Abstract
Cardiac troponins have been the biomarkers of choice for the diagnosis of acute coronary syndrome (ACS) for over a decade. There has, however, been considerable interest over the last two decades for newer biomarkers that would bring added value to the measurement of troponin such as the provision of prognosis and assistance in the choice of therapeutic interventions. In this manuscript, we review the development of heart-type fatty acid binding protein (H-FABP) in patients with ACS using the evidence-based laboratory medicine format.
Phase I studies have established that H-FABP reference intervals and pre-analytical factors influencing H-FABP. Phase II studies have confirmed a) that H-FABP is elevated in patients with established myocardial infarction; b) that its serum concentration is related to the extent of infarction using survival as a surrogate; and c) that its use in chest pain patients can identify ACS patients and also provide prognostic information on survival. Furthermore, it is an independent prognostic marker for patients with suspected ACS who are troponin negative. Phase III studies involving randomised control trials for diagnosis and prognosis have not yet been performed and Phase IV studies await uptake of H-FABP in a routine service.
Introduction
Biochemical markers have played an increasingly important role in the diagnosis of acute coronary syndrome (ACS), since the report of the release of aspartate amino-transferase (AST) from necrotic cardiac myocytes in 1955.1 The last two decades have seen considerable advances in our understanding of myocardial infarction (MI) and unstable angina which we now recognise as a single heterogeneous disease state known as Acute Coronary Syndrome. Currently, the cardiac troponins remain the most widely used biomarkers for the diagnosis of ACS by virtue of their high sensitivity for minor myocardial injury, almost absolute specificity for myocardial damage, and most importantly, their ability to risk-stratify patients with suspected ACS. Their widespread recognition was supported by the inclusion of cardiac troponin as the preferred biomarker in the diagnosis of ACS both in the New Definition of MI (1999)2 and further reinforced in the Universal Definition of Myocardial Infarction (2007).3
However, the scope of biomarkers in cardiovascular medicine has widened in recent years and a number of novel biomarkers have been evaluated as alternative markers for their incremental value, over and above troponin, in the risk stratification of patients with possible ACS. These novel biomarkers reflect different aspects of the pathophysiology in patients with ACS and therefore have the potential to identify defined pathological processes which might be amenable to specific therapies and, moreover, may provide complementary information when measured in conjunction with each other.4 There is also growing evidence of the benefits of a multi-marker strategy over the use of a single marker when evaluating patients with ACS.5–7
Fatty acid-binding proteins (FABP) are small (15 kDa) cytoplasmic proteins that are abundantly expressed in tissues with an active fatty acid metabolism such as heart and liver.8,9 The primary function of FABP is the facilitation of intracellular long-chain fatty acid transport.10 Nine distinct types have been identified, each showing a characteristic pattern of tissue distribution and named after the tissue in which they were first identified. Heart-type fatty acid binding protein (H-FABP) is found in abundance in cardiomyocytes but is also expressed (to a lesser extent) in skeletal muscle,11 distal tubular cells of the kidney,12 specific parts of the brain,13 lactating mammary glands and placenta.11 H-FABP was first shown to be released from injured myocardium in 198814 and shows rapid release and disappearance following acute MI.15 H-FABP appears to be a very stable protein in vitro for clinical diagnostic purposes.16
Evidence Based Evaluation of Diagnostic Tests
In 2005 Gluud & Gluud described a framework for evaluating diagnostic tests.17 They proposed a multi-phase model analogous to that used for the development of new pharmaceuticals. Phase I: determination of the reference intervals and pre-analytical factors through observational studies in healthy people. Phase II: a) demonstration of a relationship with disease, b) demonstration of a quantitative relationship with disease and c) demonstration of a relationship in people with suspected disease. Phase III: evaluation of the clinical utility of the diagnostic test through randomised trials; Phase IV: evaluation of the effects of introducing a new diagnostic test into clinical practice by surveillance in large cohort studies.
This contribution to the evidence-based laboratory medicine (EBLM) literature is particularly valuable as laboratory diagnostics tend to be used as the evidence point for clinical studies and it is rare for a trial to use a diagnostic test in a randomised fashion. In the case of myocardial infarction, it is now too late to consider a randomised control trial (RCT) to test the utility of troponin but many new markers have been evaluated and an RCT with outcome measures may be the only way to break into the accepted diagnostic strategy.
A further challenge in the field of myocardial infarction has been the serial redefinition of the disease over the past decade which makes it difficult to evaluate diagnostic strategies over prolonged periods of time. Moreover, the diagnosis of myocardial infarction as an end point has changed to decision points for therapeutic intervention and an acceptance that ischaemic heart disease is a continuum with adverse prognosis even before the advent of an acute ischaemic attack.
