were seen with the different lipid measures. The current prospective data contrast sharply with those of some large retrospective case-control studies that reported that apolipoproteins have much stronger associations with CHD risk than cholesterol levels.32,33 Case-control studies of acute MI may be liable to distortion of lipid levels by recent infarction, a potential bias that is minimized by prospective analyses of participants without cardiovascular disease at baseline.32,33 It remains unclear whether some residual artifact explains the apparent flattening of associations seen in the present analyses with CHD at very high HDL-C or at very low non-HDL-C concentration (whereas, by contrast, randomized statin trials indicate that LDL-C lowering below 80 mg/dL continues to lower CHD risk 10,34).
CONCLUSION
Lipid assessment in vascular disease can be simplified by measurement of either total and HDL cholesterol levels or apolipoproteins without the need to fast and without regard to triglyceride.
Supplementary Material
Acknowledgments
Funding/Support: The ERFC Coordinating Centre is underpinned by a program grant from the British Heart Foundation (RG/08/014) and supported by grants from the UK Medical Research Council and the BUPA Foundation and an unrestricted educational grant from GlaxoSmithKline. A variety of sources have supported recruitment, follow-up, and laboratory measurements in the cohorts contributing to the ERFC. Investigators of several of these studies have contributed to a list naming some of these funding sources, which can be found at http://www.phpc.cam.ac.uk/MEU/. Drs Di Angelantonio, Sarwar, Perry, and Thompson have been supported by PhD studentships of the UK Medical Research Council. Dr Ray is supported by a BHF Intermediate Research Fellowship.
Role of the Sponsor: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Authors/Emerging Risk Factors Collaboration (ERFC) Writing Committee: Emanuele Di Angelantonio, MD, University of Cambridge, Cambridge, United Kingdom; Nadeem Sarwar, PhD, University of Cambridge; Philip Perry, MBChB, University of Cambridge; Stephen Kaptoge, PhD, University of Cambridge; Kausik K. Ray, MD, University of Cambridge; Alexander Thompson, PhD, University of Cambridge; Angela M. Wood, PhD, University of Cambridge; Sarah Lewington, DPhil, University of Oxford, Oxford, United Kingdom; Naveed Sattar, FRCPath, University of Glasgow, Glasgow, United Kingdom; Chris J. Packard, DSc, University of Glasgow; Rory Collins, FMedSci, University of Oxford; Simon G. Thompson, DSc, Medical Research Council (MRC) Biostatistics Unit, Cambridge, United Kingdom; John Danesh, FRCP, University of Cambridge.
Drs Di Angelantonio, Sarwar, Perry, and Kaptoge contributed equally to this work.
Author Contributions: Drs Di Angelantonio and Danesh had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Di Angelantonio, Sarwar, Perry, Kaptoge, Ray, A. Thompson, Lewington, Sattar, Packard, S. G. Thompson, Danesh.
Acquisition of data: Di Angelantonio, Sarwar, Perry, Kaptoge, Ray, A. Thompson, Lewington, Sattar, Packard, Collins, S. G. Thompson, Danesh.
Analysis and interpretation of data: Di Angelantonio, Sarwar, Perry, Kaptoge, Ray, A. Thompson, Wood, Lewington, Sattar, Packard, Collins, S. G. Thompson, Danesh.
Drafting of the manuscript: Di Angelantonio, Sarwar, Danesh.
Critical revision of the manuscript for important intellectual content: Di Angelantonio, Sarwar, Perry, Kaptoge, Ray, A. Thompson, Wood, Lewington, Sattar, Packard, Collins, S. G. Thompson, Danesh.
Statistical analysis: Di Angelantonio, Perry, Kaptoge, Wood, S. G. Thompson.
Obtained funding: Collins, S. G. Thompson, Danesh. Administrative, technical, or material support: Di Angelantonio, Sarwar, Perry, Kaptoge, Ray, A. Thompson, Lewington, Sattar, Packard, S. G. Thompson, Danesh.
Study supervision: Danesh.
Financial Disclosures: Dr Collins reported being paid by the British Heart Foundation and UK Biobank and having received research funding and reimbursement of costs for attending scientific meetings (but no honoraria or consultancy payments) from Astra-Zeneca, Bayer, Bristol Myers Squibb, British Heart Foundation, Cancer Research UK, European Union, Kadoorie Trust, MRC, Merck, Roche, Sanofi, Schering, Solvay, and UK Biobank. Dr Danesh reported having received research funding from the British Heart Foundation; BUPA Foundation; diaDexus; European Union; Evelyn Trust; Fogarty International Center; GlaxoSmithKline; Medical Research Council; Merck Sharp and Dohme; National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke; Novartis; Roche; and Wellcome Trust. No other authors reported disclosures.
