Abstract
The objective of this study was to investigate whether stressful experience during early postnatal period may influence morphological characteristics of the rat neurogenic pathway – the rostral migratory stream (RMS) and proliferation of neuronal precursors in three successive areas of the RMS: in the vertical arm, the elbow and the horizontal arm. To induce stress, the pups were subjected to repeated maternal deprivation during the first postnatal week after birth. Brains were analyzed at the seventh postnatal day. The controls matched the age of maternally deprived animals. Observation of hematoxylin-eosin stained sections showed that maternal deprivation did not affect the general morphological appearance of the RMS. The shape of the RMS of maternally deprived rats resembles the RMS of control animals. Maternal deprivation caused slight, not significant increase in the RMS thickness in comparison with control rats. Significant difference between the control and maternally deprived rats concerns the olfactory ventricle. While in seven days old control rats the olfactory ventricle is completely closed, in maternally deprived rats of the same age the olfactory ventricle was regularly visible as a narrow lumen at the axis of the RMS horizontal arm. This finding indicates delayed maturation of the migratory pathway as a consequence of stress. Proliferation activity has been assessed by immunoreactivity of the endogenous cell cycle protein Ki-67. The results of Ki-67 immunohistochemistry showed that seven days' maternal separation for 3 h daily induces significant quantitative changes in the number of proliferating cells within the RMS. The response of Ki-67-positive cells to stress differed in individual part of the RMS, with a marked decrease in the vertical arm and a significant increase in the elbow, suggesting heterogeneity of neural stem cells along the RMS; while in the RMS vertical arm the number of dividing cells significantly decreased, there was a marked increase of Ki-67-positive cells in the RMS elbow. This suggests heterogeneity of neural stem cells along the RMS.
Key words: rat, rostral migratory stream, maternal deprivation, neurogenesis.
Introduction
One of the few areas of adult mammalian CNS that is known to be competent for neuronal proliferation is the subventricular zone (SVZ) lining the lateral ventricles.1–4 Neuronal precursors that originate in the SVZ migrate through a network of tangential pathways in the lateral wall of the lateral ventricle5 and then converge onto the rostral migratory stream (RMS), which leads toward the core of the olfactory bulb (OB).6 Cells in the SVZ network and RMS move rapidly by chain migration without axonal or radial glial guides.5,7–9 During their migration in the RMS, many cells undergo mitosis10 as well as apoptosis.11 Within the OB, these young neurons leave the RMS and migrate into the granule and periglomerular layers, where they differentiate into local interneurons, granule cells and periglomerular cells and are integrated into the olfactory functional circuitry.12,13
The neurogenic pathway
The RMS belongs to the brain structures, which undergo a remarkable anatomical reorganization during early postnatal development.14 The RMS of adult rats looks like a thick L-shaped column, which spans the anterior horn of the lateral ventricle and the olfactory bulb. Within the pathway in caudo-rostral direction, three parts topographically follow one after another: the vertical arm, elbow and horizontal arm. The vertical arm emerges from the anterior tip of the lateral ventricle and curves ventrally between the corpus callosum and striatum. Then the RMS turns in a prominent angle and continues along with the ventral forebrain surface as the horizontal arm. The early postnatal RMS is U-shaped because the elbow between the vertical and horizontal arms is not as sharp as at the later postnatal stages. The typical L-shaped column, characteristic for the adult rat RMS, develops in two weeks old rats.15 Over the first three postnatal weeks, the RMS shows a progressive reduction in thickness as well.2,14,15 Another significant morphological alteration within early postnatal stage, concerns the brain ventricle located at the most proximal part of the RMS, in the middle part of the OB. The prenatal and early postnatal RMS contains an opened olfactory ventricle, which closes after birth in rodents.16 In adult rats it is mentioned only as vestigial2 or virtual olfactory ventricle.17
During early postnatal development, besides quantitative differences related to the RMS thickness, shape and the olfactory ventricle obliteration, marked qualitative changes occur in the pattern of the RMS cell migration,18–20 proliferation activity14,15 or differentiation of neuronal precursors.20 Although neurogenesis occurs continuously throughout the entire life, the rate of proliferation and the fate of newborn cells can be influenced by wide range of exogenous factors. It is well known that the period of early postnatal life is very important for brain development, and developing brain structures are more vulnerable to the effects of stress. The early life stress has been shown to produce enduring morphological and functional changes in various brain structures.21–23 Animal models of early life stress commonly aim at the postnatal disruption of the motherinfant interaction. The two most well known models applied in laboratory rats are early handling and maternal separation.
