Abstract
The pathophysiology of airway diseases, such as asthma, is increasingly studied using transgenic mice and other mouse models of airway inflammation where allergen-induced changes in airway smooth muscle tone and mucous secretion is due, in part, to activation of preganglionic airway parasympathetic nerves. Ganglionic parasympathetic neurons located in the airways in several species, including humans, have anatomical and electrophysiological properties that limit transmission of preganglionic synaptic input. In this study, intracellular recordings were made from neurons in parasympathetic ganglia located on the trachea and bronchi of adult mice to determine electrophysiological properties associated with regulation of transmission of preganglionic input. Ganglionic neurons were characterized as having either tonic or phasic action potential accommodation patterns. Tonic neurons responded with repetitive action potentials sustained throughout a depolarizing current step, whereas phasic neurons generated one or a burst of action potential(s) and accommodated. A small subset displayed both patterns. Phasic neurons could be further differentiated as usually having either short- or long-duration afterhyperpolarizing potential following single and multiple action potentials. In most cells, stimulation of preganglionic nerves elicited one population of nicotinic fast excitatory postsynaptic potentials that were graded in amplitude, usually suprathreshold for action potential generation, and did not decrease in amplitude during higher frequency stimulation. Dye injection into the neurons revealed that dendrites were either absent or very short. These results provide evidence that in contrast to the characteristics of airway parasympathetic neurons reported in other species, including human, the electrophysiological and synaptic properties, and anatomical characteristics of mouse lower airway ganglionic neurons, are less associated with integration of presynaptic input.
Keywords: airway diseases, asthma
airway narrowing is caused, predominantly, by smooth muscle contraction and mucous secretion, responses that are normally under the control of the parasympathetic nervous system. In the parasympathetic nerve pathway, vagal preganglionic nerves that originate in the brainstem terminate at synapses on principal neurons within parasympathetic ganglia on the airway wall, and the postganglionic axons exiting these ganglia innervate the nearby effectors such as airway smooth muscle and mucous glands (2). The parasympathetic nervous system regulates the level and distribution of the signals emanating from the central nervous system and thus plays a pivotal role in the regulation of air flow to the lungs. Because of their peripheral location, parasympathetic nerves may be affected by local inflammation and airway remodeling (18). Although the active and passive (electrophysiological) membrane properties of airway ganglionic neurons have been reported for several mammalian species (13), including humans (8), no studies have focused on mice, a species commonly used in airway asthma and allergy studies (23).
In humans (9) and guinea pigs (16), airway parasympathetic ganglionic neurons display anatomical and electrophysiological characteristics that contribute to regulation of synaptic activity, including tonic and phasic action potential accommodation patterns, long- and short-action potential afterhyperpolarizations (AHPs), and multiple populations of fast excitatory postsynaptic potentials (fEPSPs). Their complexity is further increased by anatomical properties such as long and branching dendrites (the sites of synaptic inputs; reviewed in Ref. 14) and the fact that they represent two distinct populations of neurons, cholinergic and non-adrenergic non-cholinergic (NANC), that cause contraction and relaxation, respectively, of airway smooth muscle (2, 3).
Specific allergen challenge in the airway causes stimulation of afferent (sensory) nerves and consequent reflex activation of efferent parasympathetic nerves, resulting in an increase in smooth muscle tone and mucous secretion (7; reviewed in Ref. 2). In mice, the allergen-induced contraction of airway smooth muscle has a well-defined cholinergic component (7, 8) that is predominantly due to activation of cholinergic parasympathetic nerves (7, 22). However, it is not known whether ganglionic neurons in mouse airways integrate (attenuate or enhance) the reflex presynaptic input that has been reported for mice (7) and other species (reviewed in Ref. 14). In this study, intracellular recordings were made from neurons in parasympathetic ganglia in the tracheal and bronchial airways of mice to determine whether electrophysiological membrane properties may be associated with regulation of transmission of preganglionic input.
