Abstract
We combined retrograde tracing techniques with single-neuron RT-PCR to compare the expression of neurotrophic factor receptors in nodose vs. jugular vagal sensory neurons. The neurons were further categorized based on location of their terminals (tracheal or lungs) and based on expression of the ionotropic capsaicin receptor TRPV1. Consistent with functional studies, nearly all jugular neurons innervating the trachea and lungs expressed TRPV1. With respect to the neurotrophin receptors, the TRPV1-expressing jugular C-fiber neurons innervating both the trachea and lung compartments preferentially expressed tropomyosin-receptor kinase A (TrkA), with only a minority of neurons expressing TrkB or TrkC. The nodose neurons that express TRPV1 (presumed nodose C-fibers) innervate mainly intrapulmonary structures. These neurons preferentially expressed TrkB, with only a minority expressing TrkA or TrkC. The expression pattern in tracheal TRPV1-negative neurons, nodose tracheal presumed Aδ-fiber neurons as well as the intrapulmonary TRPV1-negative presumed Aβ-fiber neurons, was similar to that observed in the nodose C-fiber neurons. We also evaluated the expression of GFRα receptors and RET (receptors for the GDNF family ligands). Virtually all vagal sensory neurons innervating the respiratory tract expressed RET and GFRα1. The jugular neurons also categorically expressed GFRα3, as well as ∼50% of the nodose neurons. GFRα2 was expressed in ∼50% of the neurons irrespective of subtype. The results reveal that Trk receptor expression in vagal afferent neurons innervating the adult respiratory tract depends more on the location of the cell bodies (jugular vs. nodose ganglion) than either the location of the terminals or the functional phenotype of the nerve. The data also reveal that in addition to neurotrophins, the GDNF family ligands may be important neuromodulators of vagal afferent nerves innervating the adult respiratory tract.
Keywords: jugular ganglion, neurotrophin, brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, tropomyosin receptor kinases a and b, glial cell-derive neurotrophic factor family receptor α1
airway inflammation, in susceptible individuals, leads to a constellation of signs and symptoms that are indicative of vagal sensory nerve modulation. These symptoms include respiratory irritation, sneezing, coughing, dyspnea, and hyperparasympathetic reflex bronchospasm and secretions. In general terms, inflammation-induced modulation of sensory (afferent) nerve function can be categorized as either acute changes in electrical activity or longer term phenotypic changes that occur as a consequence of alteration in neuronal gene expression.
Both allergic and viral airway inflammation are associated with changes in gene expression in sensory neurons; for example, causing large-diameter trachea Aδ and intrapulmonary Aβ low-threshold mechanosensors to begin expressing neuropeptides that are typically limited to small-diameter C-fiber neurons (8, 9, 13, 37). An analogous phenotypic switch has been observed in models of joint inflammation that has been argued to contribute to the allodynia associated with arthritis (41, 43). Inhalation of respiratory irritants such as ozone and toluene diisocyanate also leads to increases in sensory neuropeptide production in airway sensory neurons (20, 21). The genes that are induced are not limited to sensory neuropeptides. Allergen-induced airway inflammation in rat lungs is associated with a phenotypic change in slowly adapting low-threshold mechanosensitive neurons (SARs) such that they begin to express TRPV1, the ionotropic capsaicin-receptor (58).
The mechanisms by which airway inflammation leads to changes in the gene expression in cell bodies located in distant sensory ganglia are not known. The classes of molecules known to be capable of interacting with receptors located on sensory nerve terminals in peripheral tissue that then transmit signals via retrograde transport mechanisms to the cell nucleus are neurotrophic factors (5, 16). Nerve growth factor (NGF) is the prototypical neurotrophic factor, initially described to promote neuronal growth and survival (28). Increases in NGF production have been described in inflammatory diseases related to airway dysfunction (15, 21, 34, 45, 49). The lung content of substance P is substantially elevated in transgenic mice in which NGF production is induced via the lung-specific Clara cell secretory protein promoter (17). This likely occurs via increases in tachykinin gene expression in primary vagal and dorsal root ganglion neurons. Intratracheal instillation of NGF increases substance P expression in airway nerves of guinea pigs (20) and ferrets (56). Moreover, the sensory plasticity observed in rat airway sensory neurons on inhalation of respiratory ozone is accompanied by elevations in NGF (18).
The manner in which a given type of sensory neuron will respond to neurotrophic factors depends on the type of neurotrophic factor receptors it expresses. The neurotrophin family of neurotrophic factors includes NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and NT-4. Each neurotrophin acts with high affinity on tropomyosin-receptor kinase (Trk) receptors, denoted TrkA for NGF, TrkB for BDNF and NT-4, and TrkC for NT-3 (4, 6, 44). Primary sensory nerves are also modulated by glial cell-derived neurotrophic factor (GDNF) and GDNF family ligands (GFLs). The GFLs comprise GDNF, neurturin, artemin, and persephin; these selectively interact with the GDNF family receptors GFRα1, GFRα2, GFRα3, and GFRα4, respectively (1). For complete signaling, GFRs require the cosignaling molecule RET (50, 53). Neurotrophic factor receptor expression has largely been characterized in neurons during development and in neonatal animals. Currently, there is little information regarding neurotrophic factor receptor expression in adult vagal afferent neurons of differing phenotypes, and virtually nothing is known in this regard about airway-specific neurons.
Different subtypes of sensory neurons express distinct neurotrophic factor receptors (30, 32, 36). Using an extensive electrophysiological analysis, we have characterized six distinct vagal afferent nerve phenotypes in the guinea pig respiratory tract (Table 1). This characterization is based on the location of the cell body (jugular or nodose ganglion), location of the nerve terminals (trachea or lungs), and conduction velocity (C-, Aδ-, or Aβ-fibers). As noted in Table 1, the trachea is innervated mainly by capsaicin-sensitive jugular C- and Aδ-fiber nociceptive-like neurons and capsaicin-insensitive nodose Aδ-fiber “cough receptor” neurons. The intrapulmonary tissue is innervated by capsaicin-sensitive jugular and nodose C-fiber neurons and capsaicin-insensitive nodose Aβ-fiber low-threshold stretch receptor neurons.