The objective of our study was to investigate whether stressful experience during the first postnatal week affects morphological characteristics of the rat RMS and proliferation of neuronal precursors within individual parts of this pathway. In the present experiment proliferation activity has been assessed by immunoreactivity of the endogenous cell cycle protein Ki-67. In studies employing the most common marker for proliferating cells (bromodeoxyuridine), stress is generally associated with decreased proliferation in neurogenic areas.24,25 While BrdU can be incorporated into DNA during S-phase only, Ki-67 is expressed throughout the cell cycle in proliferating cells.26 We expect that using Ki-67 immunohistochemistry could give a new prospect regarding the stress influence on the neurogenesis in the RMS.
Materials and Methods
Maternal deprivation
Twenty-four Wistar albino rats of both sexes were used in this study. The experimental procedures were approved by the Ethical Committee of the Institute of Neurobiology, Slovak Academy of Sciences, in accordance with current Slovak Republic legislation.
Rat pups from the first postnatal day (P1) were separated from the dam for a period of 3 h daily for 7 days (P7). Dams were first removed and placed in an adjacent cage. The litters were then transferred to a plastic container and placed in an incubator at the temperature consistent with nest measurements (34°C). After 3 h separation period, the pups were returned to their home cage, where they were reunited with the dam. Control rats were reared under the same conditions except maternal separation.
Tissue processing
At the age of P7 both maternally deprived (n=12) and control animals (n=12) were deeply anesthetized with mixture of xylazine and ketamine and perfused transcardially with 4% paraformaldehyde in 0.1 M PBS. Next day the brains were removed from the skulls and transferred into cryoprotective solution - 30% sucrose in 0.1 M phosphatebuffered saline (PBS) - and then 30 µm thick sagittal sections were cut on the cryostat and stored in dishes with 0.1 M PBS. The shape and the thickness of the RMS were evaluated on Gill's hematoxylin stained sections. For recognition of proliferating cells, Ki-67 immunolabeling was employed.
Ki-67 immunohistochemistry
To assess whether maternal deprivation affects cell proliferation in the RMS the sections were stained with the proliferation marker Ki-67. First, the sections were washed with PBS, and endogenous peroxidase was blocked using 3% H2O2. After 2 h of serum blocking with 5% normal goat serum, sections were incubated with anti-Ki-67 antibody (1:1000, Abcam, Cambridge, MA, USA) in PBST (PBS with 0.4% Triton) plus 1% normal goat serum at room temperature for 18 h After rinsing with PBS, sections were incubated with biotinylated goat anti-rabbit IgG (1:200, Santa Cruz, CA, USA) secondary antibody for 2 hours at room temperature, followed by the ABC kit and DAB substrate. Sections were mounted on glass slides, air-dried overnight, cleared with xylene and cover-slipped with Entellan.
Image analysis and quantification
In order to study the morphological characteristics of the RMS the sections were examined by light microscopy (Olympus BX51) equipped with camera system DP50. To evaluate these sections, images were captured. The thickness of the RMS was measured by UTH-SCSA Image Tool (version 3.0).
For quantitative analysis of cell proliferation only those sections where the whole extent of the RMS was visible (six sections per hemisphere) were used. The number of Ki-67-positive cells was counted using Disector software version 2.0,27 the method for unbiased estimation of cell density. We counted the cells along the whole RMS and individually in three anatomical parts – the vertical arm, elbow and horizontal arm (Figure 1). Differences in the number of proliferating cells between control and experimental animals were analyzed by the one-way ANOVA test, Tukey-Kramer and Student t- tests. The values were expressed as means ± S.E.M.