Emerging Risk Factors Collaboration (ERFC) Investigators: (eAppendix 3 lists the study acronyms) AFTCAPS: R. W. Tipping, MS, Merck Research Laboratories. ALLHAT: C. E. Ford, PhD; S. L. Pressel, MS, University of Texas School of Public Health. AMORIS: G. Walldius, MD; I. Jungner, MD, Karolinska Institutet, Sweden. ARIC: A. R. Folsom, MD, University of Minnesota; L. E. Chambless, PhD, University of North Carolina. ATTICA: D. B. Panagiotakos, MD, Harokopio University, Greece; C. Pitsavos, MD; C. Chrysohoou, MD; C. Stefanadis, MD, University of Athens, Greece. BHS: M. Knuiman, PhD, University of Western Australia. BIP: U. Goldbourt, PhD; M. Benderly, PhD; D. Tanne, MD, Sheba Medical Center, Israel. BRHS: P. H. Whincup, FRCP, University of London, United Kingdom; S. G. Wannamethee, PhD; R. W. Morris, PhD, University College London, United Kingdom. BRUN: S. Kiechl, MD; J. Willeit, MD, Medical University Innsbruck, Austria; P. Santer, MD, Department of Laboratory Medicine, Bruneck Hospital, Italy; A. Mayr, MD, Bruneck Hospital. BUPA: N. Wald, FRS, Wolfson Institute of Preventive Medicine, United Kingdom. BWHHS: S. Ebrahim, DM, London School of Hygiene and Tropical Medicine, United Kingdom; D. A. Lawlor, PhD, University of Bristol, United Kingdom. CAPS: J. W. G. Yarnell, MD, Queen’s University of Belfast, United Kingdom; J. Gallacher, PhD, Cardiff University, United Kingdom. CASTEL: E. Casiglia, MD; V. Tikhonoff, MD, University of Padova, Italy. CHARL: P. J. Nietert, PhD; S. E. Sutherland, PhD; D. L. Bachman, MD; J. E. Keil, DrPH, Medical University of South Carolina. CHS: M. Cushman, MD, University of Vermont; B. M. Psaty, MD, University of Washington; R. P. Tracy, PhD, University of Vermont (see http://chs-nhlbi.org for acknowledgments). COPEN: A. Tybjærg-Hansen, MD; B. G. Nordestgaard, MD; M. Benn, PhD; R. Frikke-Schmidt, MD, University of Copenhagen, Denmark. CUORE: S. Giampaoli, MD; L. Palmieri, DrStat, Istituto Superiore di Sanità, Italy; S. Panico, MD, Federico II University, Italy; D. Vanuzzo, MD; L. Pilotto, MD, Centre for Cardiovascular Prevention, Italy. DRECE: A. Gómez de la Cámara, MD, Hospital 12 de Octubre, Spain; J. A. Gómez-Gerique, PhD, Hospital Marqués de Valdecilla, Spain. DUBBO: L. Simons, MD, University of New South Wales, Australia; J. McCallum, DPhil, Victoria University, Australia; Y. Friedlander, PhD, Hebrew University, Israel. EAS: F. G. R. Fowkes, MBChB; A. J. Lee, PhD; F. B. Smith, MD, University of Edinburgh, United Kingdom. EPESEBOS: J. Taylor, MD, East Boston Neighborhood Health Center; J. M. Guralnik, MD; C. L. Phillips, MS, US National Institute on Aging. EPESEIOW: R. Wallace, MD, University of Iowa; J. Guralnik, MD; C. L. Phillips, MS, US National Institute on Aging. EPESENCA: D. G. Blazer, MD, Duke University Medical Centre; J. M. Guralnik, MD; C. L. Phillips, MS, US National Institute on Aging. EPESENHA: C. L. Phillips, MS; J. M. Guralnik, MD, US National Institute on Aging. EPICNOR: K-T Khaw, MBBChir, University of Cambridge. ESTHER: H. Brenner, MD; E. Raum, MD; H. Müller, DrScHum; D. Rothenbacher, MD, German Cancer Research Center, Germany. FIA: J. H. Jansson, MD; P. Wennberg, MD, Umeå University, Sweden. FINE-FIN: A. Nissinen, MD, National Institute for Health and Welfare, Finland. FINE-IT: C. Donfrancesco, DrStat; S. Giampaoli, MD, Istituto Superiore di Sanità. FINRISK-92, FINRISK-97: V. Salomaa, MD; K. Harald, MA; P. Jousilahti, MD; E. Vartiainen, MD, National Institute for Health and Welfare, Finland. FLETCHER: M. Woodward, PhD, Mount Sinai School of Medicine. FRAMOFF: R. B. D’Agostino, PhD, Boston University; P. A. Wolf, MD; R. S. Vasan, MD; M. J. Pencina, PhD, Boston