Abstract
In order to elucidate the relationship between androgens and the function of the muskrat (Ondatra zibethicus) scented glands during the breeding season, we investigated immunolocalization of steroidogenic enzymes P450scc, 3βHSD and P450c17 in the muskrat testes and scented glands. Nine adult muskrats were obtained in March (n=3), May (n=3) and July (n=3) 2010. Steroidogenic enzymes were immunolocalized using polyclonal antisera raised against bovine adrenal P450scc, human placental 3βHSD and porcine testicular P450c17. Histologically, all types of spermatogenic cells including mature-phase spermatozoa in seminiferous tubules were observed in all testes. Glandular cells, interstitial cells, epithelial cells and excretory tubules were identified in scented glands during the breeding season. P450scc, 3βHSD and P450c17 were only identified in Leydig cells during the breeding season; P450scc and P450c17 were observed in glandular cells of scented glands, however, 3βHSD was not found in scented glands during the breeding season. These novel findings provide the first evidence showing that scented glands of the muskrats are capable of locally synthesizing androgens and androgens acting via an endocrine, autocrine or paracrine manner may play an important role in scented gland function during the breeding season.
Key words: androgen, immunohistochemistry, muskrat, scented gland, testes.
Introduction
Sex steroid hormones regulate diverse reproductive physiological processes of target tissues and are secreted mainly by the ovary, testis, placenta and adrenal cortex. Two major families of enzymes are responsible for steroid biosynthesis. The first are the hydroxylase enzymes, encoded by genes belonging to the cytochrome P450 superfamily. The second family, the steroid dehydrogenase enzymes, belongs to one of two distinct groups, the short-chain alcohol dehydrogenase/reductase family or the aldo-keto reductase superfamily.1 Cholesterol side-chain cleavage cytochrome P450 (P450scc), located on the matrix side of inner mitochondrial membranes, catalyzes the conversion of substrate cholesterol to pregnenolone, a common precursor of all steroid hormones.2 Utilization of this universal steroid substrate is dictated by two enzymes. Thus, the absolute and relative levels of 3β-hydroxysteroid dehydrogenase (3βHSD) and 17α-hydroxylase cytochrome P450 (P450c17) direct subsequent steroidogenesis, therefore, these two enzymes occupy a pivotal position in the pathways leading to androgen and progesterone synthesis.3
In mammalian testes, P450scc converts cholesterol to pregnenolone and is located in the inner mitochondrial membrane of the Leydig cell; the other biosynthetic enzymes 3βHSD and P450c17 are situated in the endoplasmic reticulum. Androgens are synthesized in the endoplasmic reticulum of Leydig cells and 3βHSD is the key enzyme in the regulation of the production of testosterone.4–8 By contrast, there is the assertion that P450c17 is the predominant regulator of testosterone production.9–11 Thus, studies on the regulation of androgen synthesis in Leydig cells remain controversial. The functional significance of gonadal derived sex steroid hormones has been extensively studied; however, it is only recently that the importance of local extragonadal derived sex steroid hormones in cell physiology and pathophysiology is beginning to be appreciated in tissues such as brain, adipose tissue, breast, skin, bone and adrenal gland.12–14 Local metobolic pathways for testosterone and estrogen have recetly been reported in the skeletal muscle cell, implying that skeletal muscle is potentially an important extragonadal source of sex steroid hormone.15,16
The muskrat (Ondatra zibethicus) is a medium sized, semiaquatic rodent living throughout Canada and the United States and in some parts of northern Mexico. The muskrat gets its name from the two scented glands near its tail that give off a musky odor.17 The muskrat is a seasonal breeder with sexually active period of approximately 8 months from March to October. During the breeding period, the male muskrat is sexually active and produces mature spermatozoa. Additionally, their scented glands secret perfume substances that are used as expensive traditional Chinese medicines.18 In our previous studies, androgen receptor (AR), P450arom, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) were present in scented gland tissue of muskrats during the breeding season, which suggested the scented glands were the target organ for androgens, estrogens locally produced by P450arom could enhance scented gland function through autocrine/paracrine mechanism.19 To date, it is unclear if androgens acting on the scented glands are locally produced by steroidogenic enzymes. To help clarify this, we hypothesized that steroidogenic enzymes, especially P450scc, 3βHSD and P450c17, were present in the scented glands and locally synthesized androgen. Thus, the purpose of this study is to investigate immunolocalization of P450scc, 3βHSD and P450c17 in the scented glands and testicular tissues, and to further elucidate the relationship between androgens and scented gland function in the muskrats.