Figure 1.
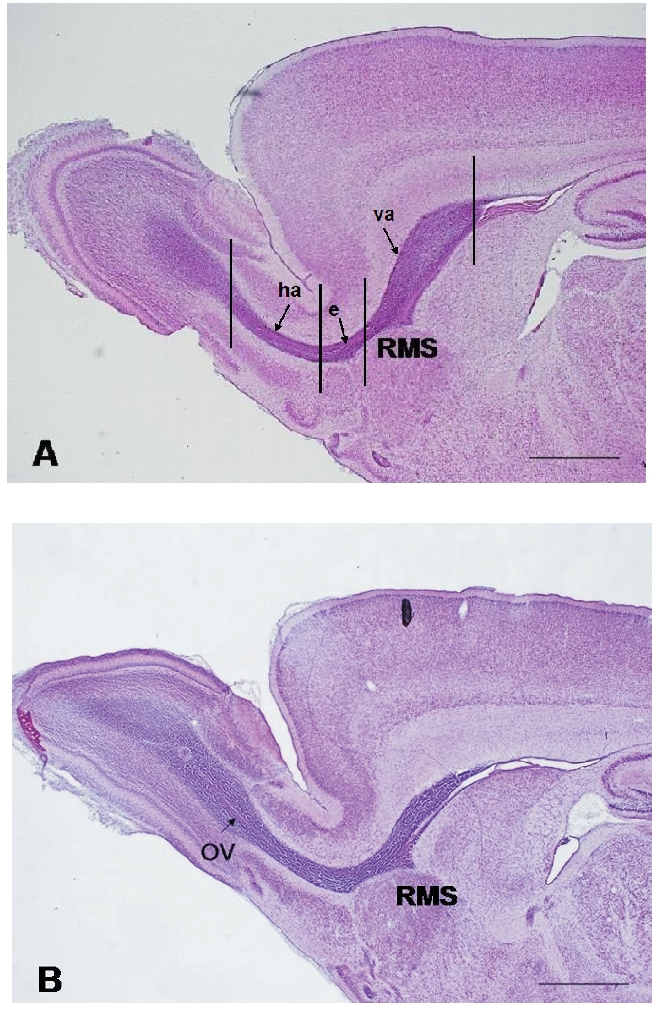
Micrographs of parasagittal sections of the RMS of P7 control (A); and maternally deprivated (B) rat stained with hematoxylin-eosin. The intense purple staining, indicative of cell density, distinguishes the migratory stream from other regions of the forebrain. Vertical lines demarcate individual parts of the RMS: (va) the vertical arm; (e) the elbow; (ha) the horizontal arm. The RMS of control rat as well as the RMS of maternally separated rat has U shape. In the P7 maternally separated rat the olfactory ventricle (OV) is visible at the most rostral part of the RMS. Scale bar: 1 mm.
Results
Morphological observations – hematoxylin staining
Under physiological conditions the rodent RMS undergoes a gradual but substantial reduction in the thickness during the first postnatal month.2,15 Our findings showed that maternal deprivation did not affect the general morphological appearance of the RMS. The shape of the RMS of maternally deprived P7 rats resembles the RMS of P7 control animals (Figure 1). Maternal deprivation caused slight, not significant increase in the RMS thickness (243±12.97 µm) in comparison with control rats of the same age (230±57.33 µm).
Significant difference between the control and maternally deprived rats concerns the presence of olfactory ventricle. In P7 control animals the RMS spans the anterior wall of lateral ventricle and OB as a compact pathway without any continuity with brain ventricles. In P7 maternally deprived rats the olfactory ventricle was regularly visible as a narrow lumen at the axis of the RMS horizontal arm (Figure 1B). This finding we consider as open olfactory ventricle.
Cell proliferation – Ki-67 immunohistochemistry
In order to obtain detailed picture on the extent of proliferation, both qualitative and quantitative analysis of Ki-67 immunohistochemistry were provided in three successive areas of the RMS in a caudo-rostral direction: in the vertical arm, elbow and horizontal arm separately.