University School of Medicine. GLOSTRUP: E. M. Bladbjerg, PhD, University of Southern Denmark; T. Jørgensen, MD, University of Copenhagen, Denmark; L. Møller, MD, World Health Organization; J. Jespersen, DSc, University of Southern Denmark. GOH: R. Dankner, MD; A. Chetrit, MSc; F. Lubin, RD, Gertner Institute for Epidemiology and Health Policy, Israel. GOTO33, GOTO43: A. Rosengren, MD; L. Wilhelmsen, MD; G. Lappas BSc; H. Eriksson, MD, Göteborg University, Sweden. GOTOW: C. Björkelund, MD; L. Lissner, PhD; C. Bengtsson, MD, Göteborg University. GRIPS: P. Cremer, MD, Klinikum der Universität München LMU, Germany; D. Nagel, PhD, University of Munich, Germany. HELSINAG: R. S. Tilvis, MD, Helsinki University Hospital, Finland; T.E. Strandberg, MD, Oulu University Hospital, Finland. HONOL: B. Rodriguez, MD, University of Hawaii. HOORN: J. M. Dekker, PhD, VU University Medical Center, the Netherlands; G. Nijpels, MD, Vrije Universiteit Medical Center, the Netherlands; C. D. A. Stehouwer, MD, Maastricht University Medical Centre, the Netherlands. HPFS: E. Rimm, ScD, Harvard University; J. K. Pai, ScD, Brigham and Women’s Hospital. IKNS: S. Sato, MD, Osaka Medical Center for Health Science and Promotion, Japan; H. Iso, MD, Osaka University, Japan; A. Kitamura, MD, Osaka Medical Center for Health Science and Promotion; H. Noda, MD, Osaka University. ISRAEL: U. Goldbourt, PhD, Sheba Medical Center. KIHD: J. T. Salonen, MD; K. Nyyssönen, PhD; T-P Tuomainen, MD, University of Kuopio, Finland. LASA: D. J. H. Deeg, PhD; J. L. Poppelaars, MA, VU University Medical Centre. LEADER: T. W. Meade, FMedSci, London School of Hygiene and Tropical Medicine; J. A. Cooper, MSc, University College London. MALMO: B. Hedblad, MD; G. Berglund, MD; G. Engstrom, MD, Lund University, Sweden. MCVDRFP: W. M. M. Verschuren, PhD; A. Blokstra, MSc, National Institute of Public Health and the Environment, the Netherlands. MOGERAUG1, MOGERAUG2, MOGERAUG3: A. Döring, MD, German Research Center for Environmental Health, Germany; W. Koenig, MD, University of Ulm Medical Center, Germany; C. Meisinger, MD, German Research Center for Environmental Health; W. Mraz, MD, Klinikum der Universität München, Institute of Clinical Chemistry, Germany. MORGEN: W. M. M. Verschuren, PhD; A. Blokstra, MSc; H. Bas Bueno-de-Mesquita, PhD, National Institute for Public Health and the Environment, the Netherlands. MOSWEGOT: L. Wilhelmsen, MD; A. Rosengren, MD; G. Lappas, MD, Göteborg University. MRFIT: L. H. Kuller, MD, University of Pittsburgh; G. Grandits, MS, University of Minnesota. NCS: R. Selmer, PhD; A. Tverdal, PhD; W. Nystad, PhD, Norwegian Institute of Public Health, Norway. NHANES I, NHANES II, NHANES III: R. Gillum, MD, US Centers for Disease Control and Prevention; M. Mussolino, PhD, US National Institutes of Health. NHS: E. Rimm, ScD, Harvard University; S. Hankinson, ScD, Harvard School of Public Health; J. Manson, MD, Harvard Medical School; J. K. Pai, ScD, Brigham and Women’s Hospital. NORTH KARELIA: V. Salomaa, MD; K. Harald, MA; P. Jousilahti, MD; E. Vartiainen, MD, National Institute for Health and Welfare, Finland. NPHS I: T. W. Meade, FMedSci, London School of Hygiene and Tropical Medicine; J. A. Cooper, MSc, University College London; C. Knottenbelt, MD, London School of Hygiene and Tropical Medicine. NPHS II: J. A. Cooper, MSc, University College London; K. A. Bauer, MD, Harvard Medical School. OSAKA: S. Sato, MD; A. Kitamura, MD, Osaka Medical Center for Health Science and Promotion; Y. Naito, MD, Mukogawa Women’s University, Japan; H. Iso, MD, Osaka University. OSLO: I. Holme, PhD, Oslo