Abstract
Diabetic gastroparesis is associated with increased oxidative stress attributable to loss of upregulation of heme oxygenase-1 (HO1), with resultant damage to interstitial cells of Cajal and delayed gastric emptying. These changes can be reversed by induction of HO1. HO1 catalyzes the breakdown of heme into iron, biliverdin and, carbon monoxide (CO). The aim of this study was to determine whether inhalation of CO can mimic the protective effects of HO1. Nonobese diabetic (NOD) mice with delayed gastric emptying were treated with CO inhalation. Serum malondialdehyde was measured as a marker of oxidative stress. Gastric emptying of solids was measured using a [13C]octanoic acid breath test. Kit expression levels were determined in immunoblots of protein extracted from the external muscle layers of the gastric body and antrum. The effect of CO on oxidative stress and gastric emptying was also determined in the presence of HO activity inhibitor chromium mesoporphyrin. CO inhalation reduced oxidative stress, restored Kit expression and reversed delayed gastric emptying in diabetic NOD mice with delayed gastric emptying. CO inhalation maintained this effect in the presence of the HO activity inhibitor, chromium mesoporphyrin, also resulting in restoration of the delay in gastric emptying. CO inhalation mimics the protective effect of upregulation of HO1 and decreased oxidative stress, increased Kit expression, and restored delay in gastric emptying. This effect of CO was independent of HO activity, suggesting that its effects were downstream of HO1. CO represents a potential therapeutic option for treatment of diabetic gastroparesis.
Keywords: macrophages, interstitial cells of Cajal, stomach, heme oxygenase, nonobese diabetic
gastroparesis is a complication of diabetes, characterized by delayed emptying of the stomach in the absence of obstruction. Diabetic gastroparesis is associated with histological changes that include loss of neuronal nitric oxide synthase and interstitial cells of Cajal (ICC) in both humans and in animal models (17, 19, 29). ICC generate electrical signals that regulate smooth muscle contraction in the stomach and help maintain normal gastric function (7). ICC can be identified in tissue by labeling with antisera to receptor tyrosine kinase Kit, a protein expressed on ICC. Kit and its ligand, stem cell factor, are also important survival factors for ICC (25).
The number of ICC in healthy tissue is not static; rather, normal ICC networks are maintained by a balance between cell death, maintenance, and ICC proliferation (14). In disease states such as diabetic gastroparesis, this balance is disturbed with eventual loss of ICC. We have previously shown that increased oxidative stress associated with diabetes can lead to loss or damage to ICC in mice (9). Upregulation of the enzyme heme oxygenase-1 (HO1) is an important cellular defense mechanism against oxidative stress (1). Upon onset of diabetes in the nonobese diabetic (NOD) mouse model of diabetes, there is upregulation of HO1, which protects ICC by lowering oxidative stress. Loss of upregulation of HO1 in these mice leads to increased oxidative stress, which in turn leads to damage and loss of ICC networks and the development of delayed gastric emptying. Induction of HO1 by hemin reverses these effects, decreases oxidative stress, increases Kit expression levels, and normalizes gastric emptying (9).
The underlying mechanism of the cytoprotective effect of HO1 is unknown, but the preponderance of data in the literature suggests that the protective effect of HO1 is mediated by carbon monoxide (CO), a product of heme metabolism by HO1 (33, 35). In the gastrointestinal tract, low-dose CO inhalation has been shown to protect rat intestinal grafts from ischemia/reperfusion injury (27), improve levels of colitis in IL10−/− mice (18), and protect against the development of postoperative ileus and necrotizing enterocolitis (26, 40). CO released from CO-releasing molecules also has similar effects in animal models and has been shown to reduce oxidative stress and reduce postoperative ileus (11).
The goal of this study was to determine whether, in the NOD mouse model of type 1 diabetes, low-dose CO inhalation mimics the protective effects of HO1 in diabetic mice with delayed gastric emptying by reducing oxidative stress, increasing Kit expression, and normalizing delay in gastric emptying.
MATERIALS AND METHODS
Animals and experimental design.