The results of Ki-67 immunohistochemistry showed that 7 days of maternal separation for 3 h daily induced obvious changes in proliferating cells density in the RMS. However, these changes were not uniform in the RMS individual parts. In the RMS vertical arm of maternally separated animals the density of dividing cells was lower than in the vertical arm of intact rats RMS (Figure 2). In the elbow we have observed noticeably increased density of Ki-67-positive cells (Figure 3). Ki-67 positivity in the horizontal arm of maternally deprived rats was similar to that in control animals.
Figure 2.
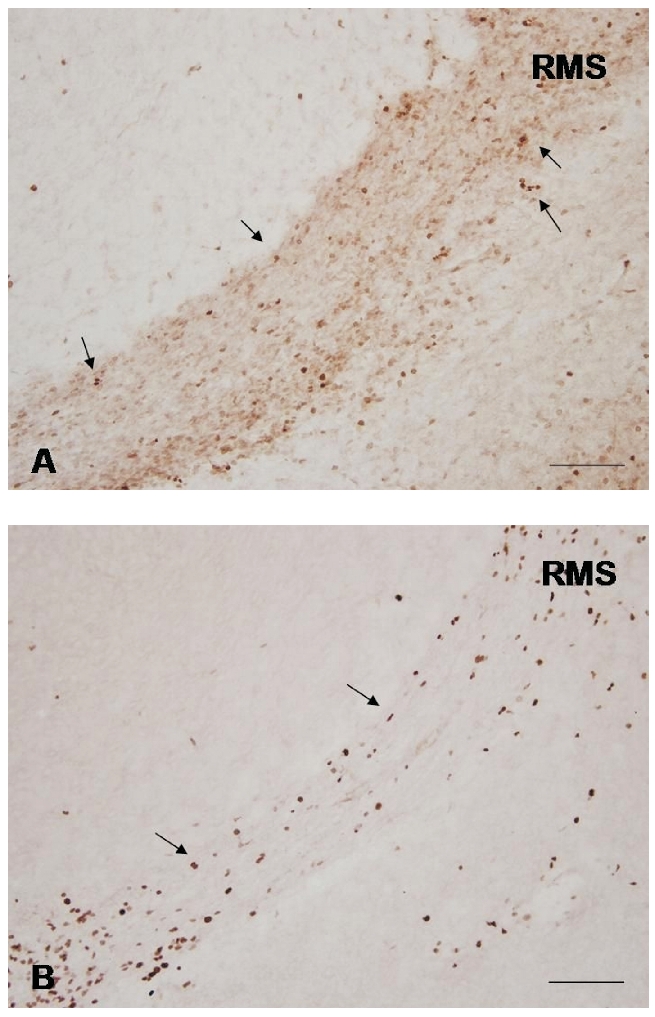
Representative photomicrographs of parasagittal sections of the RMS of P7 rats showing the distribution of Ki-67-positive cells in the vertical arm of control (A); and maternally deprived (B) rat. The Ki-67-positive cells are visible by brown stained nuclei (arrows). Scale bars = 100 µm.
Figure 3.