University Hospital, Norway; R. Selmer, PhD; A. Tverdal, PhD; W. Nystad, PhD, Norwegian Institute of Public Health. OYABE: H. Nakagawa, MD, Kanazawa Medical University, Japan; K. Miura, MD, Shiga University of Medical Science, Japan. PARIS1: P. Ducimetiere, PhD; X. Jouven, MD, INSERM, France. PRHHP: C. J. Crespo, DrPH, Portland State University; M. R. Garcia Palmieri, MD, University of Puerto Rico. PRIME: P. Amouyel, MD, Institut Pasteur de Lille, France; D. Arveiler, MD, Université de Strasbourg, France; A. Evans, MD, Queen’s University of Belfast; J. Ferrieres, MD, University of Toulouse, France. PROCAM: H. Schulte, PhD; G. Assmann, FRCP, Assmann-Stiftung für Prävention, Germany. PROSPER: J. Shepherd, MD, Glasgow Royal Infirmary, United Kingdom; C. J. Packard, DSc; N. Sattar, FRCPath; I. Ford, PhD, University of Glasgow, United Kingdom. QUEBEC: B. Cantin, MD, Institut de Cardiologie de Québec, Hôpital Laval, Canada; B. Lamarche, PhD, Laval University; J-P. Després, PhD, Centre de recherche de l’Institut universitaire de cardiologie et de pneumologie de Québec, Canada; G. R. Dagenais, MD, Institut universitaire de cardiologie et pneumologie de Québec. RANCHO: E. Barrett-Connor, MD; D. L. Wingard, PhD; R. Bettencourt, MS, University of California. REYK: V. Gudnason, MD; T. Aspelund, PhD; G. Sigurdsson, MD, University of Iceland; B. Thorsson, MD, Icelandic Heart Association, Iceland. RIFLE: M. Trevisan, MD, Nevada System of Higher Education. ROTT: J. Witteman, PhD; I. Kardys, MD; M. Breteler, MD; A. Hofman, MD, Erasmus MC, the Netherlands. SHHEC: H. Tunstall-Pedoe, MD; R. Tavendale, PhD, University of Dundee, United Kingdom; G. D. O. Lowe, DSc, University of Glasgow; M. Woodward, PhD, Mount Sinai School of Medicine. SHS: B. V. Howard, PhD, Medstar Research Institute; Y. Zhang, MD, University of Oklahoma Health Sciences Center; L. Best, MD, Missouri Breaks Industries Research Inc; J. Umans, MD, Georgetown University Medical Center. SPEED: Y. Ben-Shlomo, PhD; G. Davey-Smith, MD, University of Bristol. TARFS: A. Onat, MD, Istanbul University, Turkey. TPT: T. W. Meade, FMedSci, London School of Hygiene and Tropical Medicine. TROMSØ: I. Njølstad, MD; E. B. Mathiesen, MD; M. L. Løchen, PhD; T. Wilsgaard, PhD, University of Tromsø, Norway. ULSAM: E. Ingelsson, MD, Karolinska Institutet; L. Lind, PhD, Uppsala University, Sweden; V. Giedraitis, PhD; L. Lannfelt, MD, Uppsala University Hospital. USPHS: J. M. Gaziano, MD, Brigham and Women’s Hospital; M. Stampfer, MD, Harvard School of Public Health; P. Ridker, MD, Brigham and Women’s Hospital. USPHS2: J. M. Gaziano, MD; P. Ridker, MD, Brigham and Women’s Hospital. VHMPP: H. Ulmer, PhD, Innsbruck Medical University, Austria; G. Diem, MD; H. Concin, MD, Agency for Preventive and Social Medicine, Austria. VITA: A. Tosetto, MD; F. Rodeghiero, MD, San Bartolo Hospital, Italy. WHITE I: M. Marmot, FMedSci, University College London; R. Clarke, MD; R. Collins, FMedSci, University of Oxford; A. Fletcher, PhD, London School of Hygiene and Tropical Medicine. WHITE II: E. Brunner, PhD; M. Shipley, MSc, University College London. WHS: P. Ridker, MD; J. Buring, ScD, Brigham and Women’s Hospital. WOSCOPS: J. Shepherd, MD, Glasgow Royal Infirmary, United Kingdom; S. M. Cobbe, FMedSci, BHF Glasgow Cardiovascular Research Centre, United Kingdom; I. Ford, PhD; M. Robertson, BSc, University of Glasgow. XIAN: Y. He, MD, Chinese PLA General Hospital, China. ZARAGOZA: A. Marin Ibaez, MD, San Jose Norte Health Centre, Spain. ZUTE: E. J. M. Feskens, PhD, Wageningen University, the Netherlands; D. Kromhout, PhD, Health Council of the Netherlands, The Hague.
