Abstract
During embryogenesis, the mammalian heart develops from a primitive heart tube originating from two bilateral primary heart fields located in the lateral plate mesoderm. Cells belongings to the pre-cardiac mesoderm will differentiate into early cardiac progenitors, which express early transcription factors which are also common to the Isl-1 positive cardiac progenitor cells isolated from the developing pharyngeal mesoderm and the foetal and post-natal mice hearts. A second population of cardiac progenitor cells positive to c-Kit has been abundantly isolated from adult hearts. Until now, these two populations have been considered two different sets of progenitor cells present in the heart in different stages of an individual life. In the present study we collected embryonic, foetal and infant hearts, and we tested the hypotheses that c-Kit positive cells, usually isolated from the adult heart, are also present in the intra-uterine life and persist in the adult heart after birth, and that foetal Isl-1 positive cells are also positive to c-Kit. Using immunohistochemistry we studied the temporal distribution of Isl-1 positive and c-Kit/CD105 double positive cells, and by immunofluorescence and confocal analysis we studied the co-localization of c-Kit and Isl-1 positive cells. The results indicated that cardiomyocytes and interstitial cells were positive for c-Kit from the 9th to the 19h gestational week, that cells positive for both c-Kit and CD105 appeared in the interstitium at the 17h gestational week and persisted in the postnatal age, and that the Isl-1 positive cells were a subset of the c-Kit positive population.
Key words: Isl-1, c-Kit, human heart, embryo, foetus.
Introduction
The mechanisms involved in establishing the early heart and regulating its morphogenesis have been widely studied.1 During embryogenesis, the atrioventricular (A–V) canal and the sinu-atrial segment at the venous pole as well as the conotruncus at the arterial pole are added to the heart tube during tube looping.2,3 Most of the cells that will differentiate into the developing myocardium are derived from a population of progenitor cells located in the pharyngeal mesoderm, termed the secondary heart field (SHF).4–6
Cells belongings to the pre-cardiac mesoderm will differentiate into early cardiac progenitors which can in turn differentiate into primitive cardiomyocytes, smooth muscle cells and endothelial cells.7 Known markers of pre-cardiac cells of the primary and the secondary heart field are Nkx2.5 and GATA-4.4 The expression of these two transcription factors is common to the LIM homeobox transcription factor positive (Isl-1+) cardiac progenitor cells (CPCs) and the developing pharyngeal mesoderm8 as well as foetal and postnatal mice hearts.9 This small number of Isl-1+ cells has been identified as a residue of the migrating SHF cells and may be considered resident progenitor cells in the myocardium in the post-natal age.10
With regard to the Isl-1+ cell distribution, Genead et al.11 described the frequency of Isl-1+ cells in the early first trimester human embryonic heart. They did not observe differences among the outflow tract, atria and right ventricle, confirming what had been previously reported for the late first and early second trimester.12 Clusters of Isl-1+ cells were identified in the right atrial wall of foetal and new born hearts, whereas occasional Isl-1+ cells have been found in the ventricular regions.13 Isl-1 has been identified as a marker of CPCs in the adult rat heart,14,15 giving support to the hypothesis that some cells from the embryo may also persist into adulthood.16
Most studies concerning the localisation of CPCs in the developing heart have been performed in mice or chicks. The localisation and identification of CPCs in the human foetal and adult heart has been investigated for the first time by Limana and colleagues,17 with their analysis limited to epicardium and CD34+ or c-Kit+ cells. Cells expressing c-Kit were identified also in the human foetal and post-natal myocardium specimens. These cells were located within the connective tissue and within muscle bundles. Their number declined over time until the first post-natal month.18
The transmembrane receptor tyrosine kinase c-Kit is expressed on the cell surface of stem cells during haematopoiesis,19 dental pulp stem cells originating from the neural crest cells,20 in the developing pancreas,21 and recently as been proposed as a useful marker to differentiate primary melanoma from compound nevi.22 Apart from the many studies on the expression and localization of this receptor, c-Kit has been proposed also as the most important marker for adult CPCs.23,24 The first isolation and characterisation of adult human CPCs has been performed from percutaneous right ventricular endocardial biopsy specimens by Smith and colleagues.25 Multipotent CPCs from human cardiospheres consistently expressed c-Kit and CD105, the regulatory component of the transforming growth factor-β receptor complex that is important in angiogenesis26 and haematopoiesis.27 Therefore, it seems that c-Kit+ cells are CPCs homing the myocardium in the adulthood, while Isl-1+ cells have been proposed as a different subset of undifferentiated cells able to generate endothelial cells, cardiomyocytes, smooth muscle cells and cardiac fibroblasts but present only in the developing heart and persisting in the post-natal age.8,28,29 In the mean time another subset of cardiac undifferentiated cells (epicardial progenitors) has been identified which barely participate in atria, right ventricle and outflow tract formation and that are detectable in the murine and human foetal, but not adult hearts.30 In the present study, we analysed human hearts from embryos, foetuses and neonates at different gestational ages to determine the presence and co-localization of the Isl-1+ CPCs identified in the embryonic and postnatal heart, and the c-Kit+/CD105+ cells described in the adult heart, to understand if the c-Kit+ cells abundantly isolated from adult hearts are present also in the embryonic and foetal heart and if they are a different subset of the extensively studied Isl-1+ cells.
