Abstract
This study was conducted to correlate blood concentrations of lumefantrine with treatment outcome for patients with Plasmodium falciparum malaria when the drug was given without specific instructions for administration with food. Patients with P. falciparum malaria in the highly endemic state of Orissa, India, were enrolled during 2008 and followed-up for 28 days after admistration of artemether-lumefantrine for three days according to a World Health Organization protocol. Drug concentration in whole blood was determined by using blood spots placed on filter paper on day 7. The technology is suitable for field studies. One hundred percent of the patients had an adequate clinical and parasitological response. These results confirm the efficacy of artemether-lumefantrine in persons from poor tribal communities when given without specific instructions regarding co-administration with food, despite high inter-individual variability in blood concentrations of lumefantrine.
Artemether-lumefantrine (AL) is the most widely used artemisninin-based combination therapy globally for treatment of Plasmodium falciparum malaria.1 The drug has been registered in India since 2006 and co-formulated tablets containing 20 mg of artemether and 120 mg of lumefantrine are available. Polymerase chain reactions have detected uncorrected 28-day cure rates of 94.5% and 98.6% in India for four-dose and six-dose regimens, respectively.2,3
This drug combination is safe and well tolerated.4 Absorption of both components of artemether-lumefantrine is variable; artemether is absorbed rapidly, and lumefantrine absorption depends on its intake with fatty foods, which enhance its bioavailability.5
Malaria is common in populations with low socioeconomic status. In these patients, average intake of dietary fat may be low compared with persons in western countries, which could lead to lower blood levels of lumefantrine because of poor absorption and thus treatment failure. Treatment failure in a malnourished child suspected to be caused by poor absorption was reported by Valecha and others.3 Thus, the present study was conducted to correlate blood concentrations of lumefantrine with treatment outcome when the drug is administered for treatment of uncomplicated P. falciparum malaria without specific instructions for intake with food. Because collection and transport of samples from remote areas limits the determination of drug levels, the technique developed by Blessborn and others6 was used. This method has advantage that the samples can be collected easily in the field and stored up to 4 months at 22°C.
The study was conducted in September 2008 at one site in Sundargarh District, Orissa, India (21°36′–22°32′N, 83°32′–85°22′E), an area to which P. falciparum is endemic. The first-line treatment for malaria in this region is artesunate plus sulfadoxine-pyrimethamine (http://nvbdcp.gov.in).
The study was single arm and open label and approved by the ethics committee of the National Institute of Malaria Research. Informed consent was obtained from all adult participants and from the parents or legal guardians of minors. All patients reporting to the National Institute of Malaria Research with fever or during active survey in the field were examined for malaria parasites by using blood smears. Persons positive for P. falciparum were enrolled. On day of enrollment (day 0), a full clinical examination was conducted (temperature, body weight, and other demographic information). Reassessment for parasitological and clinical parameters was conducted on days 1, 2, 3, 7, 14, 21, and 28. Patients were treated with AL according to body weight, and the full dose was repeated if vomiting occurred within 30 minutes. The drug was supplied by the World Health Organization (WHO) (batch no. X1196; Novartis, Basel, Switzerland). Treatment response was analyzed for early treatment failure, late treatment failure, and adequate clinical and parasitological response (ACPR) according to WHO guidelines.7
For lumefantrine estimation, 100 μL of blood was collected on day 7 by venipuncture and placed on Whatman (Maidstone, United Kingdom) 31ET chr filter paper pre treated with 0.75 M tartaric acid and dried. The blood level of lumefantrine was determined at Dalarna University College, Borlange, Sweden by using high-performance liquid chromatography as described.6
Data were entered into WHO software for Kaplan Meier analysis for calculating cure rates.7 Relationship between age and drug concentration was analyzed by scatter plot. The difference of mean baseline parasitemia were analyzed by log-transformed analysis of variance. Fisher's exact test was used to determine the association between time and drug concentration.
A total of 56 patients with uncomplicated P. falciparum malaria were enrolled in the study (Table 1); five were subsequently lost to follow-up. The ACPR was 100% (95% confidence interval = 93–100%) and parasite clearance at 24, 48 and 72 hours were observed in 39.2%, 92.1%, and 100% of the patients, respectively. The mean parasite clearance time (PCT) was 40.4 hours.