MATERIALS AND METHODS
All methods were approved by the Johns Hopkins University Animal Care and Use Committee. Adult male C57BL/6 mice (20–22 g; Jackson Labs, Bar Harbor, ME) were overdosed with pentobarbital (75 mg/kg ip) and transcardially perfused with, and dissected in, oxygenated Krebs buffer (20°C). The composition of the Krebs buffer was (in mM): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO2, 14.3 NaHCO3, and 11 dextrose, equilibrated with 95% O2/5% CO2 (pH 7.4). The intrathoracic trachea and mainstem bronchi with the vagus nerves attached were removed en bloc, and the airways were cut ventrally down the midline, opened as a sheet, and pinned, lumen-side down, to a Sylgard-lined Petri dish. Unstained ganglia were located as previously described for guinea pigs (13), using a stereomicroscope at magnifications of ×20–80 with transmitted light. The ganglia were located on peritracheal and peribronchial nerve trunks emanating medially and laterally, respectively, from the vagus nerves (see results). Connective tissue overlying the ganglia was gently cleared to allow penetration by the microelectrode. Once ganglia were identified, the airway with vagus nerves was pinned to the Sylgard-lined floor of a low-volume (0.2 ml) recording chamber on a fixed-stage microscope and perfused (5 ml/min) with warmed (36–37°C) Krebs buffer.
For electrophysiological recordings, microelectrodes were fabricated on a Brown-Flaming P-87 electrode puller (Sutter Instruments, Novato, CA) from thick-wall (1 mm o.d., 0.5 mm i.d.) borosilicate capillary stock, filled with 3 M KCl and connected to a current and voltage clamp amplifier (Axoclamp 2A; Axon Instruments, Foster City, CA). The microelectrodes in the Krebs buffer-filled recording chamber had a resistance of 60 MΩ. For recording synaptic potentials, some neurons were recorded with QX314 (3 μM; Tocris Bioscience, Ellisville, MI) in the recording electrode. Intracellular recordings were performed with the electrometer in either discontinuous current clamp (3.0–4.0 kHz sampling rate) or in active bridge mode. Membrane potential monitoring, action and synaptic potential recording, current injection, and analysis were performed using a Macintosh computer equipped with an analog-to-digital translation interface and an oscilloscope simulation, recording, and analysis program (Axograph, Axon Instruments). Passive membrane properties (resting potential, input resistance, time constant), active membrane properties (action potential amplitude, duration, afterhyperpolarization duration and amplitude, accommodation), and synaptic potentials (number, amplitude, duration) were evoked and measured as previously reported (9, 16). Only neurons with stable resting membrane potentials <−40 mV, input resistances ≥20 MΩ, and action potentials ≥40 mV in amplitude were included in results. Vagus nerve-mediated fEPSPs were evoked by 1-Hz square pulses, ranging from 1–20 V and 0.02–0.8 ms in duration (pulse duration and voltage adjusted to attempt to evoke subthreshold fEPSPs), delivered to the caudal cut end of the vagus nerve 5–20 mm from the ganglion. Conduction velocity of the preganglionic axons was calculated by dividing the distance from the end of the suction electrode to the ganglion, by the time between the shock artifact and the onset of the fEPSP. For all data collection of active and synaptic membrane properties, the resting potential was current clamped to −50 mV before action potential generation and nerve stimulation. Neurobiotin ionophoresis and development were performed as previously reported (9, 13).
Results are presented as means ± 1 SE. Sample mean values were analyzed by analysis of variance, and the values comparing properties of tonic- and phasic-type neurons were compared using Student's t-statistics for two means. Means were considered to differ significantly if P values were <0.05.
All reagents used to prepare the Krebs solution were purchased from J. T. Baker Chemical (Phillipsburg, NJ). 1,1-dimethyl-4-phenylpiperazinium (DMPP), mecamylamine hydrochloride, and hexamethonium bromide were obtained from Sigma Chemical (St. Louis, MO).