Materials and Methods
Human cardiac samples
Cardiac tissue samples, comprising the endocardial, myocardial, and epicardial layers of the ventricles, were obtained by autopsy from embryos, foetuses, preterm infants, and term infants and collected, after written consent from the mother, in the Department of Human Pathology of the University of Messina, Italy. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. The Ethics Committee of the University of Messina approved the study.
Gestational age of the foetuses ranged between 9 and 40 weeks, as shown in Table 1 and Table 2. Specifically, our cohort comprised 25 samples. The causes related to the death of the analysed foetuses and of the preterm and term infants are shown in Table 1 and Table 2. Autopsies and sample collections were carried out immediately after delivery with less than a 24-hour interval since foetal death had occurred.
Table 1. Clinical and morphological data and c-Kit/CD105 expression in 1 embryo, 12 foetuses, 1 preterm infant and 1 term infant.
| Case No. | Trimester | Gestational age (wks) | Age at death | Cause of death | Heart malformations | Cardiomyocytes | Endocardium | Intestitial cells |
|---|---|---|---|---|---|---|---|---|
| Embryo | ||||||||
| 1 | I | 9 | 9 wks | VT | Endocardial cushion defect (AVC of the heart) | c-Kit+ | CD105+ | c-Kit+/CD105 |
| Foetuses | ||||||||
| 2 | I | 13 | 13 wks | SA for chorioamnionitis | Absent | c-Kit+ | CD105+ | c-Kit+/CD105− |
| 3 | II | 14 | 14 wks | TA for CRS | VSD | c-Kit + | CD105+ | c-Kit+/CD105− |
| 4 | II | 14 | 14 wks | SA for chorioamnionitis | Absent | c-Kit+ | CD105+ | c-Kit+/CD105− |
| 5 | II | 14 | 14 wks | TA for cerebral malformations | Absent | c-Kit+ | CD105+ | c-Kit+/CD105− |
| 6 | II | 15 | 15 wks | SA for chorioamnionitis | Absent | c-Kit+ | CD105+ | c-Kit+/CD105− |
| 7 | II | 16 | 16 wks | SA for abruption placentae | Absent | c-Kit+ | CD105+ | c-Kit+/CD105− |
| 8 | II | 17 | 17 wks | TA for cerebral malformations | Absent | c-Kit+/− | CD105+ | c-Kit+/CD105+ |
| 9 | II | 18 | 18 wks | TA for MCA | Absent | c-Kit+/− | CD105+ | c-Kit+/ CD105+ |
| 10 | II | 19 | 19 wks | TA for PKD, UBH, CLGP left, PEV bilateral | Absent | c-Ki+/− | CD105+ | c-Kit +/CD105+ |
| 11 | II | 22 | 22 wks | SA for chorioamnionitis | Absent | c-Kit+/− | CD105+ | c-Kit +/CD105+ |
| 12 | II | 22 | 22 wks | SA for chorioamnionitis | Absent | c-Kit − | CD105+ | c-Kit +/CD105+ |
| 13 | III | 27 | 27 wks | SA for abruption placentae | Absent | c-Kit − | CD105+ | c-Kit +/CD105+ |
| Preterm infant | ||||||||
| 14 | III | 36 | 1 day | Respiratory failure | Absent | c-Kit − | CD105+ | c-Kit +/CD105+ |
| Term infant | ||||||||
| 15 | III | 40 | 6 yrs | Disseminate intravascular coagulation | Absent | c-Kit − | CD105+ | c-Kit +/CD105+ |
TA, therapeutic abortion; SA, spontaneous abortion; CLGP, cheilo-gnatho-palatischisis; CRS, caudal regression syndrome; MCA, multiple congenital anomalies; PEV, pes equino varus; PDK, polycystic kidney disease.