In this manuscript we describe the evidence to support the use of H-FABP both as a diagnostic test in ACS and as a prognostic marker for survival by presenting the evidence according to these four phases.
Phase I Studies for H-FABP in Normal Healthy Adults
Factors Influencing H-FABP Concentration Among Healthy Adults
Age and renal function have been consistently shown to influence H-FABP concentrations in normal healthy adults.18–20 H-FABP concentrations have also been reported to be higher in men than in women in some studies.19,20 It has been shown that the mean plasma concentration of H-FABP in the patients with chronic renal failure requiring dialysis is 21 to 25 times higher than in normal adults.21 However, there is no data to quantify the extent to which mild or moderate renal dysfunction influences serum H-FABP levels in healthy adults. H-FABP levels are also influenced by physical exercise.22,23 Only one small study (n=12) reported on the intra-individual biological variation of H-FABP concentration in healthy subjects which showed a modest effect (CV 14%).20 Increased body mass index has also been shown to be associated with elevated H-FABP concentration in one study.19
Reference Values in Normal Healthy Adults
Niizeki et al.19 reported upper reference limits (95th and 97.5th centile) for H-FABP (measured using the Dainippon assay) stratified into four sub-groups by age (10-year intervals between 40 and 79 years) and further sub-divided by gender in a large cohort of healthy Japanese adults (n=2099). This study showed considerable variation in the cut-off values across the groups; with 97.5th centile value ranging from 4.8 μg/L in women aged 40–49 years to 8.3 μg/L in men aged 70–79 years. While the majority of patients in this study had normal renal function, study subjects were not excluded on the basis of abnormal renal function.
In 2009, Bathia et al.18 reported on the 99th centile values of H-FABP for males and females in a sizeable UK population spanning all age groups (median age: 57 years in males, 51 years in females). Subjects with renal dysfunction (defined as eGFR <60 mL/min) were excluded. The 99th centile values were 5.3 and 5.8 μg/L (Randox Biochip) and 8.3 and 9.1 μg/L (Dainippon MARKIT-M) in females and males respectively. These authors demonstrated a significant correlation between assays (r=0.84, p<0.001) but noted a calibration difference as shown by the measurement of recombinant H-FABP. This stresses the importance of using assay specific cut-off values when interpreting H-FABP results in clinical practice and a need for harmonisation.
Phase II Studies of H-FABP in ACS
Phase IIa: H-FABP and Proven ACS
H-FABP was first reported to be released from injured myocardium in 1988.14 The release kinetics of H-FABP following acute MI mirror those of myoglobin – typically it is detectable at 1–3 hours, peaks at around 4 hours and returns to baseline concentrations within 24 hours.15 H-FABP appears to be a very stable protein as both plasma samples and recombinant protein solutions can be subjected to repeated freezing/thawing at least eight times without loss of immunoreactivity.24
Much of the attention in the 1990s was focused on the utility of H-FABP as a superior early diagnostic marker of ACS due to its favourable release kinetics following myocardial cell necrosis as described earlier. Studies have consistently shown that H-FABP is superior to myoglobin in the early diagnosis of ACS, especially in the first 6 hours from onset of symptoms. This is probably because H-FABP is a slightly smaller molecule than myoglobin and the normal plasma value of H-FABP is much lower than that of myoglobin25 both absolutely and relative to the magnitude of change seen in ACS. Table 1 summarises all studies to date recruiting patients with suspected ACS (majority presenting <6 h from symptom onset) which have evaluated H-FABP and myoglobin as early diagnostic markers of ACS after excluding those studies that recruited less than 100 patients and those that did not report their data using receiver operating characteristic (ROC) analysis.
Table 1.
Comparison of H-FABP with myoglobin and troponin in the early diagnosis of MI.
