Abstract
Human memory is strikingly susceptible to social influences, yet we know little about the underlying mechanisms. We examined how socially induced memory errors are generated in the brain by studying the memory of individuals exposed to recollections of others. Participants exhibited a strong tendency to conform to erroneous recollections of the group, producing both long-lasting and temporary errors, even when their initial memory was strong and accurate. Functional brain imaging revealed that social influence modified the neuronal representation of memory. Specifically, a particular brain signature of enhanced amygdala activity and enhanced amygdala-hippocampus connectivity predicted long-lasting, but not temporary memory alterations. Our findings reveal how social manipulation can alter memory and extend the known functions of the amygdala to encompass socially-mediated memory distortions.
Our memories are often inaccurate. Ubiquitous sources of false recollection are social pressure and interpersonal influence (1-4). This phenomenon, dubbed “memory conformity” (4), is encountered in a variety of contexts, including social interactions, mass media exposure and eyewitness testimony. In such settings an individual may change veridical recollections of past events to match a false account provided by others (1-6). Although these social influences on memory have been extensively demonstrated (1-5), the underlying neurobiology of this process is unknown.
Conformity may present in two forms which initially convey similar explicit behavior but are fundamentally different (7-8). In one type, known as private conformity, an individual’s recollection may genuinely be altered by social influence, resulting in long lasting, persistent memory errors (1,4-5,7). In such circumstances, even when social influence is removed, the individuals will persist in claiming an erroneous memory as part of their own experience (7,9). Private conformity could hence be considered a bona fide memory change. In the second type, known as ‘public conformity’, individuals may choose to outwardly comply, providing an account that fits that of others, but inwardly maintain certitude in their own original memory. Public conformity can be dispelled when the veracity of the socially transferred information abates (7,10-11). Thus, errors induced by public conformity are transient (7,9) and appear to represent a change in behavior in the absence of lasting alterations to a memory engram.
Although private and public memory conformity are often behaviorally indistinguishable, they reflect different cognitive processes (7-8). These processes are probably mediated by distinct activation in interconnected brain circuits previously found to be active in mnemonic functions and social cognition (such as the hippocampal complex, amygdala and frontal regions) (12-18). Here, we set out to characterize the brain mechanisms that lead to both types of conformity.
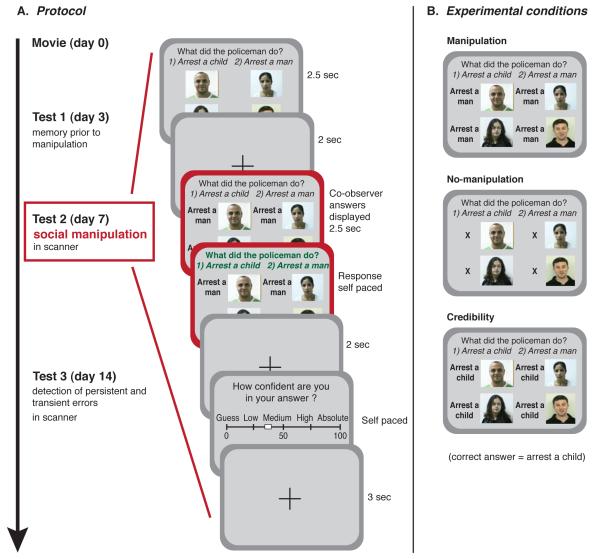
Our experimental protocol included four phases spanning a 2 week period (Fig. 1A). Thirty adult participants (12 females, age 28.6 ± 0.8, mean ± SEM) viewed an eyewitness-style documentary on a large screen in groups of five. Three days after viewing, participants returned to the lab individually and completed a memory test (Test 1). Test 1 served to assess the participants’ baseline accuracy and confidence before the manipulation stage. Four days later, participants returned to the lab and answered the same memory questions while being scanned with functional magnetic resonance imaging (fMRI) (Test 2). On this occasion a manipulation was introduced in an attempt to induce conformity.
Fig. 1. Experimental Outline.