Table 2. Clinical and morphological data and number of Isl-1 positive cells per area (mm2) in 7 foetuses, 2 preterm infants and 1 term Infant.
| Case No. | Trimester | Gestational age (wks) | Age at death | Cause of death | Growth defects | Heart malformations | Section area (mm2) | Isl-1+ cells/mm2 × 104* |
|---|---|---|---|---|---|---|---|---|
| Foetuses | ||||||||
| 16 | II | 14 | 14 wks | Abruptio placentae | Absent | Absent | 56171.5±11561.46 | 4.523±1.22 |
| 17 | II | 15 | 15 wks | TA for Prune-Belly syndrome | Absent | Absent | 46063±3055.72 | 3.543±0.15 |
| 18 | II | 16 | 16 wks | TA for trisomy 21 | IUGR | Absent | 83666.33±2011.76 | 1.394±0.17 |
| 19 | II | 19 | 19 wks | TA for trisomy 21 | Absent | Absent | 71642.66±4190.29 | 1.141±0.62 |
| 20 | II | 20 | 20 wks | TA for trisomy 21 | Absent | Absent | 186604.66±9018 | 0.735±0.10 |
| 21 | II | 21 | 21 wks | TA for trisomy 21 | Absent | Absent | 119550.33±4100.16 | 0.889±0.14 |
| 22 | II | 22 | 22 wks | TA for cerebral malformations | Absent | Absent | 162438±7750.21 | 0.738±0.02 |
| Preterm infants | ||||||||
| 23 | III | 26 | 8 days | Respiratory failure hypertrophy | IUGR | Ventricular septal | 101629±1409.49 | 1.279±0.08 |
| 24 | III | 36 | 2 hrs | Sepsis | Absent | Ventricular septal hypertrophy | 165575.33±2524.22 | 0.664±0.05 |
| Term infants | ||||||||
| 25 | III | 40 | 8 hrs | Respiratory failure | Absent | Absent | 202609±13666.33 | 0.548±0.12 |
Data for which a t-test for dependent samples was performed, P<0.05. Gestational weeks (independent variable);
Isl-1+ cells/mm2 (dependent variable); TA, therapeutic abortion; IUGR, intrauterin growth retardation; UBH, urinary bladder hypoplasia; VSD, ventricular septal defect; VT, voluntary termination; +, strongly positive; +/−, weakly positive.
All samples were fixed in 10% formalin and embedded in paraffin for morphological diagnostic evaluation (haematoxylin/eosin) and immunohistochemistry.
Immunohistochemistry
Single immunohistochemistry was performed by a streptavidin-biotin complex method using LSAB2 kit (DAKO Co., Carpinteria, CA, USA). After deparaffination and rehydration, the tissue sections were incubated with the protein blocking agent (DAKO) for 10 min and the primary antibody for 1 h. We used the anti-Isl-1 antibody (1:200, rabbit polyclonal, AB5754, Chemicon Int., Millipore Corp., Billerica, MA, USA). The linked primary antibody was detected with DAKO LSAB2 streptavidin-peroxidase system according to manufacturer's instructions. 3-Amino-9-ethylcarbazole (AEC) was used as a chromogen, whereas hematoxylin was used as a light counterstain.