RESULTS
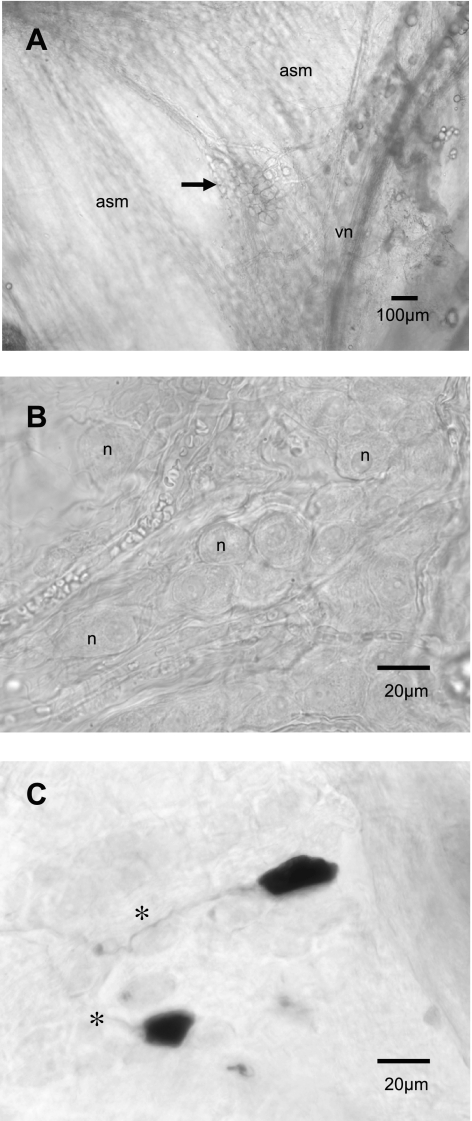
Clusters of parasympathetic neuronal cell bodies (ganglia) were randomly distributed along the dorsal surface of the trachea and primary bronchus. Ganglia in whole mount tracheas and bronchi could be easily visualized with a stereomicroscope in fresh tissue using transmitted light at low magnification (Fig. 1A), and, with a microscope at higher magnification, the somata of mouse tracheal and bronchial ganglionic neurons were ovoid or cuboidal in shape (Fig. 1B). Most neurons recorded were localized to ganglia near the tracheal bifurcation or on the adjacent right primary bronchus near the vagus nerve. Neurobiotin ionophoresis and development of the Neurobiotin revealed that the neurons (n = 8) were ovoid to cuboidal in shape, lacked significant dendritic processes, and gave rise to a single axonal process (Fig. 1C). The passive and active membrane properties of cells within the ganglia were determined in current clamp experiments. The resting membrane potential ranged from −37 mV to −68 mV with a mean of −49 ± 2.7 mV (n = 82 neurons). The membrane input resistance ranged from 20 to 80 MΩ (means = 69 ± 2 MΩ; n = 60). The input time constant and calculated input capacitance were 5.9 ± 0.5 ms and 85.5 ± 4.5 pF (n = 40), respectively. Using a brief (2 ms), suprathreshold (0.5–1.0 nA) anodal current step, action potentials were elicited with amplitudes and durations averaging 65 ± 7 mV (range of 40–81 mV) and 6.8 ± 0.2 ms (range 3.6–9.0 ms; n = 50), respectively. Most action potentials displayed an overshooting (>0 mV) depolarization phase. There was no break or “hump” in the depolarizing or repolarizing phase of the action potential (Fig. 2A). The amplitude and duration of the AHPs that followed each action potential were 7.3 ± 1 mV and 64 ± 12 ms, respectively.
Fig. 1.
Micrograph images of airway parasympathetic ganglia. All neurons recorded were localized to ganglia near the tracheal bifurcation or on the adjacent right bronchus, near the vagus nerve. In A, a low-magnification view of a cluster of parasympathetic neuronal cell bodies (ganglion, arrow) found on the dorsal surface of the trachea on the airway smooth muscle (asm) near the vagus nerve (vn) in a fresh whole mount trachea (original magnification, ×4). In B, at higher magnification (×40), another ganglion located on the right bronchus. The somata of ganglionic neurons (n) were ovoid in shape. In C, after injection with Neurobiotin and having been fixed and developed using peroxidase and DAB, the neurons were ovoid to cuboidal in shape, lacked significant dendritic processes, and gave rise to a single axonal process (*); original magnification, ×40.
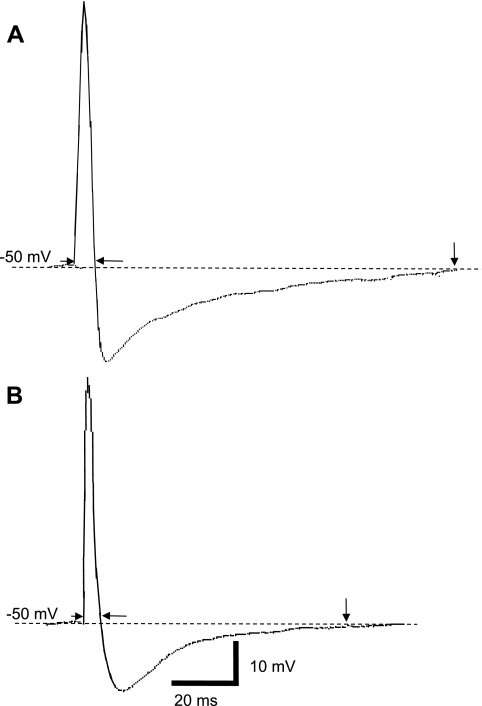
Fig. 2.
