Abstract
Our aim of this study was to compare the accuracy of three different modalities for testing sensory neuropathy in diabetic patients with and without diabetic foot problems. The three devices used included the pin-prick testing using the Neurotip® (PPT), the Semmes–Weinstein 5.07/10 g monofilament testing (SWMT), and the rapid-current perception threshold (R-CPT) measurements using the Neurometer® testing. Our study population consisted of 54 patients (108 feet) with diabetic foot problems treated at the National University Hospital in Singapore by our multi-disciplinary diabetic foot care team. Our results showed no difference in sensory neuropathy detected by PPT and 5.07/10 g SWMT in both the pathological and normal foot. In the pathological foot, there was significant increase in sensory neuropathy detected by the Neurometer® device at both the big toe and ankle sites as compared to PPT and 5.07/10 g SWMT. In the normal foot, there was a significant increase in sensory neuropathy detected by the Neurometer® device at the big toe site only as compared to PPT and 5.07/10 g SWMT. Finally, the Neurometer® measurements detected a statistically higher proportion of feet with sensory neuropathy as compared to detection by the PPT or 5.07/10 g SWMT.
Keywords: diabetic foot, neuropathy, neurometer, Semmes–Weinstein monofilament, ulcers
Diabetic sensory neuropathy is a common complication of diabetes mellitus (1). The exact cause of diabetic neuropathy is not well understood. A combination of metabolic and vascular factors is postulated to be involved (2–4). Peripheral neuropathy is associated with an increased risk of developing a foot ulceration, gangrenous changes, and/or lower extremity amputations (4–6). A recent systematic review of various risk stratification systems has also confirmed that diabetic neuropathy is one of the main risk factors for development of diabetic foot ulcers (7). Diabetic foot ulcers related to diabetic neuropathy constitutes one of the main leading causes for diabetic foot infections (8). Diabetic neuropathy has also been associated as a predictive factor for limb loss (below knee and above knee amputations) in diabetic foot patients (9). In view of the aforementioned potential complications, it is evident that diabetic neuropathy is significantly associated with a patient's increased rate of morbidity and mortality (10). Due to these reasons, it is important to detect peripheral neuropathy early in the diabetic patient so that diabetic foot care education can be provided and protective measures can be used to avoid devastating complications with the diabetic foot. Our aim of this article was to clinically compare the accuracy of three different modalities for testing sensory neuropathy in the diabetic and non-diabetic population. Our tests included the pin-prick testing (PPT); Semmes–Weinstein 5.07/10 g monofilament testing (SWMT), and the rapid-current perception threshold (R-CPT) measurements using the Neurometer®.
Materials and methods
Our study population consisted of 54 patients with diabetes mellitus and associated diabetic foot complications treated by our multi-disciplinary team at the National University Hospital in Singapore from January to June 2005. All except one patient had type 2 diabetes mellitus. The types of diabetic foot problems (DFP) were classified according to the King's classification (11). All data were documented using a study protocol including age, sex, race, type and duration of diabetes mellitus, presence of co-morbidities, laboratory values such as glycosylated hemoglobin, erythrocyte sedimentation rate, C-reactive protein, white blood cell count, blood urea nitrogen and creatinine levels, blood cultures, and soft tissue culture and sensitivities.
Pin prick testing
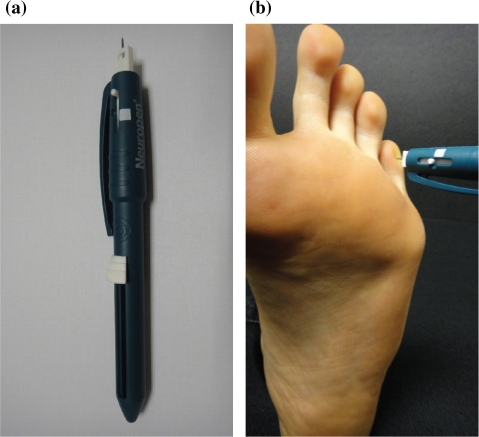
A disposable, sterile calibrated 10g Neurotip® (Owen Mumford, UK) was used for the PPT (single use only for each patient) (Fig. 1a). A constant force was applied perpendicularly on the skin of the foot until the calibrated mark was reached (Fig. 1b). A record was made as to whether the patient responded to any sensation felt in the foot. This was compared to the sensation felt on the dorsum of the hand on the same side. The area of sensory disturbance was also mapped out for both feet.
Fig. 1.
(a) Showing the Neurotip® as part of the Neuropen®. (b) Showing the Neurotip® being used on the foot.