Materials and Methods
Animals
Nine male adult muskrats were obtained in March (n=3), May (n=3) and July (n=3) 2010 from Xichuan Wangnong Muskrats Breeding Farm, Beijing, China. The muskrats were housed with one male and one female in one enclosure. All the animals were treated in accordance with the National Animal Welfare Legislation. All experimental procedures were carried out in accordance with the guidelines established by the Beijing Forestry University. After sacrifice, complete sets of testicular and scented glandular tissues were obtained from each male muskrat. The tissues were immediately fixed for 12 h in Bouin's solution or 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO, USA) in 0.05 M PBS, pH 7.4 for histological and immunohistochemical studies.
Histology
The testicular and scented glandular samples were dehydrated in ethanol series and embedded in paraffin wax. Serial sections (4 µm) were mounted on slides coated with poly-L-lysine (Sigma). Some sections were stained with hematoxylin-eosin (HE) for observations of general histology. The remaining sections were processed for immunohistochemistry.
Immunohistochemistry
The serial sections of testes and scented glands were incubated with 10% normal goat serum to reduce background staining caused by the second antibody. The sections were then incubated with primary antibodies (1:500 or 1:1000) raised against bovine adrenal cholesterol side-chain cleavage cytochrome P450 (P450scc),20 human placental 3β-hydroxysteroid dehydrogenase (3βHSD)21 and porcine testicular 17α-hydroxylase cytochrome P450 (P450c17)22 for 16 h at room temperature. The antibody to 3βHSD was kindly supplied by Dr. J.I. Mason (Edinburgh University, UK). Antibodies against P450scc and P450c17 were kindly supplied by Dr. M.J. Soares (University of Kansan Medical Center, Kansas City, KS, USA) and Dr. D.C. Johnson (Department of Gynecology and Obstetrics and Physiology, University of Kansas Medical Center, Kansas City), respectively. The sections were then incubated with a secondary antibody in a rabbit ExtrAvidinTM staining kit (Sigma), including goat anti-rabbit lgG conjugated with biotin and peroxidase with avidin, followed by visualization with 30 mg 3,3-diaminobenzidine (Wako, Tokyo, Japan) solution in 150 mL of 0.05 mol Tris-HCl l−1 buffer, pH 7.6, plus 30 µL H2O2. Finally, positively staining sections were counterstained with hematoxylin solution (Merck, Tokyo, Japan). The control sections were treated with normal rabbit serum (Sigma) instead of the primary antisera.
Results
Histology
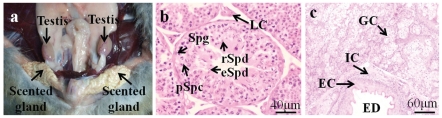
Morphological observation of testicular and scented glandular tissues were done in male muskrat during the breeding season (Figure 1a). The histological conformations of testes and scented glands were both observed in the muskrats by hematoxylin-eosin (HE) staining (Figure 1 b,c). The Leydig cells and spermatogenic cells, including spermatogonia, primary spermatocytes, round spermatids and elongate spermatids in the seminiferous tubules were identified in the testes of the muskrats collected in March, May and July 2010 (Figure 1b). Glandular cells, interstitial cells and epithelial cells of the excretory duct were observed in the scented gland of the male muskrat using hematoxylin-eosin (HE) staining. In the histological structure of scented glands, the epithelial cells of the excretory duct were idntified, interstitial cells were found in the connection between the ducts and acini or among each acinus, and glandular cells which secreted musk were the main cell type in the scented gland of male muskrat collected in March, May and July 2010 (Figure 1c).
Figure 1.