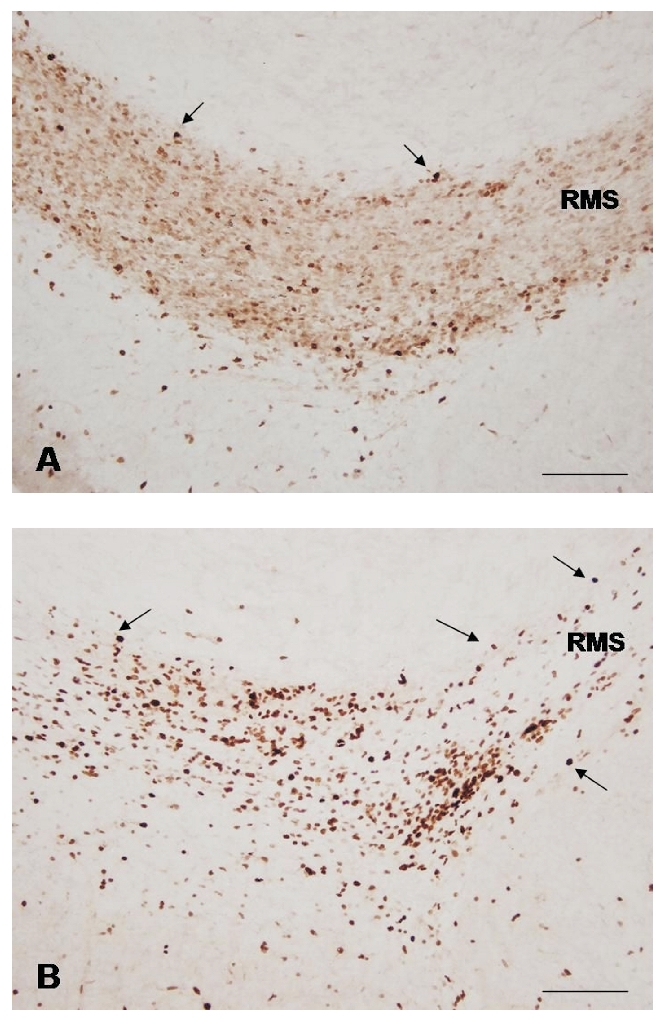
Representative photomicrographs of Ki-67-positive cells (arrows) in the elbow of P7 control (A); and maternally deprived (B) rat. Note the increased number of dividing cells after one week of maternal deprivation. Scale bars = 100 µm.
Subsequent quantitative analysis of dividing cells in the RMS was in agreement with the morphological findings. While in control rats, Ki-67-positive cells were distributed almost equally along the RMS, in maternally deprived rats the amount of labeled cells considerably varied in individual parts of the migratory pathway. In the vertical arm of the RMS we have observed a significant decrease in the number of Ki-67-positive cells in comparison with the control animals of the same age (Figure 4). In the elbow a significant increase in the Ki-67-positive cells number was noticed (Figure 4). In the RMS horizontal arm the amount of Ki-67-positive cells was also higher than in the control rats, but this increase was statistically not significant (Figure 4).
Figure 4.
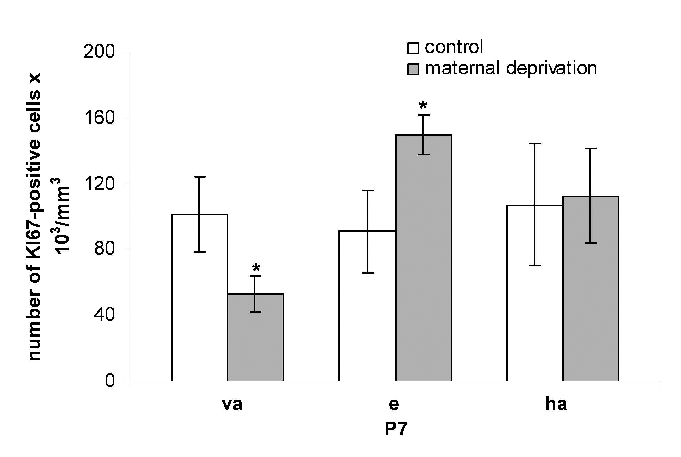
Quantitative analysis of Ki-67-positive cells in the RMS of control and maternally deprived rats. Graph shows the number of Ki-67-positive cells in the vertical arm (va), elbow (e) and horizontal arm (ha). Maternal deprivation decreased the number of dividing cells in the vertical arm and increased the number of dividing cells in elbow and horizontal arm. Statistical significance of differences between experimental and control group was P<0.05.
Discussion
Numerous studies have demonstrated that adverse early life events affect structural brain development and induce changes in adult neurogenesis.23,28 Moreover, variation in maternal care has been shown to produce changes at the molecular level, concretely at the development of the GABA system by altering GAD1 promoter methylation levels.29
Our morphological observation showed that maternal deprivation applied in newborn rats from P1–P7 caused delayed maturation of the RMS. Although, the general morphological appearance of maternally deprived rats RMS resembled the RMS of control rats of the same age, its thickness was slightly increased. According to our previous findings,15 the RMS of P7 deprived pups was approximately as thick as the RMS of control rats on the third postnatal day.