Table 1.
Operationally defined vagal afferent subtypes in the guinea pig trachea and lung
| Nodose Ganglion | Jugular Ganglion |
|---|---|
| Trachea | |
| Aδ-fibers (CV ∼6 m/s) | C-fibers (CV ∼1 m/s) |
| Touch-sensitive cough fibers | Aδ-fibers (CV ∼6 m/s) |
| Capsaicin-insensitive fibers (no TRPV1) | Capsaicin-sensitive fibers (TRPV1) |
| Intrapulmonary | |
| C-fibers (CV ∼0.7 m/s) | C-fibers (CV ∼0.8 m/s) |
| Capsaicin-sensitive (TRPV1) | Capsaicin-sensitive fibers (TRPV1) |
| Aδ/β-fibers (CV ∼14 m/s) | |
| RAR/SAR stretch-sensitive fibers | |
| Capsaicin-insensitive fibers (no TRPV1) | |
Data for nodose ganglion are based on our previous analysis of conduction velocities (CV) and capsaicin responsiveness of nodose fibers innervating guinea pig respiratory tract (25). Among 190 nodose fibers with receptive fields in the trachea, 172 (91%) were capsaicin insensitive and conducted action potentials in the Aδ range with an average of 5.6 ± 0.3 m/s; the remaining 9% conducted in the C-fiber range (1 ± 0.05 m/s). Among 263 nodose fibers with receptive fields in the intrapulmonary compartment, 114 (43%) conducted action potentials in the Aβ range with an average CV of 13.6 ± 0.7 m/s; the remaining 57% of the intrapulmonary fibers were capsaicin sensitive and conducted action potentials in the C-fiber range with CV <1 m/s. Data pertaining to jugular trachea fibers are derived from previous studies (23, 46). Among 73 jugular fibers innervating the trachea, 35 were C-fibers (CV <1 m/s) and 38 were Aδ fibers (CV 5.8 ± 2 m/s). Regardless of CVs, the vast majority (>80%) of jugular tracheal fibers innervating the trachea were capsaicin-sensitive fibers with nociceptive properties. Data pertaining to jugular neurons with receptive fields in the intrapulmonary compartment were obtained from a previous study (54). Among 36 fibers studied, 31 (86%) were C-fibers with a CV <1 m/s (0.6 ± 0.02 m/s), and the other 5 fibers had CVs of 3 ± 0.5 m/s.TRPV1, ionotrophic capsaicin receptor; RAR/SAR, rapidly and slowly adapting stretch receptor neurons.
In the present study, we combined retrograde neural tracing methods with single-neuron RT-PCR analysis of gene expression to characterize the neurotrophic factor receptors expressed in identified neuronal phenotypes innervating the respiratory tract. Our results support the conclusion that the location of the cell body (jugular vs. nodose ganglion) is more predictive of the nature of the receptor expression than either the location of the terminals or the functional phenotype of the nerve.
METHODS
Retrograde Tracing of Neurons Innervating Trachea and Lung
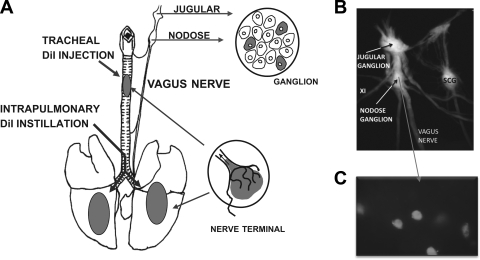
All experiments were performed with approval from the Johns Hopkins University Animal Use and Care Committee. Young male Hartley guinea pigs (200–250 g) were obtained from Hilltop Laboratory Animals (Scottsdale, PA). Animals were anesthetized by injection of ketamine (50 mg/ml) and xylazine (2.5 mg/ml) dissolved in phosphate-buffered saline (PBS). The trachea and lungs were retrogradely labeled using the fluorescent retrograde tracer DiI (Invitrogen, Carlsbad, CA) in solution (1% in DMSO, then diluted 1:10 in PBS). For tracheal labeling, a tracheotomy was performed and the dye was injected into two sites along the upper tracheal mucosa/submucosa (see Fig. 1). For lung labeling, the head and the thorax were elevated for delivery of the dye. The dye was injected into the lumen of the lower trachea with the use of a bent syringe needle angle such that the tip was positioned just rostral to the carina (see Fig. 1). The incisions were sutured, and the animal was allowed to recover for ∼2 wk for sufficient labeling of cell bodies in the vagal ganglia. All animals were closely monitored, and any animal that displayed behaviors indicating excessive pain or infection was euthanized immediately via CO2 asphyxiation.
Fig. 1.
A: diagram illustrating the method used to obtain trachea- and lung-specific vagal sensory neurons. The general method is based on the placement of DiI into the tracheal wall or instilled into the lungs. The dye was taken up by the nerve terminals and transported back to the cell bodies within about 1 wk. The ganglia were isolated, and those neurons that contained DiI were studied using single-neuron RT-PCR. B: dissection of the guinea pig vagus nerve and the nodose and jugular ganglion. The XI cranial nerve enters the nerve trunk between the 2 ganglia. Also shown is the sympathetic nerve trunk along with the superior cervical ganglion (SCG). C: isolated nodose ganglion neurons obtained from a lung-labeled guinea pig exhibiting fluorescence, indicating DiI labeling specificity.