(A) Participants viewed the movie in groups of five, and subsequently preformed three memory tests individually. Test 1 served to assess the participants’ initial memory and confidence before the social manipulation administered in Test 2. Test 3 served to identify memory errors that persisted after the social manipulation was removed. For the Test 2 scanning session, the question and possible answers were presented for 2.5 seconds, followed by the fabricated co-observers’ answers for 2.5 seconds. Subsequently, a font color change indicated that the participants were allowed to respond. Finally, confidence ratings were provided. (B) Illustration of the different experimental conditions; the manipulation condition in which all co-observers answers were incorrect, the no-manipulation condition in which the letter X was displayed instead of co-observers’ answers, and the credibility condition in which variable patterns of co-observers’ answers were displayed (SOM).
Before responding during this test, participants were presented with answers they were led to believe were given by their four fellow co-observers, whose photographs were provided with their corresponding answers (Fig. 1A). In a subset of trials, for which the target participant originally had a confident veridical memory (as identified by Test 1), the answers provided by the four co-observes were all false (manipulation condition, 80 questions). In matched control trials the letter X was presented instead of the co-observers answers (no-manipulation condition, 25 questions). Pilot data indicated that the use of manipulation and no-manipulation conditions alone would raise suspicion in the participants’ minds that the answers given by the co-observers were fabricated. Therefore we added credibility trials in which different patterns of co-observer answers were provided (Fig. 1B).
One week later the participants returned to the lab and were informed that the answers given by the co-observers during the previous fMRI session were in fact determined randomly. This rendered the socially conveyed information previously provided as uninformative. The participants were then requested to complete the memory test again (Test 3) based on their original memory of the movie. Finally, the participants were debriefed. Participants with excessive head movements in the scanner or suspected brain pathology and those that indicated suspicion of the manipulation were excluded from the analysis, resulting in a final number of participants (N) = 20.
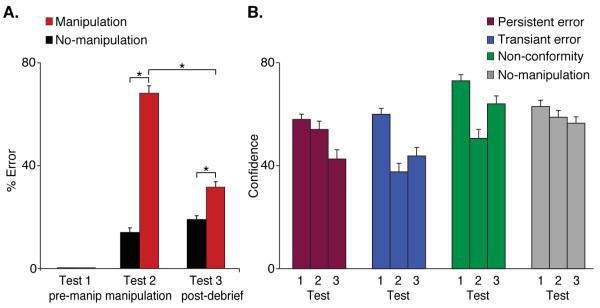
Our behavioral data revealed that our manipulation induced memory errors (Fig. 2A). Strikingly, participants conformed to the majority opinion in 68.3 ± 2.9% of manipulation trials – giving a false answer to questions they had previously answered correctly with relatively high confidence. This was not due to forgetting, because in the no-manipulation condition incorrect answers were given in only 15.5 ± 1.7% of the questions (t (19) = 16.9, p < 10−7). When social influence was removed (Test 3), participants reverted to their original, correct, answer in 59.2 ± 2.3% of the previously conformed trials (transient errors) but maintained erroneous answers in 40.8% (persistent errors). Confidence ratings in persistent and transient errors did not differ either before or after the manipulation stage (Fig. 2B). During the manipulation stage, confidence ratings in transient errors were significantly lower than in persistent errors (t = 6.9 p < 10−5). Differences in confidence levels were controlled for in the fMRI analysis by means of a covariate (Supporting Online Material, SOM).
Fig. 2. Behavioral results.