The most striking effect of maternal deprivation on the RMS morphology was manifested by the persistence of olfactory ventricle in seven days old pups. In newborn rats the olfactory ventricle disappears before the third postnatal day.15 Here, we have shown an open olfactory ventricle regularly present within the OB of maternally deprived animals. The olfactory ventricle persists in fish30 and some mammals, e.g. pig, sheep, and rabbit.31 In humans, it has been generally assumed that the olfactory ventricles become completely closed at the time of birth.32,33 Recently, Smitka et al. using magnetic resonance imaging showed high incidence of olfactory ventricles in human population.34 Although the functional consequence of the presence or absence of olfactory ventricles in humans remains unclear, it could be an important issue in studies in which the morphology of the OB is correlated with certain diseases. In spite of these findings about open olfactory ventricle under physiological conditions, we consider persistence of olfactory ventricles in rats after 7 days maternal deprivation as an adverse effect of stress.
Maternal deprivation induced striking changes also in proliferation activity of Ki-67-positive neuronal precursors within the migratory pathway. Using a similar experimental paradigm, previously we have shown that maternal deprivation35 caused significant changes in the number of proliferating cells labeled with the S-phase marker BrdU within the rat RMS. BrdU immunohistochemistry has regularly been used for estimating proliferating cells fraction in the RMS to map physiological dynamics at various postnatal ages15 and in several experimental interventions.36,37 Although BrdU-positive and Ki-67-positive cell number are often used as compatible markers of proliferation,38 our current data and other work39 indicate that cells in discrete phases of the cell cycle can be differentially influenced by stimuli.
Stress is generally associated with decreased proliferation in neurogenic areas.28 Our results of quantitative analysis showed, that the response of Ki-67-positive cells to stress was not uniform in the vertical arm, elbow and horizontal arm of the RMS. Unlike the overall decrease of BrdU labeled cells in all three parts of the RMS following maternal deprivation,35 the decrease of the number of Ki-67-positive cells was restricted only to the RMS caudal part, i.e., to the vertical arm. Surprisingly, in the elbow and in the horizontal arm of the RMS we have observed increased amount of proliferating cells. We suppose that in these areas the maternal deprivation induces a slowing down or a blockade of cycling cells before their entrance into the S-phase. If so, the cells still pertaining to the so-called growth fraction would be positive for Ki-67, while their BrdU index would decrease. Regional differences in response of the RMS anatomical parts to maternal deprivation could be a consequence of different sensitivity of dividing cells to stress in discrete phases of the cell cycle.
By means of Ki-67 immunohistochemistry, we have obtained interesting finding also in the RMS of control, intact rats. It is known, that the density of dividing cells, labeled by tritiated thymidine or BrdU, decreases following a caudo-rostral gradient, with a maximum around the lateral ventricle and minimum within the OB.2,15 However, Ki-67 labeling in P7 control rats did not reproduce the caudo-rostral reduction of cell division. Ki-67-positive cells were distributed almost equally relatively homogenously throughout the RMS individual parts. Differences between the distribution of Ki-67-positive and BrdU-positive cells in control animals could be explained by different cell cycle length along the RMS. The proliferating cells in the RMS rostral parts divide more rapidly than cells within its caudal regions,40 which explains a higher density of BrdU-positive cells within the vertical arm. On the other hand, the pattern of Ki-67 immunoreactivity suggests that the quiescent population of proliferating cells is homogenously distributed throughout the RMS. In conclusion, our findings of the olfactory ventricle persistence in P7 rats indicate that short-term maternal deprivation delayed the RMS maturation. Changes in the number of proliferating cells following maternal deprivation suggest the neurogenic pathway susceptibility to stress. Differences in response of individual parts of the pathway confirm heterogeneity of neural stem cells along the SVZ-OB pathway.