Examples of action potential recordings from mouse airway neurons. In A, the action potential of a phasic neuron (see Fig. 3) has a relatively longer duration (measured between arrowheads) compared with the durations in a tonic neuron shown in B (data summarized in Table 1). Also, note the longer action potential afterhyperpolarization (AHP; at downward arrow) by the phasic neuron (A). Scale bar in B is for traces A and B.
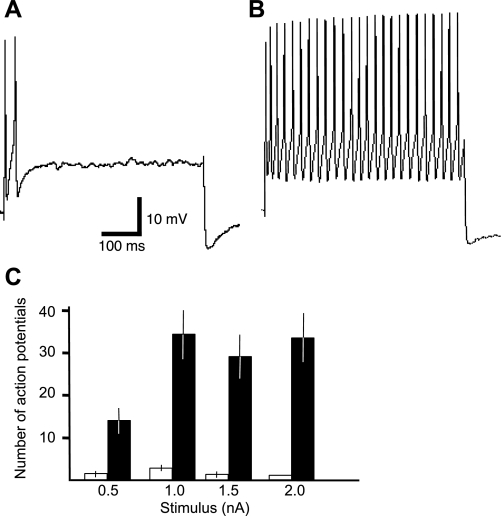
In response to prolonged (500 ms) threshold (0.5 nA) to suprathreshold (1, 1.5, 2 nA) rectangular anodal constant-current steps, ganglionic neurons generated distinct action potential discharge patterns. Of 82 neurons characterized, 51 neurons displayed accommodation (cessation of action potentials) during all steps (0.5, 1.0, 1.5, 2.0 nA; Fig. 3A), and 31 neurons were non-accommodating (repetitive action potentials) during at least one of the four test steps (Fig. 3B). These are referred to as phasic and tonic action potential patterns, respectively. Eight tonic neurons displayed phasic patterns at the higher steps (1.5, 2 nA; not shown). Phasic neurons generated 0–5 action potentials at the two lower current steps, and 0–2 at the two higher current steps (Fig. 3C); tonic neurons generated 3–32 action potentials at 0.5 nA, 2–80 at 1.0 nA, 2–60 at 1.5 nA, and 1–70 action potentials at 2.0 nA (Fig. 3C).
Fig. 3.
Two types of action potential patterns by neurons in mouse airway ganglia. In A, trace showing the action potential response by a phasic-type neuron to a prolonged (500 ms) suprathreshold (2.0 nA) depolarizing stimulus: only 1 action potential is elicited before complete accommodation to the current stimulus (2.0 nA, 500ms). In B, response by a tonic neuron that generates repetitive action potentials throughout the same stimulus as in A (2.0 nA), i.e., no accommodation to the stimulus. Calibration bars apply to all traces. In C, summary of the number of action potentials per 500-ms stimulus at the different stimulus amplitudes; open bars for phasic neurons, filled bars for tonic neurons. The number of action potentials were different for the 2 cell types at all stimuli (P < 0.05).
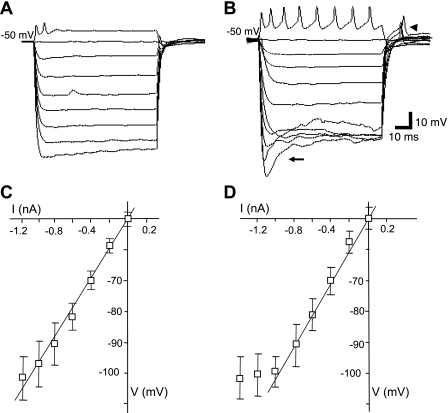
Several passive membrane properties differed between tonic and phasic neurons, and these are summarized in Table 1. The mean resting membrane potential for tonic and phasic neurons was not different; however, the mean resting input resistance (point method) for tonic neurons was ∼30% greater than that for phasic neurons (P < 0.05; Table 1), and although the time constant was slightly longer in tonic than phasic neurons, the calculated input capacitance was nearly the same (Table 1). The current-voltage (I-V) relationship was linear for most phasic (between −50 and −120 mV; Fig. 4, A and C) and tonic (between −50 to −100 mV; Fig. 4, B and D) neurons. Most tonic neurons displayed time- and voltage-dependent rectification at potentials more negative than −100 mV (Fig. 4B) with anodal break excitation evoked from potentials less than −80 mV (e.g., Fig. 4B). The input resistance calculated from the slope of the linear portion (−40 mV negative from the resting potential) of the current-voltage relationship was similar for tonic neurons (54 ± 5 MΩ; n = 7; Fig. 4C) and phasic neurons (47 ± 7 MΩ; n = 15). Outward rectification was observed in tonic and phasic neurons at depolarizing current steps (≥0.3 nA) following initiation of action potentials (not shown).