| Author and year of publication | N | Final diagnosis MI (%) | H-FABP assay | Troponin assay | Area under the ROC (final diagnosis of MI) | |||
|---|---|---|---|---|---|---|---|---|
| Myoglobin | H-FABP | Troponin | FABP + Tn | |||||
| Ishii et al. 199736 | 165 | 60 | In-house | 0.78 | 0.90 | - | - | |
| Glatz et al. 199737 | 312 | 54 | In-house | 0.82 | 0.90 | - | - | |
| Haastrup et al. 200038 | 130 | 16 | In-house | 0.84 | 0.89 | - | - | |
| Okamoto et al. 200039 | 189 | 74 | In-house | 0.84 | 0.92 | - | - | |
| Ghani et al. 200040 | 460 | 21 | In-house | Bayer Immuno 1 TnI | 0.73 | 0.80 | 0.91 | - |
| Nakata et al. 200341 | 133 | 44 | Dainippon | Roche TnT | 0.86 | 0.91 | 0.84 | - |
| Seino et al. 200342 | 371 | 49 | Dainippon | 0.76 | 0.79 | - | - | |
| Alansari et al. 200443 | 302 | 42 | Hycult | Bayer Advia centaur | 0.74 | 0.64 | 0.80 | - |
| McMahon et al. 201029 | 1128 | 10.4 | Randox | Roche TnT | 0.72 | 0.84 | 0.76 | 0.87 |
| (0–3 h) | (0–3 h) | (0–3 h) | (0–3 h) | |||||
| 0.85 | 0.89 | 0.85 | 0.92 | |||||
| (3–6 h) | (3–6 h) | (3–6 h) | (3–6 h) | |||||
| Kim et al. 201127 | 170 | 45 | Cardiodetect | Siemens Dimension TnI | 0.78 | 0.83 | 0.86 | 0.90 |
| Body et al. 201128 | 705 | 18.3 | In-house (Alere, San Diego) | In-house (Alere, San Diego) | 0.79 | 0.86 | 0.70 | - |
While the majority of studies published until 2004 showed H-FABP to be a superior early diagnostic marker, the results were not consistent across all studies. This is likely to be due to a number of factors including the heterogeneity in patient population (as evidenced by the percentage of patients diagnosed as ACS) and differences in performances of the H-FABP assays used in the various studies. Possibly most important, all studies published prior to 2003 used the old WHO definition of MI rather than the revised New Definition of MI.
There is now indirect evidence demonstrating that H-FABP is released not only during myocardial necrosis but also during acute myocardial ischaemia. Meng et al.26 demonstrated in rats that H-FABP concentration in peripheral blood rose four-fold from the baseline concentration after just 15 minutes induced myocardial ischaemia. Secondly, in human autopsy cases, they demonstrated depletion of H-FABP in the myocardium of individuals dying suddenly after the onset of chest pain. These changes were present despite the absence of myocyte necrosis on electron microscopy. This led to interest in investigating the role of H-FABP as an ischaemia marker among patients with possible ACS.
Phase IIb: Quantitative Relationship Between H-FABP and ACS
The three most recent studies of H-FABP as an early diagnostic marker in ACS27–29 (summarised in Table 1) showed that H-FABP is either superior to or adds incremental value to troponin in the early diagnosis of ACS as demonstrated by ROC analyses. Moreover, a fourth study of 664 patients with suspected ACS30 reported that the sensitivity of H-FABP (Randox Biochip assay) was superior to TnT among patients within 4 hours of symptom onset (73% vs 55%, p=0.04); the combined use of H-FABP or TnT (either one elevated) significantly improved the sensitivity to 85% (p<0.004). While no ROC analysis was reported in this manuscript, the results were consistent with the other three publications.
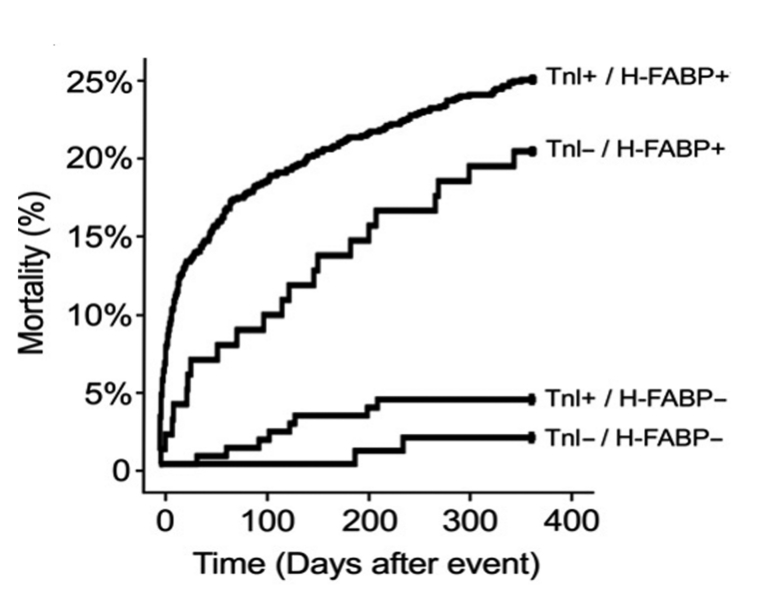
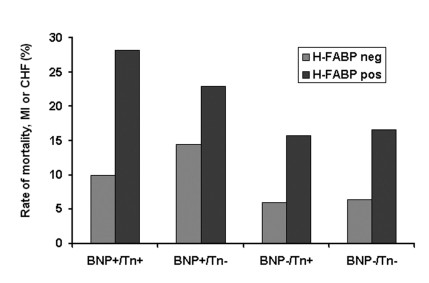
The four studies published between 2005 and 2007 which reported on the prognostic utility of H-FABP among patients with confirmed ACS are summarised in Table 2. All the studies consistently showed that elevated H-FABP is associated with an increased risk of long-term adverse outcomes (death, recurrent MI) irrespective of troponin results among patients with non-ST-elevation ACS (defined by the presence of ischaemic chest pain and at least one of either elevated troponin or ECG changes indicative of myocardial ischaemia). The prognostic value of H-FABP was shown to be independent not only of clinical risk factors and troponin but also other biomarkers such as hsCRP31 and BNP.32 Particular sub-group analyses of patients without elevated troponin in studies by O’Donoghue et al.32 and Kilcullen et al.31 showed that H-FABP was an independent predictor of long-term adverse events among troponin-negative patients. This observation would be potentially consistent with the hypothesis that H-FABP is a marker of myocardial ischaemia. In the study by Kilcullen et al., the occurrence of a negative test result for both TnI and H-FABP was associated with no deaths in the six months following the acute event (see Figure 1), and in the multimarker study by O’Donoghue et al. which evaluated H-FABP, troponin I, and BNP, it was shown that H-FABP provided incremental information for risk stratification regardless of baseline troponin or BNP status (see Figure 2).