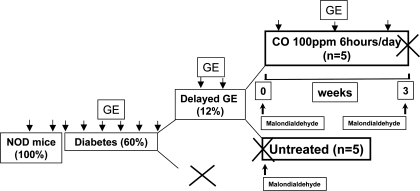
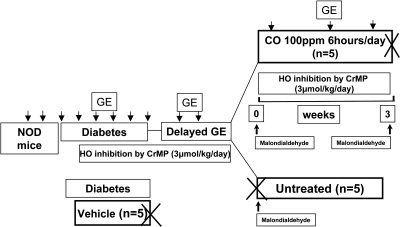
Diabetic female NOD/ShiLtJ (Jackson laboratory, Bar Harbor, ME) mice were studied as previously described (9, 10). All procedures were approved by the Mayo Clinic IACUC. The experimental designs for the two experiments in this study are outlined in Figs. 1 and 2. In the first experiment (Fig. 1), diabetic mice that developed delayed gastric emptying were assigned to two groups. One group (n = 5) was killed, and serum and gastric tissue was collected to obtain tissue as a pretreatment control. The other group (n = 5) was assigned to receive CO inhalation 100 ppm for 6 h/day for a maximum period of 8 wk or less if the mouse had two consecutive normal rates of gastric emptying. At the end of this period, mice were killed, and gastric tissue and serum were collected. In the second set of experiments (Fig. 2), diabetic mice, after 2 wk of diabetes, either received daily intraperitoneal injections of chromium mesoporphyrin (CrMP, 3 μmol/kg per day dissolved in 0.25% ammonium hydroxide, n = 10; Frontier Scientific, Logan, UT), to inhibit HO or daily intraperitoneal injections of 0.25% ammonium hydroxide (vehicle; n = 5). All mice injected with CrMP developed delayed gastric emptying as confirmed by two consecutive gastric emptying readings, and all mice injected with ammonium hydroxide alone had normal gastric emptying. Mice with delayed gastric emptying were assigned to two groups. One group (n = 5) was killed after development of delayed gastric emptying (n = 5), and serum and tissue were collected. The other group (n = 5) was assigned to receive CO inhalation 100 ppm for 6 h/day for a maximum period of 8 wk or less if the mouse had two consecutive normal rates of gastric emptying. These mice continued to receive daily intraperitoneal injections of CrMP to continually inhibit HO activity. At the end of this period, the mice were killed, and serum and tissue were collected.
Fig. 1.
Cartoon of experimental plan for carbon monoxide (CO) treatment. Eight-week-old female nonobese diabetic (NOD)/ShiLtJ mice were received from Jackson laboratory, and 2 baseline gastric emptying readings were obtained for each mouse before development of diabetes. After onset of diabetes, gastric emptying (GE) was measured every week. Mice were considered to have delayed gastric emptying if two consecutive gastric emptying t1/2 values were greater than normal. Diabetic mice with delayed gastric emptying were assigned to 2 groups; one group (n = 5) was killed, and serum and gastric tissue was collected. The other group (n = 5) was assigned to receive CO inhalation 100 ppm for 6 h/day for a maximum period of 8 wk or less if the mouse had 2 consecutive normal gastric emptying t1/2. At the end of this period, mice were killed and gastric tissue and serum was collected.
Fig. 2.
Cartoon of experimental plan for CO inhalation with heme oxygenase (HO) inhibition. Eight-week-old female NOD/ShiLtJ mice were received from Jackson laboratory, and 2 baseline gastric emptying readings were obtained before the mice became diabetic. After development of diabetes, gastric emptying was measured weekly. After 2 wk of diabetes, mice either received daily intraperitoneal injections of chromium mesoporphyrin (CrMP), 3 μmol/kg per day dissolved in 0.25% ammonium hydroxide (n = 10), or daily intraperitoneal injections of 0.25% ammonium hydroxide (Vehicle; n = 5). All mice injected with CrMP developed delayed gastric emptying as confirmed by 2 consecutive gastric emptying readings; all mice injected with ammonium hydroxide alone had normal gastric emptying. Mice with delayed gastric emptying were either killed soon after development of delayed gastric emptying (n = 5), and serum was collected, or they were assigned to receive CO inhalation (100 ppm for 6 h/day) for a maximum period of 8 wk or less if the mouse had 2 consecutive normal gastric emptying t1/2 (n = 5). These mice continued to receive daily intraperitoneal injections of CrMP to continually inhibit HO activity. At the end of this period the mice were killed and serum was collected.
Gastric emptying.
Gastric emptying of solids (baked egg yolk) was measured after an overnight fast using a [13C]octanoic acid breath test as described previously (10). Briefly, mice were fasted overnight with free access to water in a metabolic cage and placed in a chamber. After a baseline reading, mice were fed 200 mg scrambled cooked egg yolk containing 2.5 μmol [13C]octanoic acid. Air containing the exhaled breath was collected and analyzed to determine the [13C]/[12C] ratio using an Infra Red Isotope Analyzer (IRIS; Wagner Analysen Technik, Bremen, Germany).