Table 1.
Passive, active, and synaptic membrane properties of mouse airway ganglionic neurons
| Phasic Neurons | Tonic Neurons | |
|---|---|---|
| Resting potential | −48 ± 3 mV | −51 ± 4 mV |
| Input resistance | 44 ± 5 MΩ | 69 ± 8 MΩ* |
| Time constant | 5.3 ± 0.4 ms | 6.9 ± 0.8 ms* |
| Input capacitance | 6.9 ± 0.5 pF | 9.2 ± 0.8 pF* |
| Single action potential | ||
| Amplitude | 46 ± 10 mV | 55 ± 12 mV |
| Duration | 7.4 ± 0.1 ms | 6.0 ± 0.3 ms* |
| AHP amplitude | 8.0 ± 1 mV | 5.9 ± 1 mV |
| AHP duration | 70 ± 13 ms | 40 ± 9 ms* |
| AHP amplitude | 15 ± 4 mV | 7 ± 3 mV* |
| Four action potentials | ||
| AHP amplitude2 | 8.9 ± 3 mV | 7.2 ± 3 mV |
| AHP Duration | 279 ± 24 ms | 135 ± 34 ms* |
| Synaptic potentials3 | ||
| Amplitude | 16.3 ± 3 mV (13–19 mV) | |
| Duration | 7.8 ± 2 ms (5.8–10.1 ms) | |
| Threshold | 12 ± 4 mV (10–18 mV) | |
| No. Populations | 1 ± 0 | |
All values are means ± SE for 51 phasic and 31 tonic neurons. Eight neurons had a tonic response in only 1 of 4 steps and were included as tonic neurons in the table results. AHP amplitude following 4 consecutive action potentials was measured at the 4th action potential; duration from the end of the 4th action potential until the potential returned to the prestimulus resting potential.
P < 0.05 compared with phasic neurons. Vagus nerve-stimulated synaptic potentials were measured in 12 phasic and 9 tonic neurons; thus far, all fEPSP parameters are similar for both cell types. For fEPSP recordings, QX314 (3 μM) was added to the recording electrode electrolyte. AHP, afterhyperpolarization; fEPSP, fast excitatory postsynaptic potential.
Fig. 4.
Current-voltage relationship in tonic and phasic neurons. In A, current-clamp recording of a phasic neuron shows linear change in voltage from −50 to −100 mV using 200 pA, 200-ms decrementing current steps. In B, the response to the same stimuli as in A by a tonic neuron. In C, a summary of the voltage responses of phasic neurons showing a relatively linear response between −50 and −100 mV. In D, a summary of the responses by tonic neurons showing a linear response also between −50 and −100 mV; most tonic neurons displayed voltage-dependent rectification at potentials more negative than −100 mV (diversion from line) with a time-dependent change (“sag”) in the electronic voltage transients more negative than −100 mV (arrow in B). Anodal break excitation (arrowhead in B) was evoked from potentials less than −80 mV in tonic neurons. For tonic and phasic neurons, there was no difference in conductance calculated from the linear portion of the current-voltage relationship. Calibration in B applies to traces in A and B; action potentials are blunted in A and B due to recording in discontinuous current clamp (see materials and methods).
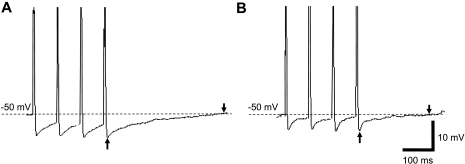
Several action potential characteristics also differed between tonic and phasic neurons. The duration of the action potential (measured at the level of the resting potential) was 20% greater in phasic compared with tonic neurons (compare Fig. 2, A and B; P < 0.05; Table 1). Threshold current for eliciting an action potential was lower in tonic than phasic neurons, as the mean current (using 150-ms steps) for evoking an action potential in phasic neurons was 0.6 ± 0.2 nA (range of 0.4 to 1.0 nA) and tonic neurons was 0.3 ± 0.1 (range of 0.1 to 0.6 nA; P < 0.05). The duration of single action potential AHP was shorter in tonic (Fig. 2A) compared with most phasic neurons (Fig. 2B; Table 1); however, 10 phasic neurons had AHP durations in the same range as tonic neurons. The duration of the spike AHP following multiple action potentials also differed between tonic and most phasic neurons (Table 1). Examples of AHP responses to four consecutive action potentials evoked by 2-ms, 3 nA steps (50 Hz) for a phasic and tonic neuron are shown in Fig. 5, A and B, respectively.