Table 2.
Prognostic value of H-FABP in studies recruiting exclusively patients with ‘confirmed’ acute coronary syndrome.
| Author and year of publication | N | H-FABP assay | Clinical setting | MI (%) | Follow-up duration | Biomarkers measured | Key findings |
|---|---|---|---|---|---|---|---|
| Ishii et al. 200544 | 328 | Dainippon | Consecutive CCU admissions, sample on admission | 73.5% | 6 mths | TnT | Increased H-FABP was independently associated with cardiac events (cardiac death or non-fatal MI) RR=9; p=0.0004 |
| Erlikh et al. 200545 | 203 | Hycult | Sample 6 h from symptom onset | NA | 12 mths | TnI, CKMB | Elevated H-FABP was independent predictor of death or non-fatal MI, OR 2.45 95% CI (1.1–5.2); p=0.02 |
| O’Donoghue et al. 200632 | 2287 | In-house (Alere San Diego) | Clinical trial subset, mean time to randomisation = 41h | 55% | 10 mths | TnI, BNP, myoglobin | Elevated H-FABP was an independent predictor of death, recurrent MI, congestive heart failure or the composite of these end points (HR, 1.9; 95% CI, 1.3 to 2.7). In a multimarker approach, H-FABP, TnI, and BNP provided complementary information |
| Kilcullen et al. 200731 | 1448 | Dainippon | Consecutive confirmed ACS, sample 12–24 h from symptoms | 85% | 12 mths | TnI, hsCRP | H-FABP predicts long-term mortality independent of GRACE clinical risk factors, troponin and hsCRP. The adjusted all-cause mortality HR among unstable angina patients (Trop-ve) was 11.35 (95% CI 2.00 to 64.34; p=0.006) |
Figure 1.
The Kaplan-Meier mortality curves in the EMMACE-2 study (Kilcullen N et al. 2007) for patients with either Beckman AccuTnI ≤60 ng/L (unstable angina) or TnI ≤60 ng/L (MI inclusive of ST elevation MI or MI with bundle branch block) according to the H-FABP cut-off value of 5.8 μg/L (Dainippon assay). One-year univariable hazard ratios for each group relative to TnI-ve /H-FABP-ve patients are: TnI+ve/H-FABP-ve 2.38 (95% CI 0.48 to 11.65; p=0.29), TnI-ve/H-FABP+ve 4.93 (95% Confidence Intervals 1.09 to 22.30; p=0.038), and TnI+ve/H-FABP+ve 7.11 (1.71 to 29.64; p=0.007). Reprinted from Kilcullen N, et al. J Am Coll Cardiol 2007;50:2061–7, with permission from Elsevier.
Figure 2.
The occurrence of death, MI, or congestive heart failure observed through 10 months of follow-up stratified by baseline concentration of BNP, troponin I, or H-FABP in the OPUS-TIMI16 trial sub-study. All assays were performed by Biosite Diagnostics, California using in house assays: BNP+ indicates BNP ≥80 ng/L; Tn+, troponin I >1.5 μg/L: H-FABP+, greater than the limit of detection. Redrawn from O’Donoghue et al. Circulation 2006;114:550–7.
Phase IIc: Utility of H-FABP in Patients with Suspected ACS
All the studies discussed above, recruited patients exclusively with confirmed ACS (hence the vast majority of patients were troponin positive), thus excluding low and intermediate risk patients who could potentially benefit the most from accurate risk stratification. It therefore became important to study the clinical utility of H-FABP in a large consecutive cohort of patients with possible ischaemic chest pain to confirm if H-FABP remains as valuable as shown in the above studies among those without troponin elevation. Two such Phase IIc studies have been published.