CO inhalation.
Mice were housed in specialized chambers for the treatment. CO was mixed with compressed air using an air gas mixer (Omega systems) to deliver 100 ppm. Random samples were obtained from the chamber and analyzed using gas chromatography to confirm the CO concentration in the chamber.
Western blotting.
Western blot analysis was used to detect and quantify Kit protein and GAPDH from protein extracts of the muscle layers of the gastric body as described previously (9). Homogenates of gastric body tissue were lysed in a solution consisting of 50 mM Tris·HCl, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM activated Na3VO4, 1 mM NaF, and the Complete Protease Inhibitors cocktail tablet (Roche, Indianapolis, IN). The total protein content was determined using a Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA) by comparison with a standard curve obtained with bovine serum albumin. From the tissue lysates, 50 μg of protein were resolved by SDS-PAGE on a 12% gel. Proteins were transferred to Immunoblot PVDF membranes (Bio-Rad Laboratories) for immunolabeling. Rabbit polyclonal anti-Kit (Cell Signaling Technology, Danvers, MA) was used as the primary antibody for Kit detection. The intensity of enhanced chemiluminescence of immunoreactive bands was quantified using the Bio-Rad Gel Doc system (Bio-Rad Laboratories). The intensities of all bands were in the linear range of detection. Kit protein expression levels are expressed as the ratio of relative OD units (rODU) for Kit-immunoreactive bands over rODU for GAPDH-immunoreactive bands.
Oxidative stress.
Oxidative stress was determined as previously reported (9) from the concentration of thiobarbituric acid-reactive substances, calculated as malondialdehyde equivalents using a commercial kit (Oxi-Tek; Zeptometrix, Buffalo, NY). Five microliters of serum sample were mixed with an equal volume of sodium dodecyl sulfate solution and 125 μl of 5% thiobarbituric acid/acetic acid reagent. Samples were incubated for 60 min at 95°C. After centrifugation at 1,600 g, supernatants from samples were read at 532 nm using a spectrophotometer (NanoDrop Technology, Wilmington, DE). A serum sample was not available for one mouse, and therefore measurement could not be performed on this mouse.
HO activity.
HO activity was measured using a modification of a previously published method (9). Spleen tissue was washed twice with phosphate-buffered saline (pH 7.4) and homogenized in 100 mM potassium phosphate buffer (pH 7.4). After sonification, the homogenate was centrifuged at 3,000 g for 15 min. The supernatant solution was centrifuged at 12,000 g for 20 min followed by ultracentrifugation at 105,000 g for 1 h. Microsomal fractions were resuspended in 100 mM potassium phosphate buffer (pH 7.4) containing 2 mM MgCl2. In 500 μl reaction mixture, 500 μg of microsome proteins were incubated with 20 μM hemin, 3 mg of liver cytosol, 0.2 U glucose-6-phosphate dehydrogenase, 2 mM glucose-6-phosphate, and 0.8 mM NADPH for 1 h at 37°C in the dark. The bilirubin produced was extracted with chloroform, and the absorbance of bilirubin at 464 nm was measured against a baseline absorbance at 530 nm (extinction coefficient, 40 mM−1/cm−1 for bilirubin). HO activity was expressed as picomoles of bilirubin produced per milligram of protein per hour.
Statistical analysis.
Data are presented as means ± SE or median ± interquartile range for all graphs. The statistical methods used were paired t-tests, unpaired t-tests, and one-way ANOVA with Tukey's posttest for parametric data and Mann-Whitney U-test for nonparametric data. A probability of <0.05 was considered significant.
RESULTS
Mice with blood glucose levels consistently higher than 250 mg/dl were considered to be diabetic. Twelve percent of NOD mice developed delayed gastric emptying (at a mean time of 4.7 wk after development of diabetes; range 3–7 wk). Of the 10 mice with delayed gastric emptying included in the study, five mice were assigned to receive low-dose CO inhalation for 6 h daily (100 ppm; mean period of 16 days; range 14–19 days) after development of delayed gastric emptying. The remaining five untreated mice were killed after development of delayed gastric emptying confirmed on two consecutive gastric emptying readings (Fig. 1).