Cell Picking
Fourteen days after injection, the animals were killed by CO2 asphyxiation. The blood from circulation was washed out by in situ perfusion with Krebs-bicarbonate solution, composed of (in mM) 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25.0 NaHCO3, and 11.1 dextrose, gassed with 95% O2-5% CO2 (pH 7.4). The jugular and nodose ganglia were harvested and studied separately. Each ganglion was incubated in the enzyme buffer (2 mg/ml collagenase and 2 mg/ml dispase II dissolved in Ca2+-, Mg2+-free Hanks' balanced salt solution) for 30 min at 37°C. The cells were gently dissociated by trituration with a glass fire-polished Pasteur pipette. Two additional enzymatic digestions were repeated at 37°C for 20 min. The cells were washed by centrifugation (3 times at 1,000 g for 2 min) and suspended in L-15 medium containing 10% fetal bovine serum (FBS). The cell suspension was transferred onto poly-d-lysine (0.1 mg/ml)-laminin (0.004 mg/ml)-coated coverslips. After the suspended neurons had adhered to the coverslips for 2 h in the incubator (37°C), the neuron-attached coverslips were flooded with the L-15 medium (10% FBS). All chemicals were purchased from Fisher Scientific Products.
Coverslips of retrogradely labeled, dissociated neurons were constantly perfused by Krebs solution bubbled with 95% O2 and 5% CO2. The cells were identified using fluorescence microscopy (see Fig. 1), and single neurons were drawn into a glass pipette (tip diameter 50–150 μm) pulled with a micropipette puller (model P-87; Sutter Instruments, Novato, CA) by applying negative pressure within 8 h of the dissection. The pipette tip was then broken in a PCR tube containing 1 μl of Invitrogen resuspension buffer and RNase inhibitor (RNaseOUT; 2 U/μl). The tubes were immediately snap frozen and stored on dry ice. Only one to four cells were collected from each coverslip, and each was placed into individual PCR tubes.
For our single-cell RT-PCR analysis, we used 50 cycles. Therefore, the major concern in these experiments is whether the superfusing fluid is contaminated by RNA/DNA from any contaminating cells. To control for this, we attempted to collect only neurons found to be accessible, clean, and not attached to other neurons or other cells or debris for single-cell RT-PCR analysis (this careful approach is the main reason why seemingly small numbers of cells are collected from 1 ganglion). In addition, our approach using constant flow perfusion of the coverslips during the cell collection repeatedly proved effective to essentially eliminate any contamination. In each experiment, the RT-PCR from the superfusing fluid from the vicinity of the collected cells (the negative control) was nearly uniformly negative (if positive, all cells from the experiment would have been discarded).
Single-Cell RT-PCR
Single cells were processed using the SuperScript III CellsDirect cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. Samples were defrosted from dry ice or −80°C, and each sample was lysed by the addition of 10 μl of resuspension buffer and incubated at 75°C for 10 min. Next, each sample was treated with 5 μl of DNase I and 1.6 μl of 10× DNase I buffer. After 5 min of room-temperature incubation of DNase treatment, 1.2 μl of 25 mM EDTA was added to each sample and placed in a thermocycler for incubation at 70°C for 5 min. Subsequently, 1 μl of oligo(dT), random primers (Invitrogen), and 10 mM dNTP mix were added to each sample and incubated at 70°C for another 5 min. The final addition of 5× RT buffer (5 μl), RNaseOUT (1 μl), and 0.1 M DTT (1 μl) was added to each sample. The total volume for each sample was divided into one-third and two-thirds portions. To account for negative RT control, we added water to one-third of the sample, whereas two-thirds of the final volume were reverse-transcribed by adding SuperScript III reverse transcriptase for cDNA synthesis. Each tube was transferred to the thermocycler preheated to 50°C for 50 min with an inactivated step set at 85°C for 5 min. The reaction was chilled at 4°C, and cDNA was stored at −20°C until PCR amplification.
The PCR mix contained 0.5 units of HotStar Taq polymerase (Qiagen, Valencia, CA) supplemented with 2.5 mM MgCl2, PCR buffer, 10 mM dNTP, custom-synthesized intron-spanning primers for β-actin, TrkA, TrkB, TrkC, GFRα1, GFRα2, GFRα3, RET, and TRPV1 (see Table 2) as well as template (sample cDNA, no-RT control, and bath control). As a positive control, whole ganglion tissue was assayed. PCR reaction conditions included a 50-cycle basis with initial activation at 95°C for 15 min, denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. Control experiments testing the amplification of the “no RT” for each individual neuron and the bath control using either one or multiple primer pairs did not produce a specific product. β-Actin, TRPV1, TrkA, TrkB, TrkC, GFRα1–3, and RET mRNA could be consistently amplified from the cDNA of a whole vagal ganglion isolated using the RNeasy Plus mini kit (Qiagen) and reverse transcribed using the OmniScript RT kit (Qiagen), which was used as positive control. Products were then visualized in ethidium bromide-stained 1.5% UltraPure agarose (Invitrogen) in an electrophoretic aperture.
Table 2.