Fig. 5.
Cumulative action potential AHP in tonic and phasic neurons. In A, the duration of the AHP (measured between arrows) following consecutive action potentials (4 stimuli, 2 ms, 3 nA steps, 50 Hz) in a phasic neuron is relatively longer compared with B, a tonic neuron (arrows). The calibration bars in B apply to traces A and B. Peak action potential amplitudes are cropped.
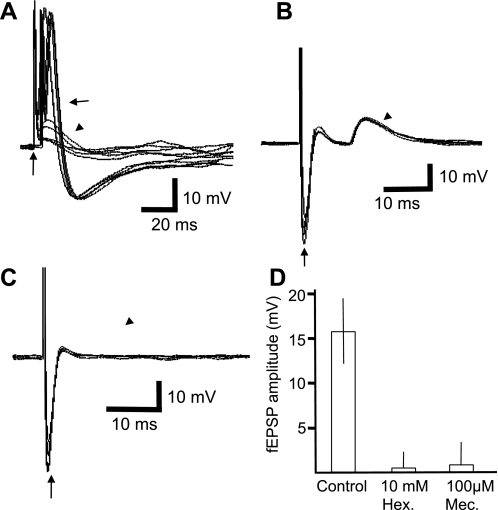
fEPSPs were evoked by stimulation of the right vagus nerve. The conduction velocity was calculated to be 6.1 ± 0.4 m/s (n = 6). For the majority of cells (17 of 21), a single fEPSP was evoked in response to a single stimulus applied to the vagal efferent fibers running to the ganglion (Fig. 6A). Two neurons had two distinct populations, and one had three populations (not shown). In most cells (16 of 21), the fEPSPs were suprathreshold for action potential initiation (Fig. 6A) and were markedly reduced or abolished by 10 mM hexamethonium (n = 6; Fig. 6C) or 100 μM mecamylamine (n = 4; Fig. 6D). The nicotinic agonist DMPP (0.1–10 mM, 10 s) caused a brief depolarization of all neurons tested (22). Excitatory synaptic potentials arose from stimulation of a single presynaptic nerve process and are typical of strong synaptic inputs. Slower potentials were not evoked by high-frequency stimulation of preganglionic (stimulus 5–40 Hz, 2- to 10-s trains, 0.1- to 0.5-ms pulses) or potential afferent (same stimuli with 0.5- to 1.2-ms pulses) vagal nerve fibers (not shown).
Fig. 6.
Vagus nerve-stimulated fast excitatory postsynaptic potentials (fEPSPs) are threshold for action potential generation and nicotinic. In A, a control neuron showing the consecutive responses in an overlay of 10 consecutive stimulations of the vagus nerve (1 Hz, 0.2 ms, 10 V, shock artifact at vertical arrow) evokes threshold fEPSPs (arrow) and some subthreshold fEPSPs (arrowhead). In B, in a different cell, overlay of traces showing a single population of fEPSPs evoked by vagus nerve stimulation (1 Hz, 0.5 ms, 20 V, shock artifact at vertical arrow). In C, overlaid traces of the same neuron as in B but in the presence of hexamethonium (10 mM, 5 min), blocking the fEPSPs; similar results were observed with 100 μM mecamylamine (summarized in D).
DISCUSSION
The present study represents the first electrophysiological and cellular evaluation of parasympathetic ganglionic neurons from tracheas and bronchi of mice. Data reported here regarding membrane properties such as the resting membrane potential, input resistance, and time constant of mouse airway neurons were within the range of those recorded in vitro for guinea pig (16), human (9), and ferret airway parasympathetic neurons (1), and in vivo for cat tracheal ganglion neurons (11). Although membrane properties of airway parasympathetic ganglionic neurons from human and other laboratory animals were similar, this may not necessarily indicate that these cells have a similar level of integration in the mammalian airways.
In this study, clusters of neurons (ganglia) were located on the mouse trachea and bronchi and could be investigated directly using intracellular microelectrodes. Thus far, it has been reported that parasympathetic neurons located in the intrinsic airway ganglia have anatomical and electrophysiological properties that allow attenuation (integration) of preganglionic signals emanating from the central nervous system (1, 9, 12, 16). However, mouse neurons do not display anatomical features such as multiple dendritic processes associated with more complex airway parasympathetic neurons, and associated with this lack of complexity, they have a somewhat simpler set of synaptic properties. Thus, anything that increases electrophysiological, dendritic, or synaptic complexity of these neurons may provide a useful model for studying how changes in these properties affect output from these ganglia, and, consequently, autonomic tone in the lower airways.