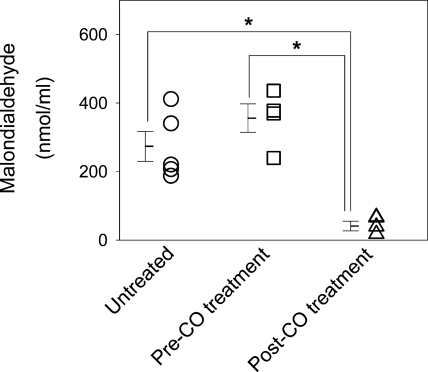
Serum malondialdehyde levels were significantly lower following administration of CO when consecutively measured in mice with delayed gastric emptying (355.7 ± 82.9 to 41.1 ± 13.9 nmol/ml; n = 4; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 3). Serum malondialdehyde levels were significantly higher in untreated mice that were killed soon after development of delayed gastric emptying compared with serum malondialdehyde levels in mice after treatment with CO inhalation (273.4 ± 48.8 vs. 41.1 ± 13.9 nmol/ml; n = 4; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 3).
Fig. 3.
Reduced oxidative stress in mice treated with CO inhalation. Levels of oxidative stress as measured by serum malondialdehyde levels were measured from diabetic mice with delayed gastric emptying killed after development of delayed gastric emptying (untreated) and diabetic mice with delayed gastric emptying before CO inhalation and after CO inhalation. Open symbols represent individual values, whereas bars represent means ± SE, *P < 0.05, one-way ANOVA with Tukey's posttest.
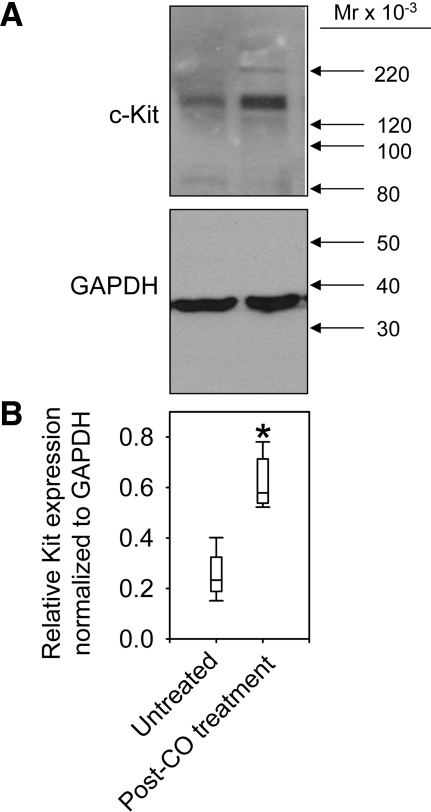
Western blot analysis of protein extracts from the gastric body of mice with delayed gastric emptying detected a protein that was the molecular weight of Kit (145 kDa). This was similar to our previously described finding using a different rabbit polyclonal antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) (9). In the gastric body, Kit expression was significantly decreased in all untreated mice with delayed gastric emptying that were killed after development of delayed gastric emptying, whereas CO inhalation increased the expression of Kit protein in mice with delayed gastric emptying compared with untreated mice (0.23;0.20–0.29 vs. 0.57;0.54–0.69 rODU; n = 5; P < 0.05, Mann-Whitney; Fig. 4).
Fig. 4.
Increased Kit expression in the stomach of mice treated with CO inhalation. Western blot analysis of Kit protein expression in the gastric body. A: representative images from Kit and GAPDH blots. B: relative protein expression (medians with interquartile ranges) obtained by densitometric analysis normalized to GAPDH. *P < 0.05, Mann-Whitney test. X-axis legends apply to both panels. Mr, relative molecular weight.
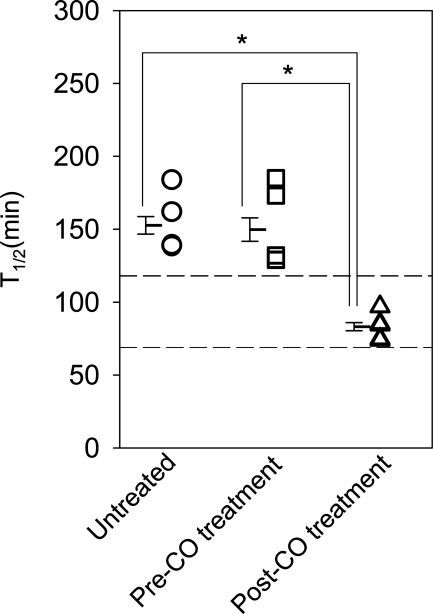
As evident by the lower time to half emptied (t1/2) values, the rate of gastric emptying was increased in mice following CO inhalation compared with both untreated mice with delayed gastric emptying and the t1/2 values recorded for the same mice before initiation of CO treatment (Fig. 5; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest). The t1/2 values following CO treatment were similar to values obtained before the onset of diabetes as well as before the development of delayed gastric emptying. Thus CO treatment normalized the rate of gastric emptying.