Table 1.
Demographic and clinical characteristics of patients enrolled in an efficacy trial of artemether-lumefantrine, Orissa, Rourkela, India, 2008
| Characteristic | Value |
|---|---|
| No. persons enrolled | 56 |
| Sex ratio (M:F) | 41:15 |
| Geometric mean ± SD age, years (range) | 21.5 ± 13 (2–70) |
| % Patients with fever on day 0 | 100 |
| Geometric mean ± SD temperature (range) | 38.9 ± 0.45 (37.8–39.6) |
| Geometric mean ± SD parasite density/μL (range) | 14,212 ± 30,820 (2,160–98,080) |
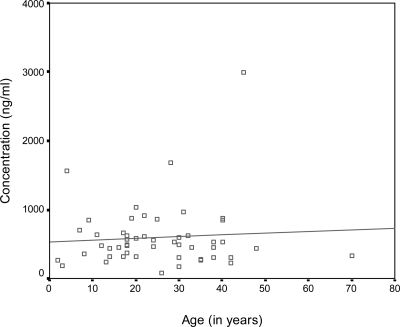
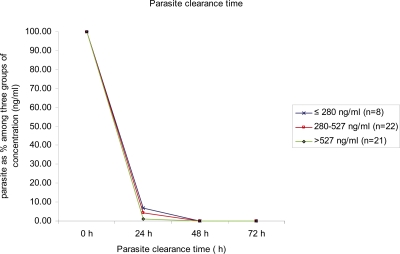
Blood concentration of lumefantrine ranged between 80 and 2,988 ng/mL and was not affected by age (r = 0.07; Figure 1). Although no data on whole blood concentration were available, a cutoff of 280 ng/mL has been used by Ezzet and others.8 Therefore, a comparison was made between groups with high (> 527 ng/mL), medium (281–527 ng/mL), and low (≤ 280 ng/mL) lumefantrine concentrations. There was no statistically significant difference in mean baseline parasitemia (P = 0.867) and PCT (P = 0.244; Figure 2) in these groups.
Figure 1.
Relationship between age and concentration of lumefantrine at day 7 of patients enrolled in the study, Orissa, India, 2008.
Figure 2.
Relationship between parasite clearance time and lumefantrine concentration in patients enrolled in the study, Orissa, India, 2008.
The lumefantrine concentration profile has been reported to be main determinant of efficacy of artemether-lumefantrine.9,10 Lumefantrine absorption and its bioavailability is highly variable and supervised treatment with fatty food is recommended (WHO, 2010).3 However, previous studies have reported that dietary fat intake is not necessarily related to bioavailability of lumefantrine.11–13 In a randomized trial in Uganda, the routine fat intake at home was considered adequate for optimal efficacy.14 In Tanzania, a 97.3% cure rate was reported regardless of food consumption.14
The main limitation of the present study is that a comparison cannot be made with other studies because ratios between plasma and whole blood concentrations have not been reported. However, eight patients with drug levels > 280 ng/mL had an ACPR and a PCT of 48 hours. Parasitologic analysis at 24-hour intervals is another limiting factor in detecting minor difference, if any, between groups.
This study demonstrates the use of a simple technique for estimating lumefantrine concentrations in whole blood in field settings. High efficacy of artemether-lumefantrine was observed, despite inter-individual variation in blood levels of lumefantrine, which was not compromised when treatment was given without specific instructions regarding co-administration with food. It is expected and reported that the food intake, especially fat content, is low in poor tribal communities. Therefore, unless specific efforts are made to supplement food/fat intake with drug, fat intake will range from 2 to 19 g/person/day in such populations compared with a recommended amount of 22 g/person/day.15 These results indirectly suggest that artemether-lumefantrine remains efficacious despite possible low fat intake in this semi-immune population. Because host immunity and high parasite sensitivity to artemether-lumefantrine may be a contributory factor, further studies are warranted among non-immune populations.
ACKNOWLEDGMENTS
We thank the World Health Organization for providing the study drug and the Indian Council of Medical Research and the National Institute of Malaria Research for permitting us to conduct this study.