Double immunohistochemistry was performed by a streptavidin-biotin complex for the goat polyclonal antibody (CD105) and labelled using polymer-alkalyne phosphatase (AP) for the rabbit polyclonal antibody (c-Kit). After deparaffination and rehydration the tissue sections were incubated with the Peroxidase Block solution (Vector Laboratories Inc., Burlingame, CA, USA) for 5 min, washed in PBS for 5 min, and incubated with the anti-CD105 goat polyclonal antibody (1:50, SC-19793, Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 30 min. After washing in PBS, sections were incubated with the biotinylated anti-goat secondary antibody (1:200, SC-2774, Santa Cruz Biotechnology Inc.) for 30 min, and washed again in PBS. For the antibody detection the streptavidin-HRP complex (LSAB2 kit DAKO) was used for 10 min. Sections were then rinsed in PBS with 0.1% Tween 20 (T-PBS) for 5 min, and incubated with 3,3′-Diaminobenzidine (DAB–Vector Laboratories) as the HRP-substrate.
After the detection of the first antigen, sections were incubated with the Doublestain Block solution (Vector Laboratories) for 3 min, washed in T-PBS for 5 min, and incubated with the anti-c-Kit rabbit polyclonal antibody (1:200, KAP-TK005, Stressgen Bioreagents, Ann Arbor, MI, USA) for 30 min. After washing with T-PBS for 5 min, sections were incubated with a labelled polymer – AP secondary antibody (Vector Laboratories) for 30 min, and washed again. Fast red (Vector Laboratories) was used as the AP substrate for 5 min. Sections were then washed in tap water and counterstained with hematoxylin for 1 min. DAKO Aqueous mounting medium was used. Each tissue section was analysed by two independent observers (CS and PC) who evaluated the number of positive cells in three paraffin embedded sections independently stained.
To analyse the number of Isl-1 positive cells the area of each section was determined. It was taken a 100%-600dpi picture of each stained slide using an Epson Perfection 1200 Scanner connected to a personal computer. The section sizes were too big to be measured with pictures taken at the microscope. Each picture was then converted to an 8-bit picture, Brightness was adjusted and Threshold applied. On the Threshold pictures the Analyze particles function of the ImageJ Software (released by the NIH, http://rsbweb.nih.gov/ij/) was applied to measure the area of the coloured sections. Data are shown in Table 2. An example of the area measurement is shown in Appendix Figure 1.
Figure 1.
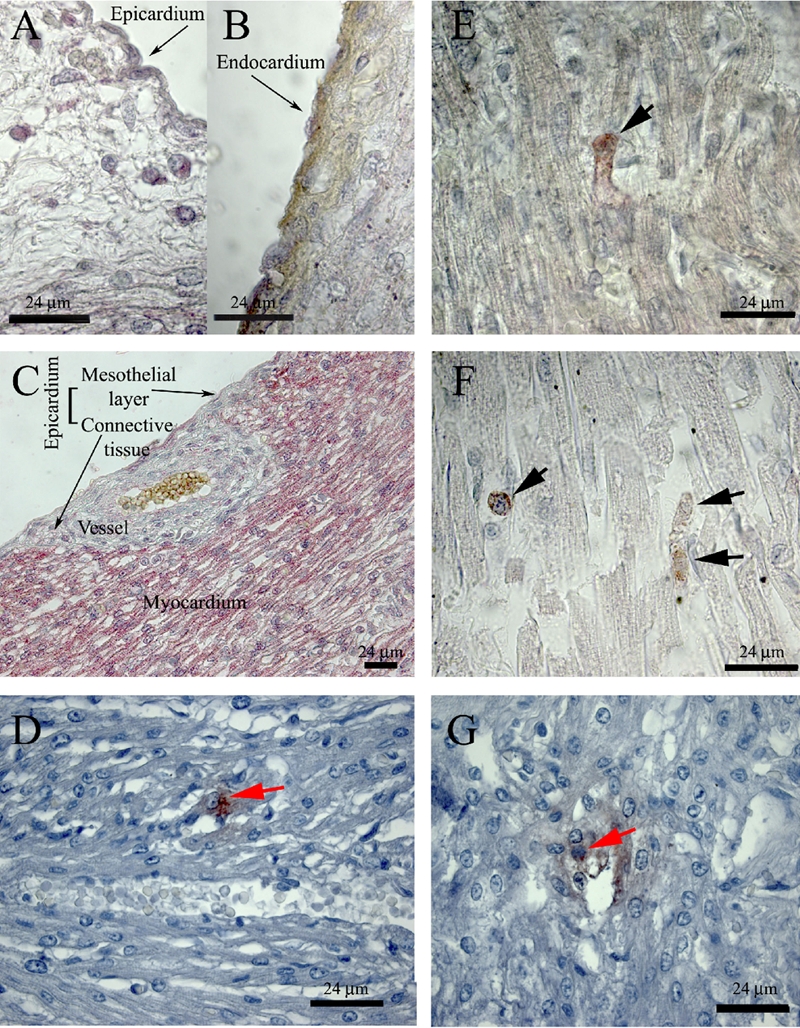
Identification of interstitial c-Kit+/CD105+ and Isl-1+ cells in human foetal and infant hearts before (A–D) and after (E–G) the 17th gestational week. A, B, C) c-Kit (Fast Red) and CD105 (DAB) double immunostaining in a 16-week foetal heart. E, F) c-Kit (Fast Red) and CD105 (DAB) double immunostaining in a preterm infant (36 weeks of gestation, E) and in a term infant (6-year-old child. F) Black arrows indicate double stained cells. D) Isl-1 (AEC) single immunostaining in a 14-week foetal heart; red arrows indicate Isl-1 positive cells. G) Isl-1 (AEC) single immunostaining in a 21-week foetal heart. Double positive cells appeared deep red; red arrow indicate Isl-1 positive cells.