Anatomic localization of the muskrat testes and scented glands, and the histolog-ic structure of the testes and scented glands by hematoxylin-eosin (HE). a) The testes and scented glands of the muskrat; b) The entire spermatogenic population from spermatogonia (Spg) to spermatozoa (Spz) was observed in seminiferous tubule, c) Glandular cells of acini, interstitial cells, and epithelial cells of large excretory duct were observed in the scented gland; LC, Leydig cells; Spg, spermatogonia; pSpc, primary spermatocytes; rSpd, round spermatids; eSpd, elongate spermatids; GC, glandular cells; EC, epithelial cells; IC, interstitial cells; ED, excretory duct. Scale bars: 2 cm (a), 40 µm (b) and 60 µm (c).
Immunohistochemistry
Immunoreactivity for P450scc, 3βHSD and P450c17 were present in both the testes and scented glands of the muskrat during the breeding seasons (Figure 2). P450scc, 3βHSD and P450c17 were immunolocalized in Leydig cells of all testes (Figure 2 a,b,c). Immunoreactivity for P450scc, 3βHSD or P450c17 was not observed in the Sertoli or germ cells. The intensities of the immunohistochemical signals for P450scc, 3βHSD and P450c17 did not appear markedly different among the testes collected in March, May and July 2010. Positive immunostainings for P450scc and P450c17 were present in the cytoplasm of glandular cells of the scented glands during the breeding season (Figure 2 e,g). Immunoreactivity for 3βHSD was not found in the scented glands of the muskrats in March, May and July 2010 (Figure 2f). No immunostaining was detected in control sections of testes and scented glands in which the primary antibody was substituted with normal rabbit serum (Figure 2 d,h).
Figure 2.
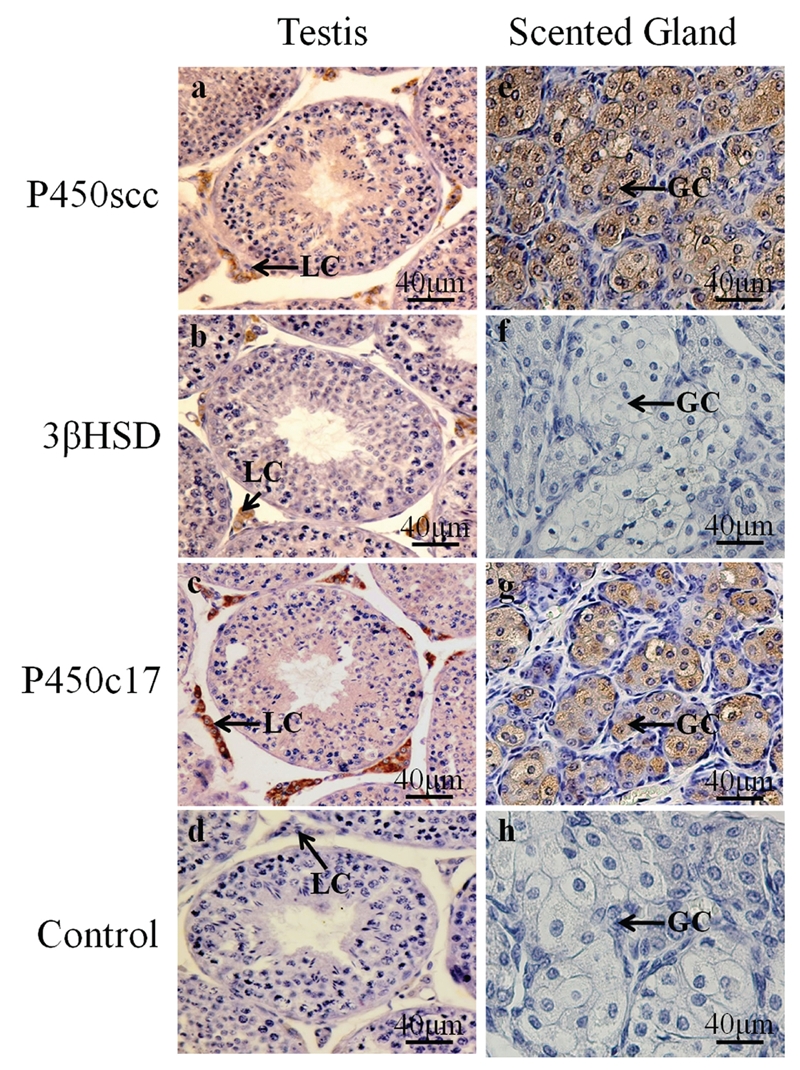
Immunolocalization of P450scc, 3 HSD and P450c17 in the muskrat testes and scented glands during the breeding season. P450scc was identified in Leydig cells of testes (a) and glandular cells of scented glands (e); immunostaining for 3 HSD was only found in Leydig cells (b), but not in glandular cells (f); immunostaining for P450c17 was present in Leydig cells (c) and glandular cells (g); No immunostaining was detected in control Leydig cells (d) or glandular cells (h) sections in which normal rabbit serum was used instead of primary antibody. LC, Leydig cells; GC, glandular cells. Scale bar: 40 µm.