Footnotes
Authors' addresses: Neena Valecha and Prakriti Srivastava, National Institute of Malaria Research, Indian Council of Medical Research, Sector 8, Dwarka, New Delhi 110077, India, E-mails: neenavalecha@gmail.com and srivastavaprakriti@rediffmail.com. Suman Mohanty, Desert Medicine Research Centre, Indian Council of Medical Research, New Pali Road, Jodhpur, Rajasthan 342005, India, E-mail: ssnimr@gmail.com. Surya Sharma and Prajesh Tyagi, National Institute of Malaria Research, Sector 5, Rourkela, Orissa 769002, India, E-mails: suryaksharma@gmail.com and mrcrkl@dataone.in. Yngve Bergqvist, School of Health and Social Studies, Dalarna University College, S-781 88 Borlänge, Sweden, E-mail: ybq@du.se. Pascal Ringwald, Antimalarial Drug Resistance, Global Malaria Programme, World Health Organization, 20Av. Appia, 1211 Geneva 27, Switzerland, E-mail: ringwaldp@who.int.
References
- 1.Manyando C, Mkandawire R, Puma L, Sinkala M, Mpabalwani E, Njunju E, Gomes M, Ribeiro I, Walter V, Virtanen M, Schlienger R, Cousin M, Chipimo M, Sullivan FM. Safety of artemether-lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar J. 2010;9:249. doi: 10.1186/1475-2875-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kshirsagar NA, Gogtay NJ, Moorthy NS, Garg MR, Dalvi SS, Chogle AR, Sorabjee JS, Marathe SN, Tilve GH, Bhatt AD, Sane SP, Mull R, Gathmann I. A randomized, double blind, parallel group, comparative safety, and efficacy trial of co-artemether versus oral chloroquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in adults in India. Am J Trop Med Hyg. 2000;62:402–408. doi: 10.4269/ajtmh.2000.62.402. [DOI] [PubMed] [Google Scholar]
- 3.Valecha N, Srivastava P, Mohanty SS, Mittra P, Sharma SK, Tyagi PK, Pradhan K, Dev V, Singh R, Dash AP, Sharma YD. Therapeutic efficacy of artemether-lumefantrine in uncomplicated falciparum malaria in India. Malar J. 2009;19:107. doi: 10.1186/1475-2875-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Guidelines for the Treatment of Malaria. Second edition. 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html Available at. [Google Scholar]
- 5.White NJ, Van MV, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Blessborn D, Romsing S, Annerberg A, Sundquist D, Bjorkman A, Lindegardh N, Bergquist Y. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J Pharm Biomed Anal. 2007;45:82–287. doi: 10.1016/j.jpba.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated falciparum Malaria. 2003. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html Available at. [Google Scholar]
- 8.Ezzet F, Vugt MV, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (Benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;45:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price RN, Uhlemann AC, Van VM, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis MB, Tsuyuoka R, Lim P, Lindegardh N, Yi P, Top SN, Socheat D, Fandeur T, Annerberg A, Christophel EM, Ringwald P. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop Med Int Health. 2006;11:1800–1807. doi: 10.1111/j.1365-3156.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 11.Ashley EA, Stepniewska K, Lindegardh N, Annerberg AK, Brockman AL, Singhasivanon P, White NJ, Nosten F. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health. 2007;12:195–200. doi: 10.1111/j.1365-3156.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 12.Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, Babigumira J, Kigozi I, Kiguli J, Kyomuhendo J, Ferradini L, Taylor W, Checchi F, Guthmann JP. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomized trial. Lancet. 2005;365:1467–1473. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 13.Mutabingwa T, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood BM, Whitty CJ. Amodiaquine alone, amodiaquine + sulfadoxine-pyrimethamine, amodiaquine + artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 14.Premji ZG, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I, Mulure N, Nwaiwu O, Kokwaro G. The content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether-lumefantrine: a review of the evidence. Malar J. 2008;25:244. doi: 10.1186/1475-2875-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigam A. Consumption of fat in Indian diet. Int J Diab Dev Countries. 2000;20:58–61. [Google Scholar]