Mouse ganglionic neurons in the airway could be classified into two types based on their response to prolonged depolarizing current steps. Many neurons displayed one, or a burst of action potentials, and accommodated in response to suprathreshold stimuli, while the remaining neurons responded with repetitive action potentials without accommodation. These neurons are referred to as phasic or tonic, respectively (4). Phasic and tonic neurons have also been reported for parasympathetic ganglionic neurons in guinea pig (13) and human bronchi (9). Unlike tonic neurons in guinea pig bronchial ganglia (12), mouse bronchial tonic neurons displayed more erratic patterns and a greater range of action potential frequencies at suprathreshold current steps (Fig. 3). The ratio of phasic to tonic cells in mouse ganglia was slightly less than human and guinea pig airway ganglia, where phasic neurons outnumber tonic neurons by up to twofold (9, 16). Accommodative properties of phasic-type neurons may greatly affect the ability of the neurons to relay preganglionic stimuli (15), and, consequently, parasympathetic tone in the airway (10, 15).
Tonic and phasic neurons in sympathetic (4), parasympathetic (12), and myenteric ganglia (21) are reported to differ in their active and passive membrane properties. In mouse airway ganglia, tonic neurons had a greater input resistance, time constant, and, thus, capacitance, compared with phasic neurons. Differences in the mean values for capacitance are due to the differences in membrane resistance and time constant. Although the lower input resistance associated with the phasic firing pattern could represent an artifact due to recording from less healthy cells (or “leaky” impalement), the fact that other membrane properties were similar (resting membrane potential, action potential properties) suggests that these two distinct firing patterns may represent normal characteristics of two populations of neurons within these ganglia. Whether tonic and phasic neurons innervate distinct target tissue and are anatomically distinct, as are tonic and phasic neurons in sympathetic ganglia (5), remains to be determined. It is unlikely that neuronal geometry underlies the differences between these two cell types, as both tonic and phasic neurons filled with Neurobiotin had similar sizes and shapes (not shown).
Single action potential properties also differed between tonic and phasic neurons. Spike width was consistently broader in phasic than in tonic cells (Fig. 2), similar to those found in human (9) and guinea pig bronchial ganglia (13, 16). Although the AHP amplitude that followed single action potentials was approximately equal in tonic and phasic neurons, the duration of the AHP was nearly twice as long in most phasic neurons. The AHP duration following four action potentials was also longer in most phasic neurons. The broader spike duration and longer AHP in phasic neurons may be indicative of a calcium-activated potassium current similar to that observed in guinea pig bronchial ganglia neurons (12). Longer AHP (≥1,000 ms AHPslow) associated with guinea pig sympathetic (5) or airway sensory (6) neurons were not observed. Nonetheless, the longer AHP in phasic neurons may be responsible for the accommodative properties in most of these neurons (5) and could limit the firing frequency, and, consequently, the output from these ganglia.
Stimulation of the vagus nerve usually evoked a single population of fEPSPs that had greater amplitudes than those reported for bronchial ganglia neurons in guinea pigs (15) or humans (9), but were similar in duration to those described in airway ganglia of other laboratory animals (1, 10) and humans (9). Preganglionic axons had conduction velocities greater than 5 m/s, similar to that observed for myelinated sympathetic preganglionic nerves, but dissimilar to the slower conducting preganglionic axons (≤1 m/s) associated with airway parasympathetic ganglia of other laboratory animals (1, 16) and some bronchial neurons in humans (9). Consistent with other airway parasympathetic ganglia neurons (1, 9, 16), fEPSPs recorded in mouse bronchial neurons were pharmacologically identified as nicotinic cholinergic (i.e., blocked by nicotinic receptor antagonists). However, unlike nicotinic synaptic transmission in other species, nearly all of the neurons had fEPSPs that reached action potential threshold following a single stimulation of preganglionic nerves, even when using a wide range of stimulus pulse durations, amplitudes, and frequencies. No differences in synaptic membrane properties were noted for tonic and phasic neurons. Neurobiotin was used to determine the presence of neuronal processes (axons and dendrites); Neurobiotin has been used in many studies to completely fill large dendrites (e.g., Ref. 23) as well as short and spiny processes (e.g., Ref. 19) on mammalian neurons. These neurons did not have multiple, branching dendrites (Fig. 1C) as reported for neurons in human (9) or guinea pig bronchial (13) or ferret tracheal ganglia (1). Complex dendritic arbors are associated with modulation of synaptic strength because long branching dendrites provide variable electrotonic distances between each synapse and the site of action potential generation (20), consequently affecting the net fEPSP amplitude.