Primer sequences for single-cell RT-PCR
| Gene | Primer | Sequence | Product Length, bp |
|---|---|---|---|
| β-Actin | Forward (5′ to 3′) | TGG CTA CAG TTT CAC CAC CA | 212 |
| Reverse (3′ to 5′) | GGA AGG AGG GCT GGA AGA | ||
| TRPV1 | Forward (5′ to 3′) | CCA ACA AGA AGG GGT TCA CA | 168 |
| Reverse (3′ to 5′) | ACA GGT CAT AGA GCG AGG AG | ||
| TrkA | Forward (5′ to 3′) | GGC TTC ATC TTC ACC CAG TT | 258 |
| Reverse (3′ to 5′) | AGG CGT TTC ATC CTT CTT CTC | ||
| TrkB | Forward (5′ to 3′) | CTG GCT AAT CCT GGG CTT CT | 215 |
| Reverse (3′ to 5′) | CGT AGG CTT CCA CGT CAT CT | ||
| TrkC | Forward (5′ to 3′) | GCA TCG CAG AGA ATG TGG T | 272 |
| Reverse (3′ to 5′) | GCC GTT GTT GTA GTG TGT GG | ||
| GFRα1 | Forward (5′ to 3′) | CTG CCT CCT CGC CTA CTC A | 215 |
| Reverse (3′ to 5′) | GCT GCC ACA CAG TCA CAT C | ||
| GFRα2 | Forward (5′ to 3′) | AGG ACA AGG AGA AGC CCA AC | 171 |
| Reverse (3′ to 5′) | CGC ATA GGA GCC CAG ACA C | ||
| GFRα3 | Forward (5′ to 3′) | GAC TCC CAA CTG CCT GGA C | 142 |
| Reverse (3′ to 5′) | CGA GCG TAG ACA TCG GAA CT | ||
| RET | Forward (5′ to 3′) | CTG CAC AGG AAC GAC TGG A | 141 |
| Reverse (3′ to 5′) | GAC AGG AAG ACG TGG AGG AA |
TrkA, TrkB, and TrkC, tropomyosin receptor kinases A–C; GFRα1-α3, glial cell-derived neurotrophic factor family (GNDF) receptors α1–α3; RET, cosignaling receptor for GNDF.
In one series of experiments, we combined whole cell patch-clamp electrophysiological recording with subsequent RT-PCR. The inward current in response to capsaicin (1 μM, 37°C) was evaluated using conventional techniques, as we have previously described (26). Once we determined whether capsaicin caused an inward current, we evaluated the same neurons for TRPV1 gene expression. In nine consecutively studied neurons, capsaicin caused a large inward current in seven neurons and had no effect in two neurons. Each of the seven responding neurons expressed TRPV1 mRNA in our single-cell RT-PCR assay, whereas neither of the nonresponding neurons expressed TRPV1 in the subsequent RT-PCR analysis.
Statistical Analysis
Data are presented as proportions in percentages, where n neurons were pooled from four to eight animals. In any given animal, the ganglia along the right and left vagus were pooled. Analysis of variance was used to compare group means (mRNA expression). Jugular and nodose neuron expression of TrkA, TrkB, TrkC, GFRα1–3, and RET receptors were compared using χ2 analysis, followed by Fisher's exact tests for pairwise comparison. A P value <0.05 was considered statistically significant. Student's t-test was used when appropriate for H&E staining of leukocytes. Data are means ± SE.
RESULTS
Location of DiI Instillation and Effect on Leukocyte Infiltration Into the Airways
Consistent with our previous studies, the DiI injected specifically into the trachea was found in histological analysis to have remained limited to the tracheal tissue; no observable dye was found in the esophagus, lungs, or heart. The DiI strongly labeled the mucosa but also diffused into the submucosal compartment. Dye infused into the lungs was found to extensively label the lung lobes. We observed minimal tracheal labeling and no observable labeling of the esophagus or the heart.
To determine whether the retrograde dye DiI used in the experiments caused an inflammatory reaction, we used hematoxylin and eosin (H&E) staining to quantify the infiltration of leukocytes into the labeled tissue. There were no significant differences in the number of leukocytes in control vs. dye-labeled tracheas and lungs. For example, the total number of H&E-stained leukocytes in the labeled region of the trachea averaged 38 ± 5/section (average of 3 sections from each animal), which was not different from the mean number of leukocytes observed in the nonlabeled trachea (39 ± 5, P > 0.5, n = 6 guinea pigs).
Single-Neuron RT-PCR Analysis of Neurotrophin Receptors: TrkA, TrkB, and TrkC
In functional studies of vagal afferent innervation of the guinea pig respiratory tract, capsaicin responsiveness is a categorical marker of C-fibers and also activates jugular tracheal Aδ-fibers. These nerves are relatively less sensitive to mechanical force and respond to other inflammatory mediators, indicating their nociceptive-like behavior. The nodose A-fiber phenotypes (tracheal Aδ-fibers and intrapulmonary Aβ-RAR/SAR fibers) are low-threshold mechanosensors and fail to respond directly to capsaicin (7, 25, 46, 54). TRPV1 expression was therefore used as a marker of nociceptive-like neurons. In a series of preliminary studies, described in methods, we combined patch-clamp electrophysiological recordings with subsequent single-cell RT-PCR analysis. In nine neurons studied, we found perfect concordance between TRPV1 mRNA expression and a given neuron's capsaicin responsiveness.
We evaluated mRNA expression of neurotrophic factor receptors for five phenotypes of vagal afferent neurons innervating the guinea pig respiratory tract that are listed in Table 1 (we cannot distinguish jugular Aδ- and C-fiber nociceptors in the trachea, because they are both TRPV1-expressing neurons). By comparing the jugular nociceptors innervating the trachea with jugular nociceptors innervating lung tissue, we could determine whether the location of the nerve terminals strongly influenced the nature of the neurotrophic factor receptor expression.
Jugular tracheal capsaicin-sensitive TRPV1-expressing neurons.
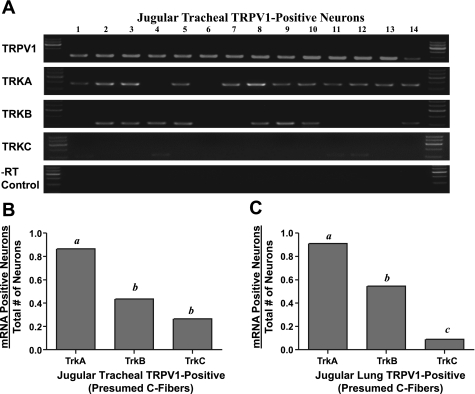
We evaluated 31 jugular neurons labeled specifically from the trachea. On the basis of our previous functional studies showing that the vast majority of jugular C-fibers innervating the trachea are capsaicin sensitive, we predicted that these neurons would express TRPV1 mRNA. Indeed, among the 6 animals studied, 70–100% of the tracheal jugular neurons expressed TRPV1 mRNA. With respect to Trk receptor expression in the TRPV1-expressing neurons, nearly all expressed TrkA. By contrast, less than one-half of the neurons (14 of 31) expressed TrkB, and only about one-third (9 of 31) expressed TrkC (Fig. 2B).