Acknowledgments:
this study was supported by projects “VEGA” grants 2/0147/09; 2/0181/11.
References
- 1.Smart I. The subependymal layer of the mouse brain and its cell production as shown by radioautography after thymidine-H3 injection. J Comp Neurol. 1961;116:325–38. [Google Scholar]
- 2.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–58. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 3.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–7. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 5.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93:14895–900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipley MT, Ennis M, Puche AC. The olfactory system. In: Paxinos G, editor. The rat nervous system. Academic Press; New York, NY, USA: 2004. pp. 921–962. [Google Scholar]
- 7.Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–96. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, García-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–81. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 9.Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–91. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 10.Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- 11.Brunjes PC, Armstrong AM. Apoptosis in the rostral migratory stream of the developing rat. Brain Res Dev Brain Res. 1996;92:219–22. doi: 10.1016/0165-3806(96)00006-5. [DOI] [PubMed] [Google Scholar]
- 12.Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. Neurosci. 2003;23:10411–8. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–18. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 14.Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- 15.Martončíková M, Račeková E, Orendáčová J. The number of proliferating cells in the rostral migratory stream of rat during the first postnatal month. Cell Mol Neurobiol. 2006;26:1453–61. doi: 10.1007/s10571-006-9039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull. 1999;49:221–43. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 17.Westergaard E. Degree Diss. University of Aarhus; Denmark: 1970. The lateral cerebral ventricles and the ventricular walls. [Google Scholar]
- 18.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and threedimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull. 1997;42:9–21. doi: 10.1016/s0361-9230(96)00116-5. [DOI] [PubMed] [Google Scholar]
- 20.Law AK, Pencea V, Buck CR, Luskin MB. Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes. Dev Biol. 1999;216:622–34. doi: 10.1006/dbio.1999.9498. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;15:721–31. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 22.Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res. 2011;216:552–60. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–45. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 25.Račeková E, Lievajová K, Danko J, Martončíková M, Flešárová S, Almášiová V, et al. Maternal separation induced alterations of neurogenesis in the rat rostral migratory stream. Cell Mol Neurobiol. 2009;29:811–9. doi: 10.1007/s10571-009-9362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Tomori Z, Krekule I, Kubínová L. Dissector program for unbiased estimation of particle number, numerical density and mean volume. Image Anal Stereol. 2001;20:119–130. [Google Scholar]
- 28.Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stressinduced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–92. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic Acid decar-boxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–7. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerman RA, Wilson JA. The fine structure of the olfactory tract in the teleost Carassius carassius L. Z Zellforsch Mikrosk Anat. 1968;91:186–99. doi: 10.1007/BF00364310. [DOI] [PubMed] [Google Scholar]
- 31.Rae AS. Nodules of cellular proliferation in sheep olfactory ventricle. Clin Neuropathol. 1994;13:17–8. [PubMed] [Google Scholar]
- 32.Humphrey T. The development of the olfactory and the accessory olfactory formations in human embryos and fetuses. J Comp Neurol. 1940;73:431–68. [Google Scholar]
- 33.Pearson AD. The development of the olfactory nerve in man. J Comp Neurol. 1941;75:199–217. [Google Scholar]
- 34.Smitka M, Abolmaali N, Witt M, Gerber JC, Neuhuber W, Buschhueter D, et al. Olfactory bulb ventricles as a frequent finding in magnetic resonance imaging studies of the olfactory system. Neuroscience. 2009;162:482–5. doi: 10.1016/j.neuroscience.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 35.Lievajová K, Martončíková M, Blaško J, Orendáčová J, Almašiová V, Račeková E. Early stress affects neurogenesis in the rostral migratory stream. Cent Eur J Biol. 2010;5:757–64. [Google Scholar]
- 36.Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–80. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bálentová S, Račeková E, Martončíková M, Mišúrová E. Cell proliferation in the adult rat rostral migratory stream following exposure to gamma irradiation. Cell Mol Neurobiol. 2006;26:1131–39. doi: 10.1007/s10571-006-9067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 39.Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–41. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev Dyn. 1998;213:220–7. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]