Data Management Team: M. Walker, PhD; S. Watson, MMath, University of Cambridge, United Kingdom. Coordinating Centre: R. Collins, FMedSci, University of Oxford, United Kingdom; E. Di Angelantonio, MD; S. Erqou, MD; S. Kaptoge, PhD, University of Cambridge, United Kingdom; S. Lewington, DPhil, University of Oxford; L. Orfei, MSc; L. Pennells, MSc; P. L. Perry, MBChB; K. K. Ray, MD; N. Sarwar, PhD; M. Alexander, MPhil; A. Thompson, PhD, University of Cambridge; S. G. Thompson, DSc, MRC Biostatistics Unit, United Kingdom; M. Walker, PhD; S. Watson, MMath; F Wensley, MSc, University of Cambridge; I. R. White, MSc, MRC Biostatistics Unit; A. M. Wood, PhD; J. Danesh, FRCP (principal investigator), University of Cambridge.
Additional Information: eFigures 1-3, eTables 1-8, and eAppendixes 1-3 are available at http://www.jama.com.
REFERENCES
- 1.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7):498–511. doi: 10.1056/NEJM199908123410707. [DOI] [PubMed] [Google Scholar]
- 2.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Blair SN, Daniels SR, et al. American Heart Association Science Advisory and Coordinating Committee AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 5.Brunzell JD, Davidson M, Furberg CD, et al. J Am Coll Cardiol; Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation; 2008; pp. 1512–1524. [DOI] [PubMed] [Google Scholar]
- 6.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 7.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300(18):2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 8.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 11.Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 12.Fibrinogen Studies Collaboration Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35(6):1570–1578. doi: 10.1093/ije/dyl233. [DOI] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors Collaboration The Emerging Risk Factors Collaboration: analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22(12):839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 14.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Emerging Risk Factors Collaboration Lipoprotein (a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Fibrinogen Studies Collaboration Correcting for multivariate measurement error by regression calibration in meta-analyses of epidemiological studies. Stat Med. 2009;28(7):1067–1092. doi: 10.1002/sim.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I Study) Am J Cardiol. 2008;101(10):1428–1436. doi: 10.1016/j.amjcard.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 20.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 21.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 22.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed October 15, 2009];AIM HIGH: niacin plus statin to prevent vascular events. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00120289.
- 24.Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [Accessed October 15, 2009];Treatment of HDL to reduce the incidence of vascular events: HPS2-THRIVE. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00461630.
- 26.Wang J, Ban MR, Kennedy BA, et al. APOA5 genetic variants are markers for classic hyperlipoproteinemia phenotypes and hypertriglyceridemia. Nat Clin Pract Cardiovasc Med. 2008;5(11):730–737. doi: 10.1038/ncpcardio1326. [DOI] [PubMed] [Google Scholar]
- 27.Frikke-Schmidt R, Nordestgaard BG, Stene MCA, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesterol ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299(23):2777–2788. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed October 15, 2009];A study of RO4607381 in stable coronary heart disease patients with recent acute coronary syndrome. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00658515.
- 30.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326(7404):1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63(10):1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 32.Parish S, Peto R, Palmer A, et al. International Studies of Infarct Survival Collaborators The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30(17):2137–2146. doi: 10.1093/eurheartj/ehp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQueen MJ, Hawken S, Wang X, et al. INTERHEART study investigators Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Danielson E, Fonseca FAH, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.