Immunofluorescence
After deparaffination and rehydration, tissue sections were blocked with 5% BSA in PBS for 30 min and incubated with the first primary antibody overnight, one night for each antibody (1:50, anti-c-Kit, KAP-TK005, Stressgen Bioreagents; 1:50, anti-Isl-1, goat polyclonal, SC-23590, Santa Cruz Biotechnology Inc.). After washing with PBS, cells were further incubated with fluorescent secondary antibodies 1 h at room temperature (1:50, TRITC-conjugated anti-rabbit secondary antibody, T5268, Sigma-Aldrich, St. Louis, MO; 1:50 FITC-conjugated donkey anti-goat secondary antibody, SC-2024, Santa Cruz Biotechnology Inc.). In single immunofluorescence experiments nuclei were stained 10 min with 10 µg/mL Hoechst33342 staining in PBS (Invitrogen Corp., Carlsbad, CA, USA). In confocal experiments nuclei were stained 15 min with 1 µM Toto-3 staining in PBS (T3604, Invitrogen Corp.). To avoid cross-reactions, sections were incubated with goat-anti-Isl-1 first and with rabbit anti-c-Kit after, and with donkey anti-goat first and goat anti-rabbit after, in four different incubations.
To avoid autofluorescence of paraffin/formalin embedded sections, the samples were incubated in 0.1% Sudan Black in 70% ethanol for 5 min after nuclei staining.31 For imaging they were both used a Zeiss LSM5 Exciter Laser Scanning Confocal Microscope and a Leica CTR5000 fluorescent microscope. During confocal microscope observations all parameters for picture acquisition were kept the same and automatically saved.
Statistical analysis
Data are shown as mean values and the standard deviation has been calculated. The hypothesis that the number of Isl-1+ cells per mm2 varied with the gestational age was tested using a t-test of dependent samples (P<0.05). The hypothesis that there was a linear correlation between the gestational age of the foetuses and the number of Isl-1+ cells per mm2 was tested using the Pearson coefficient (P<0.05). For the statistical analysis it was used the STATISTICA 6.0 software (StatSoft Italia srl, Vigonza, Italy).
Results
Expression of c-Kit on the membrane of cardiomyocytes of foetal hearts
To study the localization of c-Kit+ CPCs previously isolated and characterized in the rat adult heart by our research group,14 we performed immunohistochemistry and immunofluorescence experiments on heart samples from embryonic and foetal human hearts.
As shown in Table 1 and Figure 1 A–C, many interstitial and subepicardial cells and all cardiomyocytes were positive for c-Kit from the 9th to the 17th week of gestation, and sometimes also to the 22nd week.
The expression of c-Kit on the surface of cardiomyocytes of the first and second gestational trimesters was confirmed by immunofluorescence experiments (Figure 2 A, F). In the only embryonic sample (9th gestational week) some structures similar to the primordial myocardium of the cardiac tube were clearly positive to c-Kit (Figure 2 B–C). Later on during gestation, clusters of c-Kit+ cells were present (Figure 2 D).