Semmes–Weinstein 5.07/10 g monofilament testing
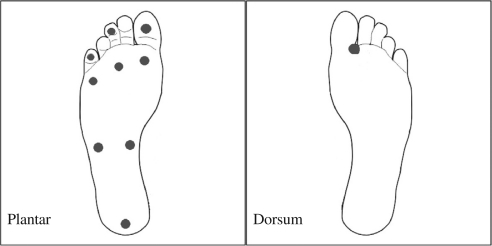
The Semmes–Weinstein 5.07/10 g monofilament was used and pressed against the skin perpendicularly until the monofilament buckled (12). This applied a consistent 10 g force to the site. The test was performed on 10 test sites – nine on the plantar surface of the foot and one on the dorsum of the foot (6). The nine plantar sites were the pulps of the first, third, and fifth toes, over the skin overlying the first, third, and fifth metatarsal heads, two in the arch of the foot and the heel (Fig. 2). The dorsal site was in the first web space. Patients who could feel 7 or more out of 10 sites were categorized as normal while patients with <7 test sites felt were classified as having loss of protective sensation. The authors in this study selected this 10 point 5.07/10 SWMT as the standard for diagnosing neuropathy in this study.
Fig. 2.
Showing the 10 sites in the foot for 5.07 Semmes–Weinstein monofilament testing (SWMT).
Neurometer® testing
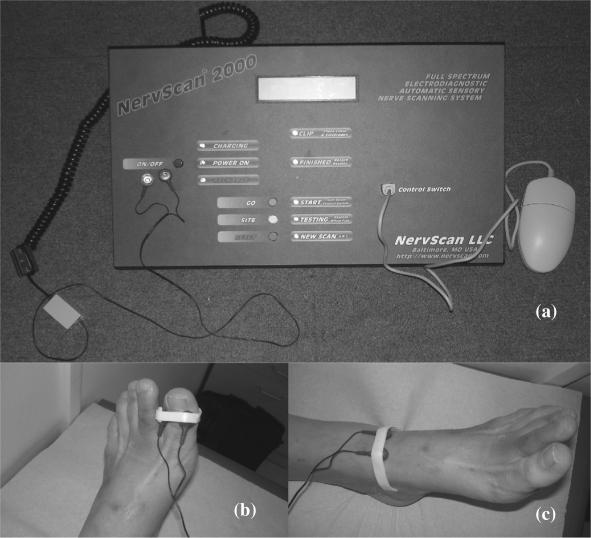
The Neurometer® (Neurotron, Baltimore, Maryland, USA) testing generated rapid current perception threshold (R-CPT) readings based on the minimal strength of alternating current stimulus that the patient could detect. The test was double-blinded and the two test sites that were used in our study included the big toe and the ankle (Fig. 3). The Neurometer® applied three different frequencies (2000, 250, and 5 Hz) of alternating current signals at levels calibrated between 0 and 10 mA. Each frequency stimulated a different group of nerve fibers; 2000 Hz stimulated alpha beta fibers, 250 Hz stimulated alpha delta fibers (both fibers being large diameter fibers), while 5 Hz stimulated small diameter C fibers. At each frequency, the current was increased over a variable time interval until the patient could detect a sensation at the test site. This was repeated until three concurrent values for current intensity were obtained. A R-CPT value was then generated for each frequency. The R-CPT values obtained for each frequency at each test site ranged from 1 to 25; 6 to 13 being normal, 1 to 5 indicating hyperesthesia, and 14 to 25 indicating hypoesthesia. Both hyperesthesia and hypoesthesia indicated the presence of sensory neuropathy. The extent of neuropathy present using the two test sites were also documented for both feet.
Fig. 3.
(a) Showing the Neurometer® Machine. (b) Electrodes applied to the big toe site. (c) Electrodes applied to the ankle site.
Statistical analysis was employed using the chi-square test to compare the results obtained using the three different methods. A p-value of <0.05 represented a significant difference while a p-value of <0.001 was highly statistically significant.
Results
Our study population ranged between 30 and 80 years old, the average being 56.7 years old. The ratio of males to females was 1:1 and our demographic distribution was 42.6% Chinese, 33.3% Malays, 20.4% Indians, and 3.7% of other races. The majority of our patients had been diagnosed with diabetes mellitus for more than 10 years; 38 out of 54 patients (70.4%). In our study cohort of 54 patients (108 feet), there were 61 feet with DFP and 47 with normal feet. Seven patients had bilateral DFP. The most common DFP that was encountered included 21 diabetic foot ulcers, 10 abscesses, 8 with cellulitis, and 6 with gangrene. In other pathological feet, the complications were combinations of an ulcer and abscess in 6 feet, ulcer and gangrene in 6 feet, and abscess with gangrene in 4 feet.