The first study by McCann et al. reported on the prognostic value of H-FABP measured on admission in 550 patients presenting to a coronary care unit with ischaemic-type chest pain recruited over a period of three years.33 Acute MI, defined as cTnT ≥30 ng/L, with or without electrocardiographic features of ischaemia or infarction, in the absence of any other cause for the chest pain, was diagnosed in 291 of 550 patients. Patients were followed up for one year from the time of admission for the primary outcome measure of death or recurrent MI which occurred in 54 of the 550 patients (9.8%). Significant univariate predictors of death or MI included elevated levels of H-FABP (defined as >5 μg/L measured using the Randox Biochip assay) as well as peak cTnT, NT-pro-BNP, fibrinogen, and D-dimer. When incorporated in a logistic regression model along with clinical risk factors and other biomarker results, elevated H-FABP remained an independent predictor of adverse outcomes, as was elevated peak cTnT and NTpro- BNP. Although the cohort size was moderate, it included only patients admitted to the coronary care unit and therefore consisted of a group with a high proportion of patients diagnosed with MI. Despite this, the study differed from previously published studies in that it did not require a clinical diagnosis of ACS as a criterion for inclusion in the study. The findings of this study were consistent with previously described studies in ACS and confirmed the prognostic value of H-FABP independent of troponin and BNP as described in these earlier studies.
Our FAB study included a consecutive unselected cohort of 1080 patients presenting with chest pain (‘suspected ACS’), all of whom had TnI measured using the sensitive Centaur TnI-Ultra assay (Siemens Healthcare Diagnostics).34 955 patients had H-FABP measured using the Randox Biochip assay on a sample taken 12–24 hours from onset of symptoms. Of these 955 patients, 199 (20.8%) with elevated TnI-Ultra (i.e. above the 99th centile value) were classified as having non-ST-segment elevation MI. After a median follow-up period of 18 months, the primary outcome measure of death or readmission with MI had occurred in 96 of 955 (10.1%) patients. There was a gradient of increasing risk across increasing concentrations of H-FABP (p<0.001). Multivariate regression analyses confirmed that age, previous MI, admission heart rate, and H-FABP concentration remained statistically significant as independent predictors of long-term risk. In the troponin-negative subgroup (n=756), there were 40 major adverse events (death or MI) during the follow-up period. Table 3 shows the event rates across each of the sub-groups based on H-FABP concentration when stratified into patients with or without elevated troponin (MI or not), confirming increasing risk with H-FABP concentration among troponin-negative patients. H-FABP remained an independent predictor of death or MI even after adjustment for age and serum creatinine in this sub-group. Particular strengths of this study were to include an unselected cohort of consecutive patients with suspected ACS and the use of a sensitive Advia TnI-Ultra assay that meets the all-important requirement of achieving adequate assay precision (<10% CV) at or below the 99th centile value, allowing the results to be extrapolated more readily to contemporary clinical practice.
Table 3.
FAB study: Long-term adverse events stratified by H-FABP and TnI results. Reproduced with permission from Viswanathan et al. J Am Coll Cardiol 2010;55:2590–8.
| H-FABP concentration | Trop <50 ng/L | Trop ≥50 ng/L | Total |
|---|---|---|---|
| Group 1 = 0.15–3.26 μg/L (n=635) | 16/573 (2.8%) | 8/62 (12.9%) | 24/635 (3.8%) |
| Group 2 = 3.27–6.48 μg/L (n=203) | 14/148 (9.5%) | 10/55 (18.2 %) | 24/203 (11.8%) |
| Group 3 = 6.49–12.77 μg/L (n=63) | 9/32 (28.1%) | 15/31 (48.4%) | 24/63 (38.1 %) |
| Group 4 = 12.78–151 μg/L (n=54) | 1/3 (33.3%) | 23/51 (45.1 %) | 24/54 (44.4%) |
| Entire cohort | 40/756 (5.3%) | 56/199 (28.1 %) | 96/955 (10.1%) |
| N=955 | p<0.001 | p<0.001 | p<0.001 |
Phase III and IV Studies
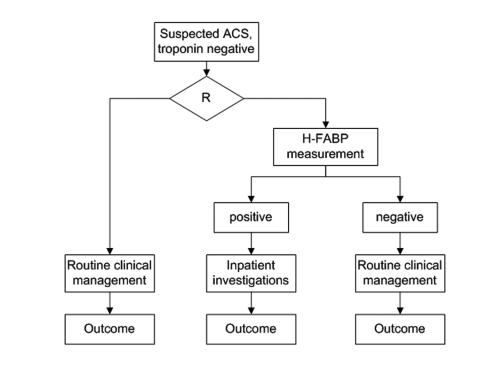
Currently there are no published Phase 3 and Phase 4 studies for H-FABP in ACS. While RCTs are commonly done for therapeutic interventions (medications), they are rarely performed for diagnostic tests. Cardiac troponin was never subjected to an adequately powered RCT and since it is now widely adopted in routine clinical practice and a component of major guidelines, it is not ethical to design such a trial. The most scientifically robust way to demonstrate additive value of novel biomarkers would be in the form of a randomised clinical trial whereby patient management is guided with or without the results of the marker or multimarker panel under evaluation. Figure 3 gives an example of one such way of designing such a trial to test the hypothesis of how the results of a novel biomarker will influence management among patients with suspected ACS who are troponin negative. Such a trial would also allow us to address the cost-effectiveness of a strategy of introducing H-FABP measurement in clinical practice. Currently H-FABP is yet to be widely adopted as a routine test in clinical practice and hence no Phase IV studies available but we expect that this will change in the years to come.