Figure 2.
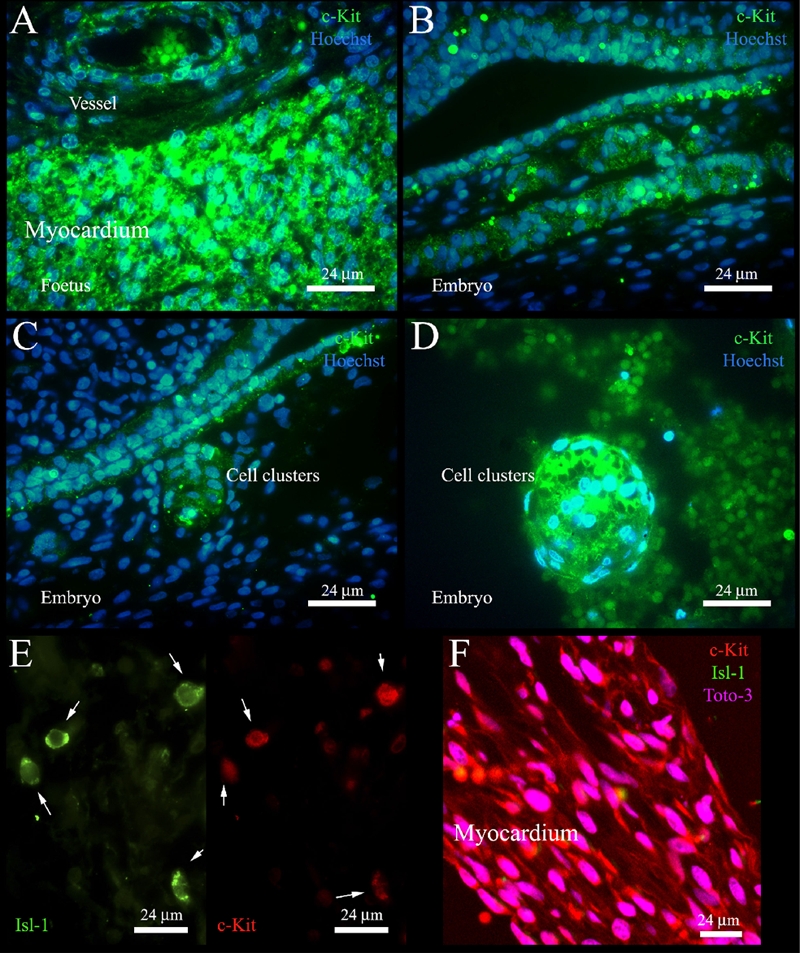
Expression of c-Kit and Isl-1 in embryonic and foetal hearts. A) Expression of c-Kit in a 16-week foetal heart. B, C) Expression of c-Kit in a 9-week human embryo. D) A cluster of c-Kit+ cells in a 15-week foetal heart. E) Co-localization of c-Kit+ and Isl-1+ cells in a 14-week foetal heart; white arrows indicate cells double positive for Isl-1 and c-Kit. F) Confocal microscopy analysis of the expression of c-Kit and Isl-1 in the myocardium of a 16-week foetal heart.
Immunolocalisation of cardiac progenitor cells
In literature there have been clearly identified two main populations of cardiac progenitor cells: human cells double positive for c-Kit and CD105 in adult hearts by Smith et al.,25 and cells positive for Isl-1 by Laugwitz et al.9 in the developing heart. To localize separately the two populations in the human foetal hearts we performed a double immunostaining for c-Kit and CD105, and a single immunostaining for Isl-1. Results were summarised in Table 1 for c-Kit/CD105 and Table 2 for Isl-1.
Interstitial c-Kit+/CD105+ cells appeared at the 17th gestational week and persisted after birth, as shown in Table 1 and Figure 1 E–F. These double positive cells were localised only in the myocardium and were never localised inside capillaries. Many dividing cells were identified in the myocardium (Figure 1F). There were no differences between normal hearts and foetuses with heart malformations (Table 1).