Fig. 5.
Gastric emptying normalizes following CO inhalation. Individual mean ± SE t1/2 values of gastric emptying for each mouse (open symbols) and the grouped data (bars) are shown. *P < 0.05, one-way ANOVA with Tukey's posttest. The 2 horizontal dashed lines indicate the normal range of gastric emptying.
The decreases in serum malondialdehyde and improvement of Kit levels and gastric emptying were not due to lowering of blood glucose levels in mice treated with CO. There was no difference in the blood glucose levels measured for three days before starting CO treatment in mice with delayed gastric emptying and blood glucose levels measured for three days after normalization of gastric emptying with CO treatment (474 ± 91 mg/dl vs. 549 ± 100 mg/dl; n = 5; P > 0.05, paired t-test). Also, there was no difference in insulin amount administered during this period (4.4 ± 2.4 U vs. 3.3 ± 1.8 U; n = 5; P > 0.05, paired t-test).
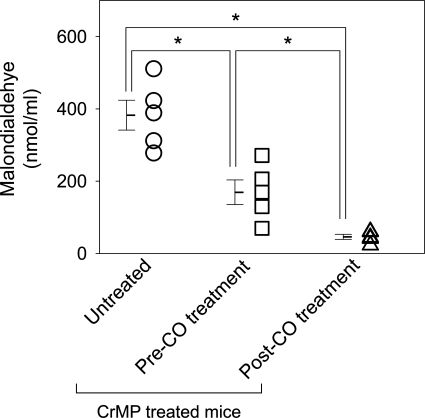
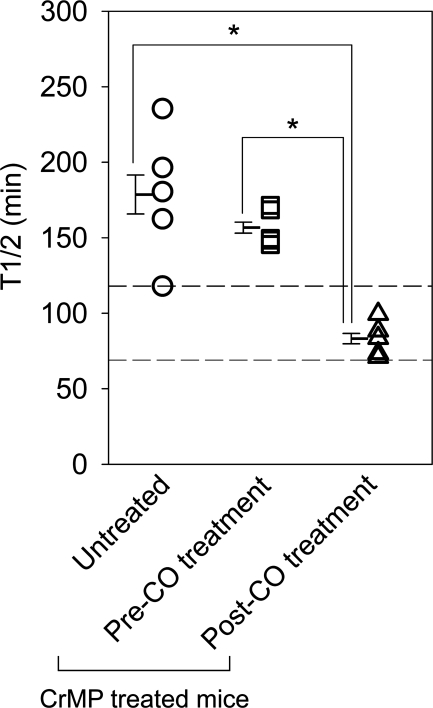
Given our previous observation that HO1 upregulation reverses delayed gastric emptying (9), we tested our hypothesis that normalized gastric emptying following CO inhalation was a direct result of CO effects, rather than a result of a potential feedback upregulation of HO1 by CO, using CrMP to inhibit any HO that might have become active following CO treatment. Diabetic mice treated daily with an intraperitoneal injection of 3 μmol/kg CrMP developed delayed gastric emptying compared with vehicle-treated mice (t1/2 values 167.5 ± 10.1 min, n = 10, vs. 95 ± 4.9, n = 5; P < 0.05, unpaired t-test) after mean period of 1.9 wk (range 1–3 wk) similar to that previously described (9). Of the 10 mice that developed delayed gastric emptying following CrMP treatment, five mice were assigned to receive low-dose CO inhalation for 6 h daily (100 ppm; mean period of 16 days; range 14–19 days). The mice continued to receive daily intraperitoneal injections of CrMP during treatment with CO to continually inhibit HO activity. The remaining five untreated mice were killed after development of delayed gastric emptying on two consecutive gastric emptying readings (Fig. 2). Mice with delayed gastric emptying had significantly lower serum malondialdehyde levels following CO inhalation (169.1 ± 38.1 to 45.8 ± 7.8 nmol/ml; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 6) in the presence of maintained HO inhibition. Serum malondialdehyde levels were significantly higher in untreated mice that were killed after development of delayed gastric emptying with CrMP treatment compared with serum malondialdehyde levels in CrMP-treated mice after CO inhalation (382.6 ± 46.1 vs. 45.8 ± 7.8 nmol/ml; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 6). CO inhalation significantly shortened t1/2 of gastric emptying and normalized gastric emptying in mice with delayed gastric emptying (t1/2 values 156.7 ± 6.1 to 83.2 ± 5.7 min; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 7) in the presence of HO inhibition. The t1/2 of gastric emptying in untreated mice killed after development of delayed gastric emptying with CrMP treatment was significantly higher than mice treated with CO inhalation (t1/2 values 178.6 ± 21.6 vs. 83.2 ± 5.7 min; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 7).