Figure 3.
A hypothetical randomised control trial design for evaluation of H-FABP in guiding clinical management amongst suspected ACS patients who are troponin negative, R = randomisation.
Questions for Future Research
Several unanswered questions need to be addressed in future research studies on H-FABP. Firstly, there is little published data on the clinical utility of serial H-FABP measurements on admission and at six hours post admission in line with the recommendations for measuring troponin as specified in the Universal Definition of MI. The choice of an absolute cut-off value to define elevated H-FABP requires more detailed consideration and serial measurements will require the determination of biological variability to determine significant changes between samples (reference change values). Secondly, age-specific cut-off values for H-FABP have been described in a Japanese population19 but similar data is not available in other ethnic groups. While 99th centile values have been described in Caucasian patients with normal renal function,18 there is little data on how to define ACS defining values of H-FABP in patients with renal impairment. Other sub-groups of patients where H-FABP has been shown to be elevated (patients with chronic heart failure, tachydysrhythmias) would also pose similar challenges. Finally as discussed earlier, future studies should address the cost-effectiveness of a strategy of using H-FABP concentration to focus resources for further investigations and management, preferably in a randomised fashion.
Assays for Measuring H-FABP
Early experimental FABP assays used polyclonal antibodies with relatively modest specificity for specific FABP subtypes. Subsequent assays for H-FABP utilise more specific anti-human H-FABP mouse antibodies and currently all contemporary commercially available H-FABP assays have high specificity with little or no cross-reactivity with other FABP types.35 Three companies manufacture laboratory immunoassays for the measurement of H-FABP in human serum samples - Hycult Biotechnology (Uden, The Netherlands), Dainippon Pharmaceutical (Osaka, Japan) and Randox Laboratories (Antrim, UK). While the first two are ELISA in format, Randox Laboratories use their proprietary Biochip immunoassay and in mid-2011 released an automated H-FABP immunoturbidimetric assay. In addition, two point-of-care tests are also available for the rapid detection of H-FABP: Rapicheck (Dainippon Pharmaceutical, Osaka, Japan) and CardioDetect (Rennesens, Germany).
Conclusion
Studies have now consistently shown that elevated H-FABP is an independent predictor of long-term outcomes among patients with suspected ACS, especially among troponin negative patients, even in conjunction with high sensitivity troponin assays. These troponin-negative patients with elevated H-FABP are at high risk of adverse outcome and their identification would enable physicians to target further investigations and appropriate pharmacotherapy to improve their survival. Commercial H-FABP assays (Randox Immunoturbidimetric Assay) are now capable of rapidly and accurately measuring H-FABP in routine clinical practice and their use is likely to further improve the outcome of patients with ACS. In addition, there is emerging data that the measurement H-FABP may be useful in other clinical settings such as chronic heart failure, pulmonary embolism as well as in the setting of percutaneous coronary intervention and cardiac surgery.
Footnotes
Competing Interests: Julian H Barth and Alistair S Hall have received research grants from Siemens Healthcare, Beckman Coulter and Dainippon for studies of acute coronary syndromes.