Discussion
To the best of our knowledge, this is the first study that describes immunolocalization of steroidogenic enzymes P450scc, 3βHSD and P450c17 in testicular and scented glandular tissues of the muskrat. The results of this study demonstrated that P450scc, 3βHSD and P450c17 were identified in the testes of the muskrat, and P450scc and P450c17 were present in the scented glands of muskrats during the breeding season. These findings suggest that both the testes and scented glands have the ability to synthesize androgens, and androgens acting via an endocrine, autocrine or paracrine manner may have an important role in scented glandular function during the breeding season.
In this study, P450scc, 3βHSD and P450c17 were only identified in Leydig cells during the breeding season. These results suggest that the synthesis of progestins and androgens occurs in Leydig cells during the breeding season (both delta 4 and delta 5 C21 steroids). These findings were similar to those observed in other seasonal breeders such as stallions,23 sika deers24 and bank voles,25 which demonstrated that Leydig cells were the predominant sites of androgen synthesis. However, other studies of seasonal breeders, such as Hokkaido brown bears,26 Japanese black bears,27 wild raccoon dogs,28 wild ground squirrels29 and bank voles,30 showed that steroidogenic enzymes were not only identified in Leydig cells, but also in spermatids during the breeding season, indicating that both of Leydig cells and spermatids had ability to synthetise androgen. Therefore, the present results suggest that in seasonal breeders, Leydig cells and spermatids may exhibit different steroidogenic capacities similar to other species.
In this study, P450scc and P450c17 were expressed in scented gland tissues of the muskrats during the breeding season (March, May and July), showing that the muskrat scented glands are potentially an important extragonadal source of androgens. Our previous studies indicated that AR, P450arom and ERs (ERα and ERβ) were present in scented glandular tissues of muskrats during the breeding season, suggesting that estrogens produced locally in the scented glands, acting as an autocrine/paracrine factor through ERα and ERβ, might play a role in the regulation of scented glandular function.19 Together with our current results, we propose the existence of a local autocrine and/or paracrine system of androgens and estrogens actions in scented glands of muskrats. Androgens and estrogens acting via endocrine, autocrine and/or paracrine mechanisms may play an important role in scented gland function during the breeding season.