Fig. 6.
Reduced oxidative stress in mice treated with CO inhalation in the presence of HO inhibition by CrMP. Serum levels of malondialdehyde were measured from CrMP-treated diabetic mice with delayed gastric emptying killed soon after development of delayed gastric emptying (untreated), with delayed gastric emptying before CO inhalation and after CO inhalation. Open symbols represent individual values, whereas bars represent means ± SE, *P < 0.05, one-way ANOVA with Tukey's posttest.
Fig. 7.
Gastric emptying normalizes following CO inhalation in the presence of HO inhibition by CrMP. Individual means ± SE t1/2 values of gastric emptying for each mouse (open symbols) and the grouped data (bars) are shown. *P < 0.05, one-way ANOVA with Tukey's posttest. The 2 horizontal dashed lines indicate the normal range of gastric emptying.
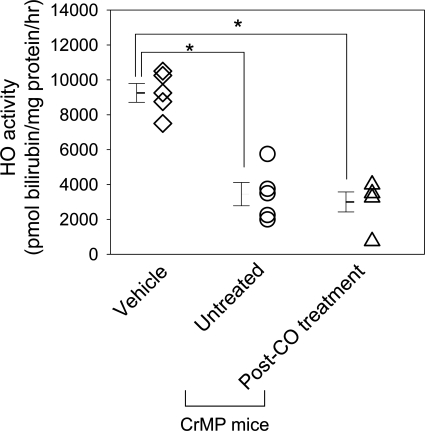
HO activity measured in the spleen was significantly inhibited in CrMP-treated mice killed soon after development of delayed gastric emptying compared with vehicle-treated mice (3,450 ± 1,494 vs. 9,250 ± 1,212 pmol bilirubin/mg per h; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest; Fig. 8). HO activity was also significantly inhibited in CrMP-treated mice that developed delayed gastric emptying and were treated with CO inhalation compared with vehicle-treated mice (3,000 ± 1,287 vs. 9,250 ± 1,212 pmol bilirubin/mg per h; n = 5; P < 0.05, one-way ANOVA with Tukey's posttest, Fig. 8). There was no statistical difference in the HO activity among the CrMP-treated mice killed soon after development of delayed gastric emptying and the CrMP-treated mice with delayed gastric emptying, which received CO inhalation (Fig. 8). There was no difference in blood glucose levels measured for three days before starting CO treatment in mice with delayed gastric emptying and blood glucose levels measured for three days after normalization of gastric emptying with CO treatment (447 ± 35 mg/dl vs. 414 ± 125 mg/dl; n = 5; P > 0.05, paired t-test). Also, there was no difference in insulin administered during this period (1.2 ± 0.9 U vs. 1.2 ± 1.0 U; n = 5; P > 0.05, paired t-test).
Fig. 8.
CrMP treatment inhibits HO activity. Individual values (open symbols) and means ± SE (bars) are shown. HO activity as measured by picomoles bilirubin per milligram of protein per hour. *P < 0.05, one-way ANOVA with Tukey's posttest.
DISCUSSION
In this study we show that administration of low-dose CO via inhalation to diabetic mice with delayed gastric emptying reduces oxidative stress, increases gastric Kit expression, and restores gastric emptying to normal rates. Therefore, CO inhalation has the same effect as upregulation of HO1 by hemin as previously described (9). Because the effects of CO inhalation persist in animals that are treated with CrMP to inhibit HO activity, the effects of CO are downstream of HO. Thus our data show that it is not necessary to have functional HO1 expressed in the muscularis propria to ameliorate the effects of diabetes on gastric function; the presence of one of the products of its activity (CO) is sufficient.