(A) Conformity level in the social manipulation condition was 68.3% versus 15.5% in the no-manipulation condition (t(19) = 16.9, p < 10−7). In Test 3 participants reverted back to their original correct answer in 59.2% of the previously conformed-to events (transient errors) and on 40.8% maintained their erroneous answer (persistent memory error). The error rate was significantly different in Test 3 between the manipulation and no-manipulation conditions (t (19) = 3.7 p < 0.002). The questions included in the manipulation and no-manipulation trials were those for which participents gave correct answers in Test 1 with medium-high confidence. (B) Confidence ratings over time for differential trial types. (* p < 0.002)
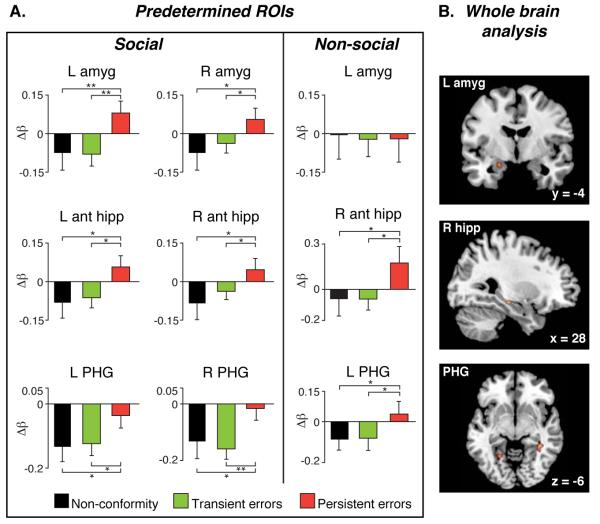
Our brain imaging data indicated that at the time of exposure to social influence, distinct brain signatures characterized instances of memory conformity that would result in persistent and transient errors. We first performed analysis on a-priori anatomically defined regions of interest (ROIs) selected by virtue of being widely implicated in memory encoding and maintenance (the bilateral anterior hippocampus, bilateral posterior hippocampus and bilateral parahippocampal gyrus) and in social-emotional processing (bilateral amygdala) (12-25). Brain activity was averaged across all voxels in each ROI for the three conditions of interest (persistent errors, transient errors and instances when participants did not conform to the erroneous information, i.e. non-conformity). In all regions, except for the left posterior hippocampus, the blood oxygen level dependent (BOLD) signal was greater during trials that subsequently resulted in persistent memory errors relative to trials that resulted in transient errors or non-conformity (Fig. 3A). No significant difference was found between transient error and non-conformity trials in these regions.
Fig. 3. MTL activation during manipulation predicts long-term socially-induced memory errors.
(A) BOLD signal in anatomically a-priori defined MTL regions. L, left; R, right; In the social manipulation, enhanced activation was found during trials that subsequently resulted in persistent errors relative to all other conditions in the bilateral hippocampal complex and amygdala. In the non-social manipulation this pattern was evident in the hippocampal complex but not in the bilateral amygdala (B) Whole brain exploratory analysis in the social manipulation (p < 0.001, k > 10) revealed greater activity in persistent error versus transient error in the left amygdala, right hippocampus, right PHG and left PHG bordering on the occipital lobe. All areas also survived small volume correction for multiple comparisons (family wise error < 0.05). The baseline in all figures is the no-manipulation condition. (* p < 0.05 ** p < 0.005)
To examine whether other brain regions differentiate between persistent and transient errors, we conducted a whole brain exploratory analysis. Greater activity during trials resulting in persistent errors versus trials resulting in transient errors was found in four regions, all in the medial temporal lobe (MTL, Fig. 3B): the left amygdala (−22,−8,−10), right hippocampus (28,−22,−12), right parahippocampal gyrus (PHG, 36,−48,−10) and a region bordering the left PHG and occipital cortex (−22,−54,−10), [p < 0.001, cluster threshold (k) > 10]. In the opposite comparison (transient vs. persistent errors) enhanced activation was found in the bilateral dorsal anterior cingulate cortex (ACC, Brodman Area 32; −12,22,42; 8,20,46).
A striking activation in both aforementioned analyses was found in the left amygdala. A behavioral control study (SOM) indicated that elevated activation in the amygdala during trials that resulted in persistent errors was not due to heightened emotional arousal during these trials. Nor were these errors related to questions associated with greater emotional content. Rather, heightened amygdala activation seemed specific to socially induced memory change.