Generally, steroid production is regulated by the relative levels and a tissue-specific array of steroidogenic enzymes. Steroidogenic organs may exhibit functional zonation or compartmentalization.31 Interestingly, the present study showed that 3βHSD was not found in scented gland tissues of muskrats during the breeding season, indicating that the muskrat scented glands were incapable of converting pregnenolone to progesterone or dehydroepiandrosterone to androstenedione. In our present study however, P450c17 was identified in glandular cells of scented glands and previous research identified P450arom in scented gland tissues,19 implying that the scented glands have the capacity to synthesize testosterone and estrogen. Thus, it is possible the progesterone or androstenedione, as a precursor of testosterone and estrogen, is synthesized by the testes or adrenal glands of the muskrats. The characteristic of compartmentalization is not unique to scented glands of muskrats, similar observations have been found in other extragonadal tissues, such as placentae of the Japanese Shiba goat and equine fetal adrenal glands. In the Japanese Shiba goat, due to a lack of a placental 3βHSD expression, androgen synthesis from placental P450c17 expression relied on androgens synthetised by 3βHSD which was expressed in the maternal ovaries.32 The source of progesterone as a precursor of androgen synthesis in equine fetal adrenal glands, was supplied by the fetal gonads or placenta since 3βHSD was absent from the equine fetal adrenal glands.33 Taken together, this suggests that the present results are comparable with the broader concept that steroid hormone synthesis is a complex process, involving the activity of several enzymes and sometimes interactions among several tissues.34
In summary, the present results show for the first time that there are clear functional distinctions in terms of steroidogenic capacity in the testes and scented glands of the muskrat. P450scc, 3βHSD and P450c17 were expressed in Leydig cells and P450scc and P450cl7 were expressed in scented glandular tissue, which suggests that the steroid synthesis in the muskrat requires cooperation between tissues and tissues expressing different steroidogenic enzymes to promote normal levels of steroid hormones synthesis for the maintenance of testicular and scented glandular functions. However, the current data demonstrate that their expression was still very distinct and region specific at the cellular level. The purpose of this compartmentalization of steroidogenic enzymes at the tissue or cell levels remains to be discovered, and the continued study of the muskrat testes and scented glands will provide a useful animal model for studying this and other aspects of androgen and estrogen synthesis.
Acknowledgments:
the authors would like to thank Dr. J.I. Mason (Edinburgh University, UK) for providing the antibodies of 3 HSD; Dr. M.J. Soares (University of Kansas Medical Center, Kansas City, KS, USA), and Dr. D.C. Johnson (The Department of Gynecology and Obstetrics and Physiology, University of Kansas Medical Center), for providing antibodies of P450scc and P450c17, respectively. They also express their great appreciation to Dr. Johanna Rochester (University of Colorado, Boulder, CO, USA) and Scott Kavanaugh (University of Colorado), who help them to revise the English form of this paper. This study is supported by a Grant-in-Aid from the Program for the Fundamental Research Funds for the Central Universities (BLYX200921), the Beijing Forestry University Young Scinentist Fund (BLX2W8004) and the Changjiang Scholars and Innovative Research Team in Universities (IRT0607) from China.
References
- 1.Hinshelwood MM. Steroidogenesis, Overview. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. Academic Press; CA, USA: 1998. pp. 644–653. [Google Scholar]