From the 9th to the 16th gestational week, they were present only c-Kit+ (in the subepicardial space, Figure 1A, and in the myocardium, Figure 1C) or CD105+ cells (endocardium and endothelium, Figure 1B). Isl-1+ cells were present in the myocardium and in the suben-docardium, with no differences between right and left ventricles. Groups of Isl-1+ cells were sometimes visible, with a number ranging between 4.523±1.22×104 at the 14th gestational week and 0.548±0.12×104 per mm2 in the post-natal age (Table 2, Figure 1 D,G). The t-test for dependent variables demonstrated that the decrease in the number of Isl-1+ cells was significant and that this difference depended on the gestational week. The number of Isl-1+ cells was higher between the 14th and the 16–19th gestational weeks and it was almost the same between the 20th gestational week and the post-natal age. The Pearson coefficient (r=0.5; P=0.14) demonstrated that there was not a linear correlation between the gestational week and the number of cells per mm2.
Colocalization of c-Kit /Isl-1 cells
To study whether these markers were labelling the same or a different cell population, we evaluated the co-expression of c-Kit and Isl-1. We found that all the interstitial and subendocardial Isl-1+ cells were positive for c-Kit (Figure 2E), while not all the cells positive for c-Kit were Isl-1+ (Appendix Figure 2). Cardiomyocytes were positive only for c-Kit (Figure 2F). The co-localization of the expression of c-Kit and Isl-1 on the same cell was confirmed by confocal microscopy (Appendix Figure 3).
Discussion
In this study we described the localization and the temporal distribution of c-Kit+/CD105+ and Isl-1+ cells from the embryonic to the post-natal heart, and we tested the hypothesis if c-Kit+ cells abundantly isolated from adult hearts were also present in the embryonic and foetal heart and if they represented a subset of cells different from the Isl-1+ cell population.
Cardiac precursors can be isolated from adult and post-natal hearts,9,32 and are characterized by several markers among which c-Kit both in foetal17 and adult heart,16 Isl-1 in early cardiogenesis,9,28 and CD105 in human endomyocardial biopsy specimens.25
With regard to these three CPC markers, the present study shows that Isl-1+ cells are present in the human hearts in foetuses, preterm infants and term infants, that their number decreases with the gestational week particularly after the 16–19th gestational week, and that there is a difference in the temporal distribution of c-Kit+ cells. These data also show that cardiomyocytes and some interstitial cells are c-Kit+ from the 9th to the 16th–19th gestational weeks, and that after this time period, only a few interstitial cells express c-Kit. Interstitial cells, which express both c-Kit and CD105 on their surfaces, are present only from the 17th week to the 6th year of age. The presence of c-Kit+/CD105+ cells in the different stages of gestation does not demonstrate that c-Kit+ cells are resident cardiac cells. But the fact that many structures in the embryonic heart and foetal cardiomyocytes are positive to c-Kit in the early weeks of gestation provides evidence that c-Kit+ cells are present in the heart from the early stages of cardiogenesis, and their number decreases during gestation until the postnatal age.
We also demonstrated that the interstitial and subendocardial Isl-1+ cells are positive also to c-Kit. The co-localization of the two CPC markers let us suppose that Isl-1+ cells are a subset of the c-Kit+ CPCs population usually isolated and identified in the adult heart.
The hypothesis that c-Kit+ human CPCs are more abundant in the early stages of gestation and that their number decreases over time is supported by a recent paper, where the authors isolated and grown human CPCs from neonate and infant specimens.33 The c-Kit+ cells that the authors isolated and expanded were also positive to Isl-1 in culture inside cardiospheres, supporting our hypothesis that the postnatal Isl-1+ cells may be a subpopulation of c-Kit+ human CPCs, which originate from the embryonic pre-cardiac mesoderm and probably persist in the adulthood.
In conclusion, we have demonstrated that foetal cardiomyocytes are positive to c-Kit, and that foetal Isl-1+ cells are a subset of foetal c-Kit+ CPCs. A better understanding of the localisation and migration of CPCs during cardiogenesis may be achieved using mouse or zebrafish transgenic models.
Acknowledgements:
this research was supported by MIUR ex-60% Dott. Valentina Di Felice (Ministero dell'Università e della Ricerca) 2007 and “Ministero della Salute” Ricerca Finalizzata 2007 Prof. Giovanni Zummo.