Fig. 2.
Single-neuron RT-PCR analysis of gene expression in ionotropic capsaicin receptor TRPV1-expressing jugular neurons labeled from the trachea. A: gel results of gene expression for TRPV1 and tropomyosin receptor kinase A–C (TrkA, TrkB, and TrkC) mRNA are shown as an example using 14 picked neurons. Negative reverse transcriptase control (−RT control) was done for each neuron. The TrkC expression was faint, so the contrast was adjusted; among these 14 neurons, TrkC was clearly evident but faint in neurons 1, 4, 11, and 12. B: mRNA expressions of 31 TRPV1-positive, trachea-specific jugular neurons were analyzed for TrkA, TrkB, and TrkC and expressed as the number of mRNA-positive neurons divided by the total number of neurons. C: mRNA expressions of 11 TRPV1-positive lung-specific jugular neurons were also analyzed in the same manner as described in B. All data are presented as proportions. a,b,cP < 0.01, where different letters denote statistical significance based on χ2 analysis or Fisher's exact test (groups with the same letter denote no statistical significance). Neurons were isolated from 8–12 ganglia from 4–6 guinea pigs.
TRPV1-expressing jugular neurons labeled from lungs.
Jugular neurons project axons to the lung compartment. We evaluated 11 jugular neurons retrogradely labeled with DiI from the lung compartment. Again, consistent with our observation that jugular neurons innervating the lungs tissue are capsaicin-sensitive C-fibers, all 11 neurons expressed TRPV1. The overall expression of TrkA (91%), TrkB (55%), and TrkC (9%) was not significantly different from that described above for jugular tracheal neurons (Fig. 2C). Thus the location of the jugular C-fiber nerve terminals (tracheal epithelium vs. intrapulmonary structures) was not a relevant variable with respect to Trk receptor expression by these neurons.
Nodose tracheal TRPV1-insensitive neurons (nodose Aδ cough receptors).
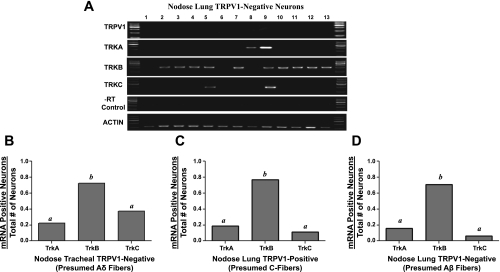
The trachea mucosa and submucosa are innervated by two distinct sensory nerve phenotypes: jugular nociceptive fibers (as described above) and nodose Aδ-fibers. Our previous electrophysiological studies predicted that the vast majority of nodose neurons labeled specifically from the trachea would be capsaicin-insensitive Aδ-fiber neurons (see Table 1 legend). We evaluated gene expression in 56 nodose neurons labeled from the trachea. Consistent with our physiological studies, 74% of these neurons did not express TRPV1. We focused our attention on these TRPV1-negative (presumed Aδ-fiber) neurons (Fig. 3B). By contrast to the jugular neurons innervating the trachea, the nodose Aδ cough receptors in the trachea rarely expressed TrkA (22%), whereas the vast majority (76%) expressed TrkB and a modest number expressed TrkC (35%) (Fig. 3B). The difference in the proportion of TrkB- and TrkA-expressing neurons between the nodose and jugular trachea-labeled TRPV1-expressing neurons was significant (P < 0.01).
Fig. 3.
Single-neuron RT-PCR analysis of trachea- and lung-specific nodose neurons. A: gel results of gene expression for TRPV1, TrkA, TrkB, and TrkC mRNA are shown as an example using 13 picked nodose neurons labeled from the lungs. A negative control (−RT control) was done for each neuron, and β-actin was used as a positive control for each neuron. B: Trk receptor expression, presented as the proportion of TRPV1-negative tracheal nodose neurons (presumed Aδ-fiber) expressing TrkA, TrkB, and TrkC (n = 41 neurons evaluated). C: Trk receptor expression in TRPV1-expressing nodose neurons labeled from the lung (presumed lung C-fiber neurons). Data are expressed as proportions of 32 neurons studied. D: Trk receptor expression in TRPV1-negative nodose neurons labeled from the lung (presumed lung Aβ fiber neurons). Data are expressed as proportions of 33 neurons studied. a,bP < 0.01, where different letters denote statistical significance in the proportion of neurons expressing a given Trk receptor, based on χ2 analysis or Fisher's exact test. Neurons were obtained from 12 ganglia of 6 guinea pigs.
Nodose intrapulmonary C-fiber neurons and Aβ-fiber neurons.
Nodose ganglion neurons also project axons to the intrapulmonary compartment. In a previous histochemical analysis, we noted that 64% of the intrapulmonary nodose neurons were small-diameter neurofilament-negative C-fiber-type neurons. In our electrophysiological analysis, we found about 50% to be C-fibers, with the remainder being fast-conducting Aβ-type fibers, as discussed in the Table 1 legend (54).
We evaluated gene expression in a total of 65 nodose ganglion neurons retrogradely traced from the lung compartment. Consistent with predictions based on our previous functional and histological analyses, 32 of 65 neurons were TRPV1-expressing nodose neurons (presumed C-fiber neurons) and 33 were TRPV1 negative (presumed low-threshold Aβ mechanosensors). The overall expression pattern of Trk receptors was not significantly different between these two functionally distinct sensory nerve subtypes and in both cases was analogous to the nodose Aδ tracheal fibers (cough receptors). Thus only a small percentage (<20%) of neurons expressed TrkA or TrkC, whereas the vast majority (∼80%) of these neurons expressed TrkB (Fig. 3, C and D).