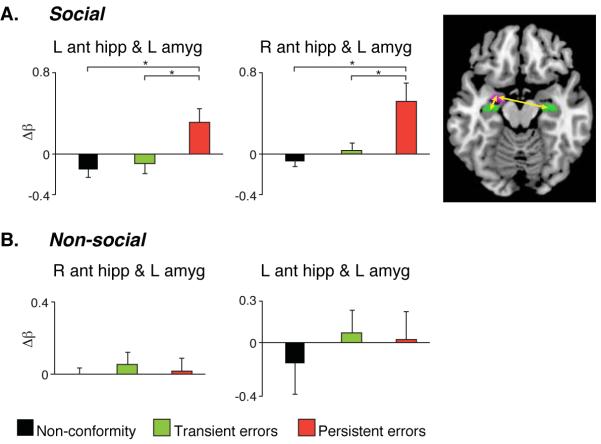
The amygdala plays a key role in social and emotional processing and modulates memory related hippocampal activity (13-23). It is strategically placed for this function, having rich anatomical connections with the hippocampal complex (the anterior hippocampus in particular) as well as with neocortical areas (13-16,23,26). The amygdala is thus a prime candidate for mediating social effects on memory, most likely involving its interactions with other brain regions (13-14). This consideration motivated us to carry out a functional connectivity analysis, using a psychophysiological interaction (PPI) approach (27). This analysis showed heightened functional connectivity between the left amygdala and bilateral anterior hippocampus within anatomically defined ROIs, during trials that subsequently resulted in persistent memory errors as opposed to transient errors and non-conformity (Fig. 4A).
Fig. 4. Amygdala-hippocampal functional connectivity during manipulation predicts long-term socially induced memory errors.
(A) Social manipulation. Functional connectivity between the left amygdala and bilateral anterior hippocampus was heightened in the persistent error condition relative to all other conditions. (B) Non-social manipulation. No condition dependent difference in functional connectivity between the left amygdala and bilateral anterior hippocampus was found. The baseline in all figures is the no-manipulation condition (*p < 0.05). The inset depicts the anatomical ROIs used in the aforementioned analyses..
We also sought to identify which brain regions responded to the information presented by the co-observers (SOM). To this end, trials in which misleading information was presented (the manipulation condition) were contrasted with the no-manipulation condition. Five regions (Fig. S1A) were identified in the frontal and occipital cortex. Further analysis of brain activity in these regions (Fig. S1B) suggests that they are involved in non-mnemonic processes such as conflict monitoring (28-31) in the face of competing memories (32-34).
Were our findings driven merely by the presentation of additional information regardless of social context? To answer this question we performed a control fMRI experiment using a non-social medium to convey misinformation (SOM). Participants underwent a similar protocol to that of our main experiment. However, in memory Test 2, instead of receiving answers from co-observers, participents were told that the information originated from four different computer algorithms, a common technique used to control for social effects (30). Conformity in this case was significantly lower (45.3 ± 4.7%) than in the social manipulation described earlier (68.3 ± 2.9%) but significantly higher than with no-manipulation at all (15.0 ± 2.4%) (t (38) = 4.2 and t (19) = −5.7 respectively; p < 0.0002).
Analysis of BOLD signal in the a-priori MTL ROIs revealed an interaction between memory (persistent errors and transient errors) and experimental manipulation (social and non-social) in the bilateral amygdala (p < 0.05). This interaction was driven by greater activation in trials resulting in persistent memory errors relative to transient errors in the social manipulation, but not in the non-social manipulation (Fig. 3A). These results suggest that enhanced activity in these regions is related specifically to socially-induced persistent memory errors. In contrast the right anterior and posterior hippocampus and left PHG revealed a main effect of memory (p < 0.05) where there was greater activity during trials resulting in persistent relative to transient errors regardless of manipulation type (p < 0.05) (Fig. 3A). Thus the BOLD signal in these regions was associated with long-lasting memory errors irrespective of the medium by which information was conveyed. Results of a functional connectivity analysis between the left amygdala and bilateral anterior hippocampus showed a significant interaction (p < 0.05). Heightened connectivity was seen during trials that resulted in persistent errors relative to transient errors, a pattern specific to the social manipulation (Fig. 4B). Our control experiment’s results hence indicate that heightened amygdala activation and enhanced connectivity with the hippocampus are specific to socially-induced memory changes, whereas hippocampal complex activation differentiates between persistent and transient errors regardless of the source of influence.