The therapeutic effect of CO is consistent with previous studies demonstrating the anti-inflammatory and antiapoptotic roles of this product of heme metabolism (23, 28, 30, 34). There are studies indicating that the expression of both HO1 (33) and HO2 (36) proteins protects cells from injury. Although bilirubin, one of the other products of heme metabolism, is an antioxidant with cytoprotective properties (13, 33), our study shows that CO alone was sufficient to restore gastric emptying to normal in diabetic mice. Normalization of gastric emptying by CO inhalation was observed even in the presence of an inhibitor of HO activity. By demonstrating that HO activity was significantly inhibited in the spleen by CrMP, regardless of CO treatment status, we established that the ability of CO to induce HO1 expression by itself (22) is not necessary for the positive response to CO administration. Therefore, the beneficial effects of CO are independent of HO1 activity, and CO is likely the product of HO1 activity responsible for HO1-mediated restoration of normal gastric function in diabetes. These results of the independent action of CO are similar to the action of CO in suppression of rejection in mouse to rat cardiac transplant (35) and protection from the effects of hyperoxic lung injury (31).
CO is a well-known environmental toxin. However, CO was used in this study at doses of 100 ppm for 6 h per day, and this dose is known to result in low levels (3%) of carboxyhemoglobin in humans (32). By comparison, smokers commonly have carboxyhemoglobin levels of 6% (39), and the toxic effects attributable to hypoxia are observed in humans when levels exceed 20% although some ill effects can be seen at 10–20% (8). Other studies have administered CO at doses around 1,000 ppm and have reported no significant adverse effects (39). Thus there seems to be a reasonable window of safety between doses of CO that are potentially therapeutic and the doses that are toxic, and the dose of CO used in this study can be considered a low dose.
The relative safety of the effective dose of CO, together with the independence of CO effects from activation of HO, makes CO a potential therapy for restoring normal gastric emptying in diabetic gastroparesis. An alternative approach would be to use compounds that induce HO1. These compounds include hemin, aspirin, and statins (2), with only hemin currently being shown to be effective in humans at standard doses (6). Hemin is FDA approved for treatment of intermittent porphyria (4), also making it attractive as a potential drug for the treatment of gastroparesis. Potential but not absolute limitations for use of hemin include the need for slow venous infusion and its side-effect profile (12, 16, 21). CO can be administered by inhalation safely in humans as shown in previous clinical trials, making it an attractive therapeutic option (5). In addition, an alternative is also available in the form of CO-releasing molecules (CORMs), which slowly release the CO in aqueous solution or in response to changes in pH (3). CO released from CORMs has been shown to reduce oxidative stress and reduce the development of postoperative ileus (11), but CORMs are not available for clinical use yet.
The cellular mechanism of action for CO was not established in this study. We focused on the changes in Kit protein in response to CO, as we and others have previously reported that, although changes in other markers, particularly nNOS expression, are observed in most models of diabetic gastroparesis (9, 17, 19, 38), it is the expression of Kit, the marker for ICC, that tracks most closely with development of delayed gastric emptying (9). Normalization of gastric emptying and Kit expression was clearly associated with a reduction in overall oxidative stress as indicated by malondialdehyde levels, a reporter molecule for lipid peroxidation (20). Thus, although blood glucose levels were not different between the groups and insulin was only administered to maintain the glycemia levels between 400 and 600 mg/dl, the lowering of oxidative stress was sufficient to restore Kit levels and gastric emptying to normal. The amount of insulin required to maintain the level of blood glucose between 400 and 600 mg/dl before and after administration of CO inhalation was not different, making it unlikely that CO altered insulin sensitivity.
Because Kit expression is the marker that best correlates with the changes in gastric emptying during diabetes in NOD mice (9), we propose that CO may be acting on ICC or ICC progenitors to restore gastric emptying to normal. The mechanism for this effect might be via modulation of p38 MAPK (30). A recent study demonstrated that oxidative stress and the development of postoperative ileus are reduced primarily because of activation of p38 MAPK following treatment of the animals with CORMs (11). In this respect, CO inhalation may act to correct an imbalance between the processes that injure ICC networks and the processes that generate and maintain ICC (14). This could be mediated by increased differentiation of committed ICC progenitors (24), enhanced proliferation of mature ICC (37), or inhibition of death or dedifferentiation of ICC (15).