GDNF Family Ligand Receptors: GFRα1, GFRα2, and GFRα3
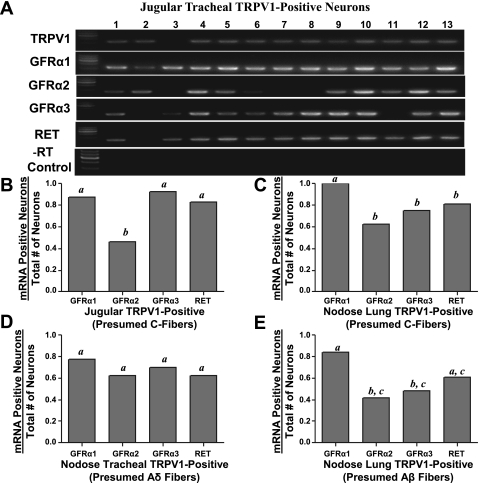
We first evaluated the GFRs in 42 jugular TRPV1-positive neurons (results were similar between the tracheal and lung compartments, so we pooled the data). We found the GDNF receptor GFRα1 was expressed in nearly all (∼90%) of the jugular neurons (Fig. 4, A and B). Likewise, virtually all of these jugular neurons also expressed GFRα3 and RET. We found GFRα2 was not as often expressed in jugular C-fiber neurons (∼50%) (Fig. 4B; P < 0.01 compared with either GFRα1 or GFRα3).
Fig. 4.
Expression of glial cell-derived neurotrophic factor (GDNF) family receptor mRNA in trachea- and lung-labeled vagal sensory neurons. A: an example of single-neuron RT-PCR results from 13 jugular neurons labeled from the trachea. −RT control was done for each neuron. Negative bath controls were also conducted (not shown). In B–E, RET and GFRα mRNA expression in different nerve subtypes is expressed as a proportion of neurons expressing the gene of interest. B: gene expression in TRPV1-positive jugular neurons labeled from lungs and trachea (n = 42 neurons evaluated). C: gene expression in TRPV1-expressing nodose neurons labeled from the lungs (n = 32 neurons evaluated). D: gene expression in TRPV1-negative nodose neurons labeled from the trachea (n = 41 neurons evaluated). E: gene expression in TRPV1-negative nodose neurons labeled from the lungs (n = 33 neurons evaluated). All data are presented as proportions. a,b,cP < 0.05, where different letters denote statistical significance, based on χ2 analysis or Fisher's exact test. Neurons were obtained from 12 ganglia of 6 guinea pigs.
By contrast to the expression of the neurotrophin receptors, there was little significant difference in the pattern of GFR expression between nodose and jugular neurons. We evaluated 41 nodose trachea Aδ-fiber neurons (TRPV1-negative neurons) from 6 guinea pigs and found that similarly to the jugular neurons, nearly all the neurons expressed GFRα1 and the majority of neurons also expressed GFRα2, GFRα3, and RET (Fig. 4D). A significantly lower percentage of intrapulmonary nodose neurons expressed GFRα3 (Fig. 4, C and E; P < 0.01) compared with jugular neurons, and this was especially noted in the intrapulmonary TRPV1-negative (presumed Aβ) neurons (Fig. 4E).
DISCUSSION
Sensory neuroplasticity (phenotypic changes due to alteration in gene expression) plays an important role in the pathophysiology of inflammatory diseases. Although this topic has received more attention from those interested in diseases associated with pain, it is likely important in airway inflammatory diseases, as well (8, 9, 11, 13, 17, 19, 21, 37, 58). In this study, we have characterized the pattern of neurotrophic factor receptors in distinct vagal sensory nerve phenotypes innervating the adult respiratory tract. The data obtained support two principal conclusions. First, the pattern of expression of neurotrophin receptors (TrkA, TrkB and TrkC) was not constant among the different nerve phenotypes studied. The key predictor of Trk expression, however, had little to do with sensory nerve function or location of the nerve terminals, but rather was based largely on the location of the cell bodies. Those neurons situated in the nodose ganglion expressed mainly TrkB, whereas those situated in the jugular ganglion expressed TrkA. Second, the GFRs (GFRα1, GFRα2, and GFRα3), as well as the coreceptor RET, are commonly expressed in the majority of vagal sensory neurons innervating the respiratory tract regardless of phenotype. This implicates a heretofore underappreciated role of GFLs (GDNF, neurturin, artemin, persephin) as important mediators in sensory neuroplasticity in the adult respiratory tract.
This study depended on retrograde tracing techniques to define the ganglion neurons specifically innervating the trachea vs. intrapulmonary tissue compartments. It is possible that the placement of dye into these tissue compartments could evoke an inflammatory response that in turn could lead to changes in neurotrophic receptor expression. Although this caveat must be kept in mind, we also found no evidence of an overt inflammatory reaction at the injection sites. This study also depended on single-neuron RT-PCR analysis of neurotrophic receptor gene expression. One must be cautious when extrapolating from mRNA expression data to conclusions regarding the production of functional proteins. It is worth noting, however, that in our past studies we have found very tight concordance between the gene expressions of various receptors in single identified sensory neurons with functional end points. Our single-neuron RT-PCR analysis correlated with function with respect to protease-activated receptors, TRPV1 (this study, see methods), as well as TRPA1, adenosine receptors, and MrgA receptors (10, 26, 29, 39).