References
- 1.Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest. 1955;34:126–31. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 4.Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J, et al. Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51:810–24. doi: 10.1373/clinchem.2004.046292. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–3. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 6.Bodí V, Sanchis J, Llàcer A, Fácila L, Núñez J, Pellicer M, et al. Multimarker risk strategy for predicting 1-month and 1-year major events in non-ST-elevation acute coronary syndromes. Am Heart J. 2005;149:268–74. doi: 10.1016/j.ahj.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Tello-Montoliu A, Marín F, Roldán V, Mainar L, López MT, Sogorb F, et al. A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Intern Med. 2007;262:651–8. doi: 10.1111/j.1365-2796.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- 8.Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res. 1996;35:243–82. doi: 10.1016/s0163-7827(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 9.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 10.Schaap FG, Binas B, Danneberg H, van der Vusse GJ, Glatz JF. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ Res. 1999;85:329–37. doi: 10.1161/01.res.85.4.329. [DOI] [PubMed] [Google Scholar]
- 11.Zschiesche W, Kleine AH, Spitzer E, Veerkamp JH, Glatz JF. Histochemical localization of heart-type fatty-acid binding protein in human and murine tissues. Histochem Cell Biol. 1995;103:147–56. doi: 10.1007/BF01454012. [DOI] [PubMed] [Google Scholar]
- 12.Maatman RG, van de Westerlo EM, van Kuppevelt TH, Veerkamp JH. Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction. Biochem J. 1992;288:285–90. doi: 10.1042/bj2880285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelsers MM, Hanhoff T, Van der Voort D, Arts B, Peters M, Ponds R, et al. Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin Chem. 2004;50:1568–75. doi: 10.1373/clinchem.2003.030361. [DOI] [PubMed] [Google Scholar]
- 14.Glatz JF, van Bilsen M, Paulussen RJ, Veerkamp JH, van der Vusse GJ, Reneman RS. Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988;961:148–52. doi: 10.1016/0005-2760(88)90141-5. [DOI] [PubMed] [Google Scholar]
- 15.Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116:155–62. doi: 10.1007/BF01270583. [DOI] [PubMed] [Google Scholar]
- 16.Wodzig KW, Pelsers MM, van der Vusse GJ, Roos W, Glatz JF. One-step enzyme-linked immunosorbent assay (ELISA) for plasma fatty acid-binding protein. Ann Clin Biochem. 1997;34:263–8. doi: 10.1177/000456329703400307. [DOI] [PubMed] [Google Scholar]
- 17.Gluud C, Gluud LL. Evidence based diagnostics. BMJ. 2005;330:724–6. doi: 10.1136/bmj.330.7493.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bathia DP, Carless DR, Viswanathan K, Hall AS, Barth JH. Serum 99th centile values for two heart-type fatty acid binding protein assays. Ann Clin Biochem. 2009;46:464–7. doi: 10.1258/acb.2009.009055. [DOI] [PubMed] [Google Scholar]
- 19.Niizeki T, Takeishi Y, Takabatake N, Shibata Y, Konta T, Kato T, et al. Circulating levels of heart-type fatty acid-binding protein in a general Japanese population: effects of age, gender, and physiologic characteristics. Circ J. 2007;71:1452–7. doi: 10.1253/circj.71.1452. [DOI] [PubMed] [Google Scholar]
- 20.Pelsers MM, Chapelle JP, Knapen M, Vermeer C, Muijtjens AM, Hermens WT, et al. Influence of age and sex and day-to-day and within-day biological variation on plasma concentrations of fatty acid-binding protein and myoglobin in healthy subjects. Clin Chem. 1999;45:441–3. [PubMed] [Google Scholar]
- 21.Górski J, Hermens WT, Borawski J, Mysliwiec M, Glatz JF. Increased fatty acid-binding protein concentration in plasma of patients with chronic renal failure. Clin Chem. 1997;43:193–5. [PubMed] [Google Scholar]
- 22.Sorichter S, Mair J, Koller A, Pelsers MM, Puschendorf B, Glatz JF. Early assessment of exercise induced skeletal muscle injury using plasma fatty acid binding protein. Br J Sports Med. 1998;32:121–4. doi: 10.1136/bjsm.32.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G, Schena F, Montagnana M, Salvagno GL, Guidi GC. Influence of acute physical exercise on emerging muscular biomarkers. Clin Chem Lab Med. 2008;46:1313–8. doi: 10.1515/CCLM.2008.250. [DOI] [PubMed] [Google Scholar]
- 24.Wodzig KW, Pelsers MM, van der Vusse GJ, Roos W, Glatz JF. One-step enzyme-linked immunosorbent assay (ELISA) for plasma fatty acid-binding protein. Ann Clin Biochem. 1997;34:263–8. doi: 10.1177/000456329703400307. [DOI] [PubMed] [Google Scholar]
- 25.Glatz JF, van der Vusse GJ, Simoons ML, Kragten JA, van Dieijen-Visser MP, Hermens WT. Fatty acid-binding protein and the early detection of acute myocardial infarction. Clin Chim Acta. 1998;272:87–92. doi: 10.1016/s0009-8981(97)00255-6. [DOI] [PubMed] [Google Scholar]