Neurons in mouse bronchial parasympathetic ganglia display somewhat less diverse and complex electrophysiological and anatomical properties compared with other neurons in airway ganglia. This may indicate that these neurons are less likely to integrate information received from the central nervous system, making them distinct from other mammalian autonomic ganglionic neurons, most of which integrate input from the central nervous system (14). In mouse tracheal and bronchial ganglia, preganglionic stimuli evoked threshold fEPSPs, resulting in an input to output ratio near unity, with rare multiple or subthreshold fEPSPs that could amplify or attenuate, respectively, preganglionic input. The existence of tonic and phasic firing patterns and relatively long, additive action potential AHPs may contribute to the integration of signals from the central nervous system. Changes in the integrative properties, as reported for other airway parasympathetic ganglia neurons (17), may exert some effect on the output from these ganglia, and, consequently, on airway caliber (10).
GRANTS
This work was supported by NIH Grant HL-088608 (A. C. Myers).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Holly K. Rohde for technical help.
REFERENCES
- 1. Cameron AR, Coburn RF. Electrical and anatomic characteristics of cells of ferret paratracheal ganglion. Am J Physiol Cell Physiol 246: C450–C458, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol 101: 971–985, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Canning BJ, Undem BJ. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea pig trachealis. J Physiol 471: 25–40, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cassell JF, Clark AL, McLachlan EM. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea pig. J Physiol 372: 457–483, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christian EP, Weinreich D. Long-duration spike afterhyperpolarizations in neurons from the guinea pig superior cervical ganglion. Neurosci Lett 84: 191–196, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Christian EP, Togo JA, Naper KE, Koschorke G, Taylor GA, Weinreich D. A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. J Neurosci Meth 47: 147–160, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol 182: 7420–7439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eum SY, Norel X, Lefort J, Labat C, Vargaftig BB, Brink C. Anaphylactic bronchoconstriction in BP2 mice: interactions between serotonin and acetylcholine. Br J Pharmacol 126: 312–316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kajekar R, Rohde HK, Myers AC. The integrative membrane properties of human bronchial parasympathetic ganglia neurons. Am J Respir Crit Care Med 164: 1927–1932, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Kessler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol 518: 843–855, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell RA, Herbert DA, Baker DG, Basbaum CB. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res 437: 157–160, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Myers AC. Ca2+ and K+ currents regulate accommodation and firing frequency in guinea pig bronchial parasympathetic ganglia neurons. Am J Physiol Lung Cell Mol Physiol 275: L357–L364, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Myers AC. Anatomical characteristics of tonic and phasic postganglionic neurons in guinea pig bronchial parasympathetic ganglia. J Comp Neurol 419: 439–450, 2000 [PubMed] [Google Scholar]
- 14. Myers AC. Transmission in autonomic ganglia. Respir Physiol 125: 99–111, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Myers AC, Undem BJ. Analysis of preganglionic nerve evoked cholinergic contractions of the guinea pig bronchus. J Auton Nerv Syst 35: 175–184, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Myers AC, Undem BJ, Weinreich D. Electrophysiological properties of neurons in guinea pig bronchial parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol 259: L403–L409, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Myers AC, Undem BJ, Weinreich D. Influence of antigen on membrane properties of guinea pig bronchial ganglion neurons. J Appl Physiol 71: 970–976, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Pan J, Rohde HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by NT-3 and repeated allergen challenge. Am J Respir Cell Mol Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson BB, Dacey DM. Morphology of wide-field bistratified and diffuse human retinal ganglion cells. Vis Neurosci 17: 567–578, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Rall W. Core conductor theory and cable properties of neurons. In: Handbook of Physiology–The Nervous System, edited by Kandel ER. Bethesda, MD: American Physiological Society, 1977, p. 39–97 [Google Scholar]
- 21. Wade PR, Wood JD. Electrical behavior of myenteric neurons in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 254: G522–G530, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell-cholinergic nerve interaction in mouse airways. J Physiol 587: 3355–3362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright LL, Vaney DI. The type 1 polyaxonal amacrine cells of the rabbit retina: a tracer-coupling study. Vis Neurosci 21: 145–155, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy 37: 973–989, 2007. [DOI] [PubMed] [Google Scholar]