It should also be stated that once a neuron is isolated from a ganglion to study gene expression, its conduction velocity and functional phenotype cannot be determined. For this study we have taken advantage of our extensive experience with vagal innervation of the guinea pig lung and trachea to make assumptions regarding the phenotype of the isolate neuron. We know that jugular neurons project nearly uniformly capsaicin-sensitive nociceptive-like fibers to trachea and lungs. Nodose neurons are more complex in that they project mainly capsaicin-insensitive Aδ cough receptors to the trachea and both capsaicin-insensitive Aβ-fiber and capsaicin-sensitive C-fiber to the lungs (see Table 1 legend). Therefore, on the basis of the location of the cell body (jugular or nodose ganglion), location of terminal (trachea or intrapulmonary), and expression of the capsaicin receptor TRPV1, one can draw meaningful inferences regarding phenotypes.
The nature of the neurotrophic factor receptor expressed by a given neuron may be influenced by molecular cues received during embryonic development and/or by cues from the tissue of innervation. With respect to Trk receptor expression in adult respiratory afferent neurons, our data favor the idea that embryonic environment may be more important. The neurons comprising the jugular ganglion are derived from the neural crest, whereas the nodose ganglion is derived from the epibranchial placodes; it is known that these two disparate structures receive different transcriptional signals during development (2, 3, 22). With respect to the neurotrophin receptors, we found that the placodal neurons innervating the respiratory tract preferentially expressed TrkB. This was the case for the TRPV1-positive (presumed nociceptive C-fibers) as well as the TRPV1-negative tracheal Aδ cough fibers in the trachea and the intrapulmonary Aβ-RAR/SAR fiber neurons. This supports the hypothesis that the Trk receptor expression is likely determined early in embryological development irrespective of the functional phenotype or ultimate location of the terminals within the respiratory tract. Likewise, the observation that jugular neurons preferentially expressed TrkA, regardless of the location of their nerve terminals, supports the same viewpoint. In the trachea, jugular C-fibers innervate the epithelium, whereas many in the lungs innervate structures at the lung margins (54).
The general idea that the ganglion of origin (jugular vs. nodose) predicts receptor expression is consistent with our functional studies on C-fiber subtypes. With respect to the activation profile and neuropeptide content, it matters less where the location of the terminals are (extra- or intrapulmonary) but more on whether the C-fiber neuron is placode (nodose ganglion) or neural crest (jugular ganglion) derived (24, 54). We are not aware of other studies comparing neurotrophin receptor expression in identified A- and C-fiber vagal sensory neurons. However, we recently evaluated the neurotrophin receptor expression in nodose and jugular ganglion C-fiber neurons innervating the mouse lungs and obtained data in agreement with those produced presently; the placodal C-fibers preferentially expressed TrkB, whereas the neural crest C-fibers preferentially expressed TrkA (40). The expression of TrkB by nodose neurons is also consistent with studies showing the developing nodose neurons in general often express TrkB and is dependent on BDNF for survival (57, 59). Likewise, it may not be surprising that the jugular C-fiber neurons innervating the airways express TrkA, since nearly all jugular C-fiber neurons innervating the airways also express sensory neuropeptides (54). Although Trk receptor expression changes during different stages of development, postnatally it is known that most peptidergic nociceptive neurons in the dorsal root ganglion often express TrkA (30, 55), and these neurons, like jugular neurons, are also derived from the neural crest.
Studies with human subjects and laboratory animals have revealed that both NGF and BDNF are elevated during airway inflammation (33, 34, 38, 42, 47, 49, 52). Our data predict that NGF, the high-affinity agonist for TrkA, may be an important mediator in phenotype maintenance and neuroplasticity for jugular C-fibers, whereas BDNF and NT4, the high-affinity agonists for TrkB, may be more relevant for nodose C-, Aδ-, and Aβ-fibers. We previously evaluated the extent to which we could mimic allergen-induced neuropeptide gene expression in nodose trachea Aδ neurons with exogenously applied NGF. Whereas allergen challenge often influenced >50% of the tracheal neurons, we found NGF capable of phenotypically altering only about 10% of the neurons (20). In the present light, this finding is not surprising, since we noted in this study that only a small percentage of the nodose tracheal Aδ neurons expressed TrkA.
Although Trk receptor expression differentiated placode- vs. neural crest-derived vagal sensory neurons innervating the respiratory tract, this was not the case for the GFRα receptor expression. There is relatively little information regarding the expression of receptors for GFLs in vagal sensory neurons. In the somatosensory system, RET and GFRα expression has been used in the subclassification of nociceptors (30, 35, 55). The RET-positive nociceptors correspond with the subclass of nociceptors that can be stained with IB4 and fail to express neuropeptides. The RET-negative nociceptors correspond with the peptidergic, IB4-negative nociceptors. We were therefore somewhat surprised that virtually all vagal sensory neurons, including both nodose and jugular nociceptive neurons, expressed RET. Moreover, virtually all vagal sensory neurons innervating the adult guinea pig respiratory tract expressed mRNA for multiple GFRα receptors. Although for technical reasons we did not evaluate GFRα4, we found GFRα1, the receptor for GDNF, to be expressed in nearly all jugular and nodose neurons innervating the adult respiratory tract. We also noted that GFRα3 was nearly uniformly expressed in jugular neurons and largely expressed in nodose C-fiber and trachea Aδ neurons. Although GFRα2 was not as uniformly expressed as GFRα1 and α3, it was expressed in nearly 50% of the neurons irrespective of phenotype.