- 2.Boerboom D, Sirois J. Equine P450 cholesterol side-chain cleavage and 3 beta-hydroxysteroid dehydrogenase/delta(5)-delta(4) isomerase: molecular cloning and regulation of their messenger ribonucleic acids in equine follicles during the ovulatory process. Biol Reprod. 2001;64:206–15. doi: 10.1095/biolreprod64.1.206. [DOI] [PubMed] [Google Scholar]
- 3.Conley AJ, Kaminski MA, Dubowsky SA, Jablonka-Shariff A, Redmer DA, Reynolds LP. Immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase and P450 17 alpha-hydroxylase during follicular and luteal development in pigs, sheep, and cows. Biol Reprod. 1995;52:1081–94. doi: 10.1095/biolreprod52.5.1081. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Henry HL, Norman AW. Androgen effects in mammals. In: Henry HL, Norman AW, editors. Encyclopedia of Hormones. Academic Press; CA, USA: 2003. pp. 70–83. [Google Scholar]
- 5.Samuels LT, Helmreich ML. The influence of chorionic gonadotropin on the 3beta-ol dehydrogenase activity of testes and adrenals. Endocrinology. 1956;58:435–42. doi: 10.1210/endo-58-4-435. [DOI] [PubMed] [Google Scholar]
- 6.Levy H, Deane HW, Rubin BL. Visualization of steroid-3beta-o1-dehydrogenase activity in tissues of intact and hypophysectomized rats. Endocrinology. 1959;65:932–43. doi: 10.1210/endo-65-6-932. [DOI] [PubMed] [Google Scholar]
- 7.Niemi M, Ikonen M. Cytochemistry of oxidative enzyme systems in the Leydig cells of the rat testis and their functional significance. Endocrinology. 1962;70:167–74. doi: 10.1210/endo-70-2-167. [DOI] [PubMed] [Google Scholar]
- 8.Niklowitz P, Khan S, Bergmann M, Hoffmann K, Nieschlag E. Differential effects of follicle-stimulating hormone and luteinizing hormone on Leydig cell function and restoration of spermatogenesis in hypophysectomized and photoinhibited Djungarian hamsters (Phodopus sungorus) Biol Reprod. 1989;41:871–80. doi: 10.1095/biolreprod41.5.871. [DOI] [PubMed] [Google Scholar]
- 9.Payne AH, Sha LL. Multiple mechanisms for regulation of 3 beta-hydroxysteroid dehydrogenase/delta 5----delta 4-isomerase, 17 alpha-hydroxylase/C17–20 lyase cytochrome P450, and cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology. 1991;129:1429–35. doi: 10.1210/endo-129-3-1429. [DOI] [PubMed] [Google Scholar]
- 10.Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–25. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- 11.Wing TY, Ewing LL, Zirkin BR. Effects of luteinizing hormone withdrawal on Leydig cell smooth endoplasmic reticulum and steroidogenic reactions which convert pregnenolone to testosterone. Endocrinology. 1984;115:2290–6. doi: 10.1210/endo-115-6-2290. [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–30. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 13.Maia HJ, Casoy J, Valente J. Testosterone replacement therapy in the climacteric: benefits beyond sexuality. Gynecol Endocrinol. 2009;25:12–20. doi: 10.1080/09513590802360744. [DOI] [PubMed] [Google Scholar]
- 14.Czajka-Oraniec I, Simpson ER. Aromatase research and its clinical significance. Endokrynol Pol. 2010;61:126–34. [PubMed] [Google Scholar]
- 15.Aizawa K, Iemitsu M, Maeda S, Jesmin S, Otsuki T, Mowa CN, et al. Expression of steroidogenic enzymes and synthesis of sex steroid hormones from DHEA in skeletal muscle of rats. Am J Physiol Endocrinol Metab. 2007;292:577–84. doi: 10.1152/ajpendo.00367.2006. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:961–8. doi: 10.1152/ajpendo.00678.2007. [DOI] [PubMed] [Google Scholar]
- 17.Van Dorp. New macrocyclle compounds for the secretions of the civet cat and the muskrat. Recuei. 1973;92:9–15. [Google Scholar]
- 18.Chen YS, Zhao WG, Zhao M, Chang ZJ, Zhang Y, Ma DW. Histological Observation on Musk-secreting Scenteded Gland in Muskrat. Chin J Zoology. 2007;42:91–5. [Google Scholar]
- 19.Lu L, Zhang H, Lv N, Ma X, Tian L, Liu S, et al. Zool Sci. 2011. Immunolocalization of androgen receptor, aromatase cytochrome P450, estrogen receptor alpha and estrogen receptor beta proteins during the breeding season in scenteded glands of muskrats (Ondatra zibethicus) (In press) [DOI] [PubMed] [Google Scholar]