- 26.Meng X, Ming M, Wang E. Heart fatty acid binding protein as a marker for postmortem detection of early myocardial damage. Forensic Sci Int. 2006;160:11–6. doi: 10.1016/j.forsciint.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Lee HJ, Kim K, Jo YH, Kim TY, Lee JH, et al. Heart-type fatty acid binding protein as an adjunct to cardiac troponin-I for the diagnosis of myocardial infarction. J Korean Med Sci. 2011;26:47–52. doi: 10.3346/jkms.2011.26.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Body R, McDowell G, Carley S, Wibberley C, Ferguson J, Mackway-Jones K. A FABP-ulous ‘rule out’ strategy? Heart fatty acid binding protein and troponin for rapid exclusion of acute myocardial infarction. Resuscitation. 2011;82:1041–6. doi: 10.1016/j.resuscitation.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 29.McMahon CG, Lamont JV, Curtin E, McConnell RI, Crockard M, Kurth MJ, et al. Diagnostic accuracy of heart-type fatty acid-binding protein for the early diagnosis of acute myocardial infarction. Am J Emerg Med. 2011 doi: 10.1016/j.ajem.2010.11.022. (in press) [DOI] [PubMed] [Google Scholar]
- 30.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29:2843–50. doi: 10.1093/eurheartj/ehn363. [DOI] [PubMed] [Google Scholar]
- 31.Kilcullen N, Viswanathan K, Das R, Morrell C, Farrin A, Barth JH, et al. EMMACE-2 Investigators Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50:2061–7. doi: 10.1016/j.jacc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 32.O’Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–7. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 33.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol. 2009;103:22–8. doi: 10.1016/j.amjcard.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Viswanathan K, Kilcullen N, Morrell C, Thistlethwaite SJ, Sivananthan MU, Hassan TB, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55:2590–8. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 35.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Ishii J, Wang JH, Naruse H, Taga S, Kinoshita M, Kurokawa H, et al. Serum concentrations of myoglobin vs human heart-type cytoplasmic fatty acid-binding protein in early detection of acute myocardial infarction. Clin Chem. 1997;43:1372–8. [PubMed] [Google Scholar]
- 37.Glatz JF, Haastrup B, Hermens WT, Zwaan CD, Barker J, McNeil C, et al. Fatty acid-binding protein and the early detection of acute myocardial infarction: The EUROCARDI multicenter trial [Abstract] Circulation. 1997;96:I–215. [Google Scholar]
- 38.Haastrup B, Gill S, Kristensen SR, Jørgensen PJ, Glatz JF, Haghfelt T, et al. Biochemical markers of ischaemia for the early identification of acute myocardial infarction without ST segment elevation. Cardiology. 2000;94:254–61. doi: 10.1159/000047326. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto F, Sohmiya K, Ohkaru Y, Kawamura K, Asayama K, Kimura H, et al. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin Chem Lab Med. 2000;38:231–8. doi: 10.1515/CCLM.2000.034. [DOI] [PubMed] [Google Scholar]
- 40.Ghani F, Wu AH, Graff L, Petry C, Armstrong G, Prigent F, et al. Role of heart-type fatty acid-binding protein in early detection of acute myocardial infarction. Clin Chem. 2000;46:718–9. [PubMed] [Google Scholar]
- 41.Nakata T, Hashimoto A, Hase M, Tsuchihashi K, Shimamoto K. Human heart-type fatty acid-binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology. 2003;99:96–104. doi: 10.1159/000069726. [DOI] [PubMed] [Google Scholar]
- 42.Seino Y, Ogata K, Takano T, Ishii J, Hishida H, Morita H, et al. Use of a whole blood rapid panel test for heart-type fatty acid-binding protein in patients with acute chest pain: comparison with rapid troponin T and myoglobin tests. Am J Med. 2003;115:185–90. doi: 10.1016/s0002-9343(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 43.Alansari SE, Croal BL. Diagnostic value of heart fatty acid binding protein and myoglobin in patients admitted with chest pain. Ann Clin Biochem. 2004;41:391–6. doi: 10.1258/0004563041731565. [DOI] [PubMed] [Google Scholar]
- 44.Ishii J, Ozaki Y, Lu J, Kitagawa F, Kuno T, Nakano T, et al. Prognostic value of serum concentration of heart-type fatty acid-binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clin Chem. 2005;51:1397–404. doi: 10.1373/clinchem.2004.047662. [DOI] [PubMed] [Google Scholar]
- 45.Erlikh AD, Katrukha AG, Trifonov IR, Bereznikova AV, Gratsianskiĭ NA. Prognostic significance of heart fatty acid binding protein in patients with non-ST elevation acute coronary syndrome: results of follow-up for twelve months. Kardiologiia. 2005;45:13–21. [PubMed] [Google Scholar]