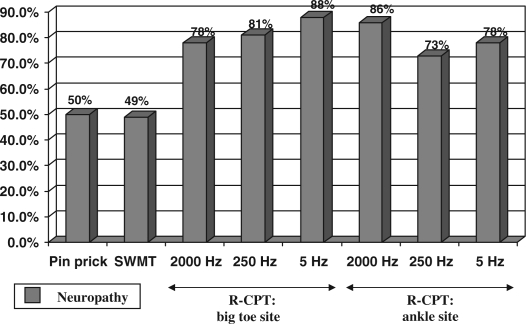
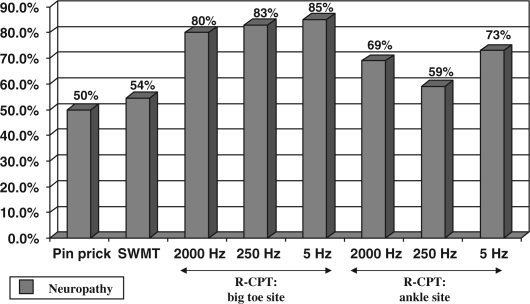
Fig. 4 shows the results of PPT, 5.07/10 g SWMT, and R-CPT testing in the pathological foot. There was no significant difference in sensory neuropathy detected by PPT compared to neuropathy detected by SWMT (p=0.93). There was a statistically significant increase in sensory neuropathy detected by Neurometer® testing at the big toe site for all three frequencies as compared to PPT (2000 Hz: p=0.014, 250 Hz: p=0.006, 5 Hz: 0.0004). There was also a statistically significant increase in sensory neuropathy detected by Neurometer® testing at the ankle site for all three frequencies as compared to PPT (2000 Hz: p=0.0002, 250 Hz: p=0.029, 5 Hz: p=0.013). When the R-CPT readings were compared with the results of SWMT, there was a significant increase in sensory neuropathy detected by Neurometer® testing at the big toe site for all three frequencies as compared to SWMT (2000 Hz: p=0.016, 250 Hz: p=0.007, 5 Hz: p=0.0005). There was also found a significant increase in sensory neuropathy detected by Neurometer® testing at the ankle site for all three frequencies as compared to SWMT (2000 Hz: p=0.0003, 250 Hz: p=0.032, 5 Hz: p=0.015).
Fig. 4.
Comparison of pin-prick testing, 5.07 Semmes–Weinstein monofilament testing, and rapid current perception threshold (R-CPT) Testing in the pathological foot.
Fig. 5 shows the results of PPT, 5.07/10 g SWMT, and R-CPT testing in the normal foot. There was no significant difference in sensory neuropathy detected by PPT compared to neuropathy detected by SWMT (p=0.80). There was a statistically significant increase in sensory neuropathy detected by Neurometer® testing at the big toe site for all three frequencies as compared to PPT (2000 Hz: p=0.007, 250 Hz: p=0.001, 5 Hz: p=0.001. However, there was no significant increase in sensory neuropathy detected by Neurometer® testing at the ankle site for all three frequencies as compared to PPT (2000 Hz: p=0.098, 250 Hz: p=0.46, 5 Hz: p=0.52). When the Neurometer® readings were compared with the results of 5.07/10 g SWMT, we found a significant increase in sensory neuropathy detected by Neurometer® testing at the big toe site for all three frequencies as compared to SWMT (2000 Hz: p=0.017, 250 Hz: p=0.004, 5 Hz: p=0.003). However, there was no significant increase in sensory neuropathy detected by Neurometer® testing at the ankle site for all three frequencies as compared to SWMT (2000 Hz: p=0.20, 250 Hz: p=0.74, 5 Hz: p=0.81).
Fig. 5.
Comparison of pin-prick testing, 5.07 Semmes–Weinstein monofilament testing, and rapid current perception threshold (R-CPT) Testing in the normal foot.
Discussion
The 5.07/10 g SWMT has been widely preferred as a screening tool for detection of peripheral diabetic neuropathy in view of its cost, portability, ease of administration, and higher acceptance by patients (13). In this study, we have compared PPT and R-CPT with the 5.07/10 g SWMT. Our study demonstrated no significant difference between neuropathy detected by PPT compared to neuropathy detected by 5.07/10 g SWMT. We found high sensitivity of the R-CPT test in comparison with the SWMT and PPT. However, it must be noted that R-CPT had not been validated for diagnosing diabetic neuropathy in this study.
The Neurotip® has been described by Paisley et al. to be a sensitive and inexpensive device for assessing nerve function, especially when combined with the 10 g monofilament test in the Neuropen® (14). The single use and sterile neurological examination pins provide an advantage in terms preventing any cross-contamination of infections from one patient to another.