Our results indicate that memory is highly susceptible to alteration due to social influence, creating both transient and persistent errors. After over a century of intensive behavioral research into social influences on memory (35) this study now provides a brain account of this phenomenon. Our findings suggest a mechanism by which social influence produces long-lasting alterations in memory, and they highlight the critical role of the amygdala in mediating this influence.
Although at the time of social influence on memory overt behavior was indistinguishable, transient and persistent errors nevertheless induced distinct brain signatures. Heightened activation in the hippocampal complex was seen when false information induced a long-lasting change in the participants’ memories regardless of social context. The hippocampal complex activation we observed may represent a process of reconsolidation (36) or encoding of new stable representations (e.g. gist, 37). In contrast transient changes did not activate areas known to be crucial for memory processing. Our findings provide neurobiological evidence for the classic assertion that private conformity is accompanied by actual changes in beliefs whereas public displays of conformity are not (7-8,10,38).
Enhanced activation in the bilateral amygdala and heightened functional connectivity with anterior hippocampus were a signature of long-term memory change induced by the social environment. This indicates that the incorporation of external social information into memory may involve the amygdala’s intercedence, in accordance with its special position at the crossroads of social cognition and memory (13-14,16).
Multiple formal models have proposed trace attributes that might contribute to memory distortion in different false memory protocols (37, 39-41). These postulated attributes refer, for example, to potential heterogeneity in episodic content and the persistence of memory trace elements. Our laboratory analogue to socially induced memory distortion was not intended to distinguish between specific models. However, further exploitation of our protocol, combined with cross-fertilization of behavioral and brain data, might contribute to the refinement of current models and better understanding of the biological and cognitive mechanisms of memory conformity.
Altering memory in response to group influence may produce untoward effects. For example, social influence such as false propaganda can deleteriously affect individuals’ memory in political campaigns and commercial advertising (1-2,6) and impede justice by influencing eyewitness testimony (2,4,5). However, memory conformity may also serve an adaptive purpose, because social learning is often more efficient and accurate than individual learning (42). For this reason humans may be predisposed to trust the judgment of the group, even when it stands in opposition to their own original beliefs. Such influences and their long-term effects, the neurobiological basis of which we describe here, may contribute to the extraordinary levels of persistent conformity seen in authoritarian cults and societies.
Supplementary Material
Acknowledgments
M.E. was supported by a Weizmann Institute-UK Grant. T.S. is supported by British Academy Postdoctoral Fellowship. R.J.D is supported by a Wellcome Trust Program Grant. Y.D. is supported by the Nella and Leon Benoziyo Center for Neurological Diseases. We thank A. Ben-Yakov, J.G. Edelson, T. Fitzgerald, O. Furman, S. Fleming, D. Levi, M. Guitart-Masip, A. Mendelsohn, U. Nili, A. Pine, J.S. Winston and N. Wright for helpful comments and the support teams of the Norman and Helen Asher Center for Brain Imaging at the Weizmann Institute and the Imaging Neuroscience & Theoretical Neurobiology unit in the Wellcome Trust Center for Neuroimaging.