In summary, we show that low-dose CO inhalation reversed damage to ICC in diabetic mice by countering the effects of increased oxidative stress associated with diabetes. These data, if confirmed in humans, hold promise as a new treatment in disorders associated with loss of ICC, such as diabetic gastroparesis, by preventing or reversing loss of ICC.
GRANTS
This work was supported by NIH grants DK57061, DK68055, and P30DK084567.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Mr. Peter Strege for help with the figures, Mr. Gary Stoltz for technical assistance, and Ms. Kristy Zodrow for secretarial assistance.
REFERENCES
- 1. Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase -1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther 7: 89–108, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Alberto R, Motterlini R. Chemistry and biological activities of CO-releasing molecules (CORMs) and transition metal complexes. Dalton Trans: 1651–1660, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 142: 439–450, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J 30: 1131–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther 87: 187–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camborova P, Hubka P, Sulkova I, Hulin I. The pacemaker activity of interstitial cells of Cajal and gastric electrical activity. Physiol Res 52: 275–284, 2003 [PubMed] [Google Scholar]
- 8. Choi IS. Carbon monoxide poisoning: systemic manifestations and complications. J Korean Med Sci 16: 253–261, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 135: 2055–2064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi KM, Zhu J, Stoltz GJ, Vernino S, Camilleri M, Szurszewski JH, Gibbons SJ, Farrugia G. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol 293: G1039–G1045, 2007 [DOI] [PubMed] [Google Scholar]
- 11. De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut 58: 347–356, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Dhar GJ, Bossenmaier I, Cardinal R, Petryka ZJ, Watson CJ. Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Med Scand 203: 437–443, 1978 [DOI] [PubMed] [Google Scholar]
- 13. Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA 96: 2445–2450, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 20, Suppl 1: 54–63, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gibbons SJ, De Giorgio R, Pellegrini MS, Garrity-Park MM, Miller SM, Schmalz PF, Young-Fadok TM, Larson DW, Dozois EJ, Camilleri M, Stanghellini V, Szurszewski JH, Farrugia G. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil 21: 85–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green D, Ts'ao CH. Hematin: effects on hemostasis. J Lab Clin Med 115: 144–147, 1990 [PubMed] [Google Scholar]
- 17. He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 121: 427–434, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 202: 1703–1713, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol 41: 1076–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9: 515–540, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Khanderia U. Circulatory collapse associated with hemin therapy for acute intermittent porphyria. Clin Pharm 5: 690–692, 1986 [PubMed] [Google Scholar]
- 22. Lee BS, Heo J, Kim YM, Shim SM, Pae HO, Kim YM, Chung HT. Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem Biophys Res Commun 343: 965–972, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Liu DM, Sun BW, Sun ZW, Jin Q, Sun Y, Chen X. Suppression of inflammatory cytokine production and oxidative stress by CO-releasing molecules-liberated CO in the small intestine of thermally-injured mice. Acta Pharmacol Sin 29: 838–846, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ordog T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology 134: 1083–1093, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development 116: 369–375, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology 124: 377–391, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, Murase N. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 163: 1587–1598, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakao A, Moore BA, Murase N, Liu F, Zuckerbraun BS, Bach FH, Choi AM, Nalesnik MA, Otterbein LE, Bauer AJ. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut 52: 1278–1285, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 49: 1731–1739, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 276: L688–L694, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Rhodes MA, Carraway MS, Piantadosi CA, Reynolds CM, Cherry AD, Wester TE, Natoli MJ, Massey EW, Moon RE, Suliman HB. Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am J Physiol Heart Circ Physiol 297: H392–H399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol 41: 251–260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 166: 4185–4194, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shotton HR, Lincoln J. Diabetes only affects nitric oxide synthase-containing myenteric neurons that do not contain heme oxygenase 2. Brain Res 1068: 248–256, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Tharayil VS, Wouters MM, Stanich JE, Roeder JL, Lei S, Beyder A, Gomez-Pinilla PJ, Gershon MD, Maroteaux L, Gibbons SJ, Farrugia G. Lack of serotonin 5-HT(2B) receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil 22: 462–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest 106: 373–384, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zevin S, Saunders S, Gourlay SG, Jacob P, Benowitz NL. Cardiovascular effects of carbon monoxide and cigarette smoking. J Am Coll Cardiol 38: 1633–1638, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, Ford HR. Carbon monoxide protects against the development of experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 289: G607–G613, 2005 [DOI] [PubMed] [Google Scholar]