The Neurometer® has been described as a useful tool for detection of sensory neuropathy (15, 16). It is interesting to note that in a study by Cheng et al. in 1999 assessing peripheral neuropathy by 5.07/10 g SWMT, 128 Hz graduated tuning fork testing, and Neurometer® testing in 558 Type II diabetics, 59 were found to be positive on 5.07/10 g SWMT, 45 positive on Vibration Perception Threshold, and 189 positive on Neurometer® measurements (17). In this study, Cheng et al. (17) found that the Neurometer® detected neuropathy in more patients as compared to 5.07/10 g SWMT and Vibration Perception Threshold (the differences being found to be statistically significant).
Lastly, there has been a great variation in both the reference test and methodology when using the 5.07/10 SWMT (18). A recent systematic review of the accuracy of 5.07/10 SWMT revealed a sensitivity ranging from 57 to 93% and specificity from 75 to 100% with a three-site test being the ideal methodology to maximize the diagnostic value (18). A limitation of our study would be that we used the 10-site test for our investigations using the 5.07/10 SWMT, which may have under diagnosed patients with peripheral neuropathy.
Conclusion
Comparison of the three different modalities of testing in this study showed that there was no difference between neuropathy detected by PPT compared to neuropathy detected by 5.07/10 g SWMT. However, Neurometer® measurements detected a statistically higher proportion of feet with sensory neuropathy compared to detection by PPT or by 5.07/10 g SWMT.
Acknowledgements
The authors would like to thank Mr. Jefferson J Katims, Research Director, Neurotron, Incorporated and Mr. Lee Poh Seng, Technical Marketing Executive, All Eights (Singapore) Private Limited, for the use of the Neurometer® machine.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy: results of the Seattle prospective Diabetic Foot Study. Diabet Care. 1997;20:1273–8. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 2.Allen C, Shen G, Palta M. Long-term hyperglycemia is related to peripheral nerve changes at a diabetes duration of 4 years. Diabet Care. 1997;20:1154–8. doi: 10.2337/diacare.20.7.1154. [DOI] [PubMed] [Google Scholar]
- 3.Greene DA, Lattimer SA, Sima AAF. Sorbitol, phosphoinositides and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316:599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 4.Abbot CA, Vileikyte L, Williamson SH, Carrington AL, Boulton AJM. Multicentre study of the incidence and predictive factors for diabetic foot ulceration. Diabet Care. 1998;21:1071–5. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 5.Vuorisalo S, Venermo M, Lepantalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg. 2009;50:275–91. [PubMed] [Google Scholar]
- 6.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabet Care. 1998;21:1714–9. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Risk stratification systems for diabetic foot ulcers: a systematic review. Diabetologia. 2011;54:1190–9. doi: 10.1007/s00125-010-2030-3. [DOI] [PubMed] [Google Scholar]
- 8.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006;117:212S–38S. doi: 10.1097/01.prs.0000222737.09322.77. [DOI] [PubMed] [Google Scholar]
- 9.Nather A, Bee CS, Huak CY, Chew JL, Lin CB, Neo S, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22:77–82. doi: 10.1016/j.jdiacomp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA. Diabetic neuropathy: an intensive review. Am J Health Syst Pharm. 2004;15:160–73. doi: 10.1093/ajhp/61.2.160. [DOI] [PubMed] [Google Scholar]
- 11.Edmonds ME, Foster AVM. Managing the diabetic foot. 2nd ed. London: Blackwell; 2005. [Google Scholar]
- 12.Sosenko JM, Sparling YH, Hu DS, Welty T, Howard BV, Lee E, et al. Use of the Semmes–Weinstein Monofilament in the Strong Heart Study. Diabet Care. 1999;22:1715–21. doi: 10.2337/diacare.22.10.1715. [DOI] [PubMed] [Google Scholar]
- 13.Mayfield JA, Sugarman JR. The use of the Semmes–Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract. 2000;49:S17–29. [PubMed] [Google Scholar]
- 14.Paisley AN, Abbot CA, van Schie CH, Boulton AJ. A comparison of the Neuropen against standard quantitative sensory-threshold measures for assessing peripheral nerve function. Diabet Med. 2002;19:400–5. doi: 10.1046/j.1464-5491.2002.00706.x. [DOI] [PubMed] [Google Scholar]
- 15.Masson EA, Veves A, Fernando D, Boulton AJ. Current perception thresholds: a new, quick and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia. 1989;32:724–8. doi: 10.1007/BF00274531. [DOI] [PubMed] [Google Scholar]
- 16.Masson EA, Boulton AJ. The neurometer: validation and comparison with conventional tests for diabetic neuropathy. Diabet Med. 1991;8:S63–6. doi: 10.1111/j.1464-5491.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, et al. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. 1999;246:394–8. doi: 10.1007/s004150050370. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Schlosser FJ, Sumpio BE. The Semmes–Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50:675–82. doi: 10.1016/j.jvs.2009.05.017. [DOI] [PubMed] [Google Scholar]