- 20.Roby KF, Larsen D, Deb S, Soares MJ. Generation and characterization of antipeptide antibodies to rat cytochrome P-450 side-chain cleavage enzyme. Mol Cell Endocrinol. 1991;79:13–20. doi: 10.1016/0303-7207(91)90090-f. [DOI] [PubMed] [Google Scholar]
- 21.Doody KM, Carr RR, Rainy WE, Byed W, Murry BA, Strickler RC, et al. 3β-hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology. 1990;126:2487–92. doi: 10.1210/endo-126-5-2487. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DC. Cellular localization and factors controlling rat placental cytochrome P45017 alpha (CYP17): 17 alpha-hydroxylase/C17, 20-lyase activity. Biol Reprod. 1992;46:30–8. doi: 10.1095/biolreprod46.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Tsunoda N, Nagamine N, Tanaka Y, Taniyama H, Nambo Y, et al. Testicular inhibin in the stallion: cellular source and seasonal changes in its secretion. Biol Reprod. 1998;59:62–8. doi: 10.1095/biolreprod59.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa D, Sasaki M, Suzuki M, Tsubota T, Igota H, Kaji K, et al. Immunohistochemical localization of steroidogenic enzymes in the testis of the sika deer (Cervus nippon) during developmental and seasonal changes. J Reprod Dev. 2010;56:117–23. doi: 10.1262/jrd.09-102t. [DOI] [PubMed] [Google Scholar]
- 25.Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohisto-chemistry. Mol Cell Endocrinol. 2001;178:189–98. doi: 10.1016/s0303-7207(01)00427-0. [DOI] [PubMed] [Google Scholar]
- 26.Tsubota T, Nitta H, Osawa Y, Mason J, Kita I, Tiba T, et al. Immunolocalization of steroidogenic enzymes, P450scc, 3βHSD, P450arom in the Hokkaido Brown Bear (Ursus arctos yesoensis) testis. Gen Comp Endocrinol. 1993;92:439–44. doi: 10.1006/gcen.1993.1180. [DOI] [PubMed] [Google Scholar]
- 27.Okano T, Murase T, Tsubota T. Spermatogenesis, serum testosterone levels and immunolocalization of steroidogenic enzymes in the wild male Japanese black bear (Ursus thibetanus japonicus) J Vet Med Sci. 2003;65:1093–9. doi: 10.1292/jvms.65.1093. [DOI] [PubMed] [Google Scholar]
- 28.Weng Q, Murase T, Tsubota T. Seasonal changes in spermatogenesis and testicular steroidogenesis in wild male raccoon dogs. J Vet Med Sci. 2003;65:1087–92. doi: 10.1292/jvms.65.1087. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Sheng X, Hu X, Li X, Xu H, Zhang M, et al. Seasonal changes in spermatogenesis and immunolocalization of cytochrome P450 17alpha-hydroxylase/c17–20 lyase and cytochrome P450 aromatase in the wild male ground squirrel (Citellus dauricus Brandt) J Reprod Dev. 2010;56:297–302. doi: 10.1262/jrd.09-078t. [DOI] [PubMed] [Google Scholar]
- 30.Bilinska B, Drag E, Schmalz-Fraczek B. Immunolocalization of androgen receptors in testicular cells during postnatal development of bank vole (Clethrionomys glareolus, S.) Tissue Cell. 1999;31:621–6. doi: 10.1054/tice.1999.0078. [DOI] [PubMed] [Google Scholar]
- 31.Schuler G, Ozalp GR, Hoffmann B, Harada N, Browne P, Conley AJ. Reciprocal expression of 17alpha-hydroxylase-C17,20-lyase and aromatase cytochrome P450 during bovine trophoblast differentiation: a two-cell system drives placental oestrogen synthesis. Reproduction. 2006;131:669–79. doi: 10.1530/rep.1.01033. [DOI] [PubMed] [Google Scholar]
- 32.Weng Q, Medan MS, Ren GQ, Watanabe G, Tsubota T, Taya K. Immunolocalization of steroidogenic enzymes in the corpus luteum and placenta of the Japanese shiba goat. J Reprod Dev. 2005;51:247–52. doi: 10.1262/jrd.16081. [DOI] [PubMed] [Google Scholar]
- 33.Weng Q, Tanaka Y, Taniyama H, Tunoda N, Nambo Y, Watanabe G, et al. Immunolocalization of steroidogenic enzymes P450scc, 3betaHSD, P450c17, and P450arom in fetal adrenal glands during mid-late gestation of equine. J Reprod Dev. 2007;53:1093–8. doi: 10.1262/jrd.18159. [DOI] [PubMed] [Google Scholar]
- 34.Conley AJ, Head JR, Stirling DT, Mason JI. Expression of steroidogenic enzymes in the bovine placenta and fetal adrenal glands throughout gestation. Endocrinology. 1992;130:2641–50. doi: 10.1210/endo.130.5.1374010. [DOI] [PubMed] [Google Scholar]