There have been relatively few studies published in which GFRα expression has been analyzed in organ-specific sensory neurons. In a histochemical analysis of dorsal root ganglion (DRG) neurons retrogradely labeled from the rat bladder, only a small percentage of neurons expressed GFRα receptors: ∼15, 1, and 8% of bladder-specific neurons expressed GFRα1, GFRα2, and GFRα3, respectively (14). By contrast, the majority of DRG neurons labeled from the mouse pancreas were found to express a GFRα receptor, notably GFRα3 (12). We recently found GFRα receptors are also expressed in nodose and jugular C-fibers innervating mouse lungs. Interestingly, in the mouse, GFRα3 receptors were expressed virtually exclusively in neural crest C-fibers and not in placode-derived nodose C-fibers (40). This was consistent with the aforementioned study on pancreas-specific sensory neurons, where nearly all the neural crest-derived DRG neurons expressed GFRα3, whereas none of the nodose neurons expressed this receptor (12). On the basis of the present data, it is clear that the hypothesis that GFRα3 is selectively expressed only in neural crest-derived sensory neurons cannot be extrapolated to mammalian airway neurons in general. Although overall, a lower percentage of guinea pig nodose neurons expressed GFRα3 compared with jugular neurons, the difference was not categorical as seen in the mouse.
The data obtained with GFRα receptor expression should raise the awareness of GFLs such as GDNF, neurturin, and artemin as important neuromodulators during inflammatory processes. The effect of GFL on vagal sensory neurons is not known, but in addition to altering neural phenotype, GFLs may also have acute effects on excitability. Studies on isolated DRG neurons, for example, have found that GDNF, neurturin, and artemin can substantively enhance the response of sensory C-fiber neurons to capsaicin and elicit hyperaglesia (31, 48). It will be important to learn what cell types, and under what circumstances, GFLs are expressed in the respiratory tract, especially during various types of airway inflammation. At present this is a relatively unstudied area. GDNF and neurturin have been localized to airway smooth muscle cells, where, along with RET, they have been implicated in the development of airway parasympathetic ganglion neurons (27, 51).
GRANTS
This work was funded and supported by the National Institutes of Health, Bethesda, MD Grants R01HL038095 and R01HL062296.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol 232: 1–61, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Baker CVH. The embryology of vagal sensory neurons. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich D. Boca Raton, FL: CRC, 2005, p. 2–21 [Google Scholar]
- 4. Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol 25: 1386–1403, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci 17: 7007–7016, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bothwell M. Keeping track of neurotrophin receptors. Cell 65: 915–918, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med 165: 1071–1075, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chuaychoo B, Hunter DD, Myers AC, Kollarik M, Undem BJ. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 116: 325–331, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Chuaychoo B, Lee MG, Kollarik M, Pullmann R, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol 575: 481–490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dey RD. Controlling from within: neurophysiological plasticity of parasympathetic airway neurons. Am J Physiol Lung Cell Mol Physiol 284: L578–L580, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol 509: 42–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest 98: 2284–2291, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol 506: 989–1002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freund-Michel V, Frossard N. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol Ther 117: 52–76, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Helke CJ, Adryan KM, Fedorowicz J, Zhuo H, Park JS, Curtis R, Radley HE, Distefano PS. Axonal transport of neurotrophins by visceral afferent and efferent neurons of the vagus nerve of the rat. J Comp Neurol 393: 102–117, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hoyle GW, Graham RM, Finkelstein JB, Nguyen KPT, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18: 149–157, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunter DD, Castranova V, Stanley C, Dey RD. Effects of silica exposure on substance P immunoreactivity and preprotachykinin mRNA expression in trigeminal sensory neurons in Fischer 344 rats. J Toxicol Environ Health A 53: 593–605, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 161: 1985–1990, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Hunter DD, Wu Z, Dey RD. Sensory neural responses to ozone exposure during early postnatal development in rat airways. Am J Respir Cell Mol Biol 43: 750–757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnston MC. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat Rec 156: 143–155, 1966 [DOI] [PubMed] [Google Scholar]
- 23. Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther 289: 682–687, 1999 [PubMed] [Google Scholar]
- 24. Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol 586: 1321–1336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langsdorf A, Radzikinas K, Kroten A, Jain S, Ai X. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol 44: 293–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev 48: 534–569, 1968 [DOI] [PubMed] [Google Scholar]
- 29. Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54: 739–754, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12: 1161–1171, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Mittal RA, Simbruner G, Smith J, Simbruner B, Holzinger A. Mechanical ventilation with high tidal volume or frequency is associated with increased expression of nerve growth factor and its receptor in rabbit lungs. Pediatr Pulmonol 44: 713–719, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Mohtasham L, Auais A, Piedimonte G. Nerve growth factor mediates steroid-resistant inflammation in respiratory syncytial virus infection. Pediatr Pulmonol 42: 496–504, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci 13: 4029–4041, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 282: L775–L781, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A. Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma. J Allergy Clin Immunol 118: 597–605, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, MacGlashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588: 4769–4783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384: 360–364, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol 117: 67–71, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Ohtori S, Takahashi K, Moriya H. Inflammatory pain mediated by a phenotypic switch in brain-derived neurotrophic factor-immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints in rats. Neurosci Lett 323: 129–132, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Raap U, Fokkens W, Bruder M, Hoogsteden H, Kapp A, Braunstahl GJ. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy 63: 468–475, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496: 521–530, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rochlitzer S, Nassenstein C, Braun A. The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem Soc Trans 34: 594–599, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Schmutzler BS, Roy S, Hingtgen CM. Glial cell line-derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons. Neuroscience 161: 148–156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scuri M, Samsell L, Piedimonte G. The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets 9: 173–180, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 12: 361–373, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants. A role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol 26: 420–429, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med 172: 233–237, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature 382: 80–83, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905–917, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron 55: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol 95: 742–750, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Zeeni N, Chaumontet C, Moyse E, Fromentin G, Tardivel C, Tome D, Jean A, Darcel N. A positive change in energy balance modulates TrkB expression in the hypothalamus and nodose ganglia of rats. Brain Res 1289: 49–55, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhuo H, Helke CJ. Presence and localization of neurotrophin receptor tyrosine kinase (TrkA, TrkB, TrkC) mRNAs in visceral afferent neurons of the nodose and petrosal ganglia. Brain Res Mol Brain Res 38: 63–70, 1996 [DOI] [PubMed] [Google Scholar]