Footnotes
Supporting Online Material www.sciencemag.org Materials and Methods Fig. S1 Table S1 References
References and Notes
- 1.Meade ML, Roediger HL., III Explorations in the social contagion of memory. Mem. Cognition. 2002;30:995–1009. doi: 10.3758/bf03194318. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EF. Planting misinformation in the human mind: A 30 year investigation of the malleability of memory. Learn. Memory. 2005;12:361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- 3.Schacter DL. The Seven sins of memory: how the mind forgets and remembers. Houghton-Mifflin; New York: 2001. [Google Scholar]
- 4.Wright DB, Memon A, Skagerberg EM, Gabbert F. When eyewitnesses talk. Curr. Dir. Psychol. Sci. 2009;18:174–178. [Google Scholar]
- 5.Shaw JS, Garven S, Wood JM. Co-witness information can have immediate effects on eyewitness memory reports. Law Human Behav. 1997;21:503–523. doi: 10.1023/a:1024875723399. [DOI] [PubMed] [Google Scholar]
- 6.Perkins HW, Linkenbach JW, Lewis MA, Neighbors C. Effectiveness of social norms media marketing in reducing drinking and driving: A statewide campaign. Addict. Behav. 2010;35:866–874. doi: 10.1016/j.addbeh.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith E, Mackie D. Social psychology. ed. 3 Psychology Press; London: 2007. [Google Scholar]
- 8.Allen V. In: Advances in Experimental and Social Psychology. Berkowitz L, editor. Academic Press; New York: 1965. pp. 133–170. [Google Scholar]
- 9.Reysen MB. The effects of conformity on recognition judgments. Memory. 2005;13:87–94. doi: 10.1080/09658210344000602. [DOI] [PubMed] [Google Scholar]
- 10.Asch SE. In: Groups, Leadership and Men. Guetzkow H, editor. Carnegie Press; Pittsburgh: 1951. pp. 39–76. [Google Scholar]
- 11.Festinger L. A theory of cognitive dissonance. Peterson; Evanston, IL: 1957. pp. 99–100. [Google Scholar]
- 12.Dudai Y. Memory from A to Z. Keywords, Concepts and Beyond. Oxford Univ. Press; Oxford: 2002. [Google Scholar]
- 13.Adolphs R. Cognitive neuroscience of human social behavior. Nat. Rev. Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 14.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu. Rev. Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 15.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 16.Dolan RJ. Emotion cognition and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 17.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat. Neurosci. 2010;14:163. doi: 10.1038/nn.2724. (doi:10.1038/nn.2724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochsner KN. In: Social Neuroscience: People thinking about people. Cacioppo JT, editor. MIT Press; Cambridge, MA: 2005. pp. 245–268. [Google Scholar]
- 19.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. R. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 20.Squire LR. memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Aggleton JP. The Amygdala: Second Edition. A Functional Analysis. Oxford University Press; Oxford: 2000. [Google Scholar]
- 22.Kluver H, Bucy PC. An analysis of certain effects of bi-lateral temporal lobectomy in the rhesus monkey with special reference to ‘psychic blindness’. J. Psychol. 1938;5:33–54. [Google Scholar]
- 23.Richardson M, Strange B, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 24.Okado Y, Stark CEL. Neural activity during encoding predicts false memories created by misinformation. Learn. Mem. 2005;12:3–11. doi: 10.1101/lm.87605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 26.Stefanacci L, Amaral DG. Some observations in cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J. Comp. Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 28.Cabeza R, Nyberg RL. Image cognition II. An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 29.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat. Neurosci. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- 33.Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn. Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JP, Dodson CS, Schacter DL. fMRI evidence for the role of recollection in suppressing misattribution errors: The illusory truth effect. J. Cogn. Neurosci. 2005;17:800–810. doi: 10.1162/0898929053747595. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett FC. Remembering. Cambridge University Press; Cambridge: 1932. [Google Scholar]
- 36.Dudai Y. The neurobiology of consolidations, or, how stable is the engram. Ann Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 37.Reyna VF, Brainerd CJ. Fuzzy-trace theory: An interim synthesis. Learn. Individ. Diff. 1995;7:1–75. [Google Scholar]
- 38.Sherif M. The psychology of social norms. Harper Collins; New York: 1936. [Google Scholar]
- 39.Roediger HL, 3rd, Watson JM, McDermott KB, Gallo DA. Factors that determine false recall: A multiple regression analysis. Psychon. B. Rev. 2001;8:385–407. doi: 10.3758/bf03196177. [DOI] [PubMed] [Google Scholar]
- 40.Arndt J. The role of memory activation in creating false memories of encoding context. J. exp. Psychol. Learn. 2010;36:66–79. doi: 10.1037/a0017394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howe ML. What is false memory development the development of? Comment on Brainerd, Reyna, and Ceci. Psycholo. Bull. 2008;134:768–772. doi: 10.1037/0033-2909.134.5.768. [DOI] [PubMed] [Google Scholar]
- 42.Boyd R, Richardson PJ. In: social learning: psychological and biological perspectives. Zentall RR, Galef BJ, editors. Erlbaum; Hillsdale, NJ: 1988. pp. 29–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.