References
- 1.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–19. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- 2.de la Cruz MV, Sanchez Gomez C, Arteaga MM, Arguello C. Experimental study of the development of the truncus and the conus in the chick embryo. J Anat. 1977;123:661–86. [PMC free article] [PubMed] [Google Scholar]
- 3.Di Felice V, Zummo G. Tetralogy of fallot as a model to study cardiac progenitor cell migration and differentiation during heart development. Trends Cardiovasc Med. 2009;19:130–5. doi: 10.1016/j.tcm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 5.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–6. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 7.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circ Res. 2009;104:933–42. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- 11.Genead R, Danielsson C, Wardell E, Kjaeldgaard A, Westgren M, Sundstrom E, et al. Early first trimester human embryonic cardiac Islet-1 progenitor cells and car-diomyocytes: Immunohistochemical and electrophysiological characterization. Stem Cell Res. 2010;4:69–76. doi: 10.1016/j.scr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 13.Amir G, Ma X, Reddy VM, Hanley FL, Reinhartz O, Ramamoorthy C, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg. 2008;86:1311–9. doi: 10.1016/j.athoracsur.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Di Felice V, Ardizzone NM, De Luca A, Marciano V, Gammazza AM, Macaluso F, et al. OPLA scaffold, collagen I, and horse serum induce an higher degree of myogenic differentiation of adult rat cardiac stem cells. J Cell Physiol. 2009;221:729–39. doi: 10.1002/jcp.21912. [DOI] [PubMed] [Google Scholar]
- 15.Forte G, Carotenuto F, Pagliari F, Pagliari S, Cossa P, Fiaccavento R, et al. Criticality of the biological and physical stimuli array inducing resident cardiac stem cell determination. Stem Cells. 2008;26:2093–103. doi: 10.1634/stemcells.2008-0061. [DOI] [PubMed] [Google Scholar]
- 16.Di Felice V, De Luca A, Colorito ML, Montalbano A, Ardizzone NM, Macaluso F, et al. Cardiac stem cell research: an elephant in the room? Anat Rec. 2009;292:449–54. doi: 10.1002/ar.20858. [DOI] [PubMed] [Google Scholar]
- 17.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–65. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 18.Amir G, Miller L, Shachar M, Feinberg MS, Holbova R, Cohen S, et al. Evaluation of a peritoneal-generated cardiac patch in a rat model of heterotopic heart transplantation. Cell Transplant. 2009;18:275–82. doi: 10.3727/096368909788534898. [DOI] [PubMed] [Google Scholar]
- 19.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–74. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 20.Riccio M, Resca E, Maraldi T, Pisciotta A, Ferrari A, Bruzzesi G, et al. Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures. Eur J Histochem. 2010;54:e46–e46. doi: 10.4081/ejh.2010.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Li J, Saleem S, Yee SP, Hardikar AA, Wang R. c-Kit and stem cell factor regulate PANC-1 cell differentiation into insulin- and glucagon-producing cells. Lab Invest. 2010;90:1373–84. doi: 10.1038/labinvest.2010.106. [DOI] [PubMed] [Google Scholar]
- 22.Calo PG, Esu F, Tatti A, Pilloni L, Madas F, Pisano G, et al. Isolated inguinal endometriosis. Case report with ultra-sonographic preoperative diagnosis. G Chir. 2011;32:263–5. [PubMed] [Google Scholar]
- 23.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 24.Di Nardo P, Forte G, Ahluwalia A, Minieri M. Cardiac progenitor cells: potency and control. J Cell Physiol. 2010;224:590–600. doi: 10.1002/jcp.22165. [DOI] [PubMed] [Google Scholar]
- 25.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 26.Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18–18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierelli L, Bonanno G, Rutella S, Marone M, Scambia G, Leone G. CD105 (endoglin) expression on hematopoietic stem/progenitor cells. Leuk Lymphoma. 2001;42:1195–206. doi: 10.3109/10428190109097744. [DOI] [PubMed] [Google Scholar]
- 28.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- 30.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008 3;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cyto-chem. 2001;49:1565–72. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 32.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, et al. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 33.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–73. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]


