Abstract
Visceral leishmaniasis (VL) is responsible for substantial morbidity and mortality and current available treatments have many limitations. The ability of VL infection to generate life-long immunity offers promise for the development of a VL vaccine. A VL vaccine candidate has recently completed phase I clinical trials. We constructed a computer simulation model to determine the potential economic value of a VL vaccine in the endemic region of Bihar state, India. Results found a potential vaccine to be cost-effective (and in many cases economically dominant, i.e., saving costs and providing health benefits) throughout a wide range of vaccination costs and vaccine efficacies, and VL risks. Overall, our study strongly supports the continued development of a VL vaccine.
Introduction
Although visceral leishmaniasis (VL) infection causes substantial global morbidity and mortality, ∼2.1 million disability adjusted life years (DALYs) annually, currently available treatment strategies are associated with various limitations. 1–4 Over 90% of the world's cases occur in five countries, India, Nepal, Bangladesh, Sudan, and Brazil, with at least half arising in India, of which the eastern rural state of Bihar (90% of all Indian cases) is particularly afflicted. 5,6 India and its neighboring countries of Nepal and Bangladesh are committed to fighting VL, and in collaboration with the Special Program for Research and Training in Tropical Diseases (TDR) initiative supported by the World Health Organization (WHO) have signed a memorandum of understanding with the ultimate goal of achieving VL elimination by 2015. 7
Current control measures in this region include indoor residual spraying (IRS) and drug treatment. In Bihar state, VL is resistant to the cheapest universal first-line treatment option (pentavalent antimonials); patients are therefore subjected to a more costly and rigorous treatment regimen 2,8,9; additionally, the effect of ongoing vector control efforts on regional disease incidence and transmission have yielded mixed results. 10–12
The ability of a one-time infection to grant lifelong immunity has made the idea of a potential vaccine promising 13; although first-generation whole-killed vaccines have been unsuccessful in clinical trials, second-generation recombinant polyprotein vaccines have shown more promise. Specifically, recombinant vaccine candidate LEISH-111f + MPL-SE (now LEISH-F1), 14 developed by a Bill and Melinda Gates Foundation funded initiative through the Infectious Disease Research Institute at Banaras Hindu University, has recently completed phase I clinical trials in India. The vaccine aims to prevent cases of VL and post-kala-azar dermal leishmaniasis (PKDL), a later emerging effect of infection characterized by skin lesions. 15–19
Although computer models have been used to compare the cost-effectiveness of various treatment strategies, the economic benefit of a vaccine has yet to be evaluated. 2,17 Policy makers, public health officials, scientists, and manufacturers distributing a VL vaccine in Bihar state and the surrounding endemic regions could benefit greatly from an analysis of the cost-effectiveness of the introduction of a vaccine against VL in this region as well as its implications. The construction of a stochastic computer simulation model can help delineate the benefit of vaccination while considering various environmental and vaccine scenarios. Evaluating the potential determinants of the success of a vaccine during its development is beneficial, because results may be used to guide vaccine pricing, distribution, and marketing strategies. 20
Methods
Model structure
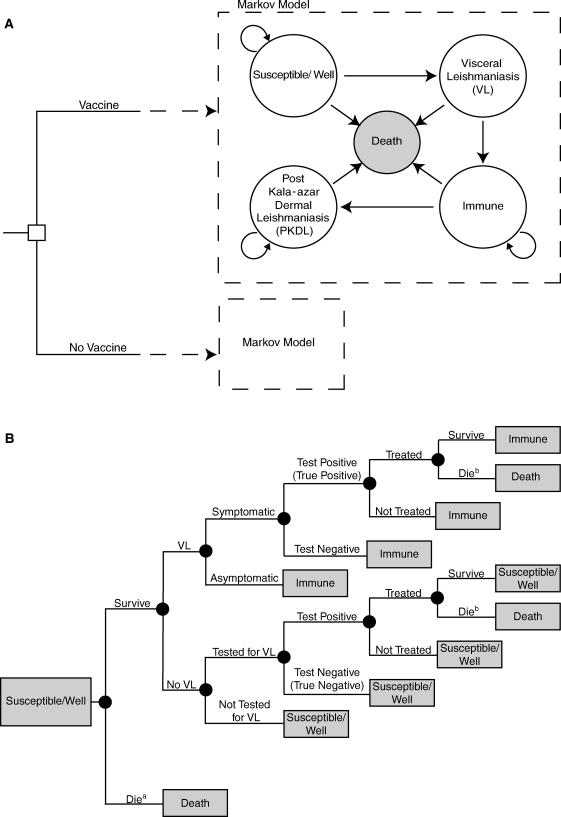
Using TreeAge Pro 2009 (TreeAge Software, Williamstown, MA), we constructed a Markov decision analytic computer simulation model to evaluate the potential cost-effectiveness of the introduction of a vaccine for VL into Bihar state and surrounding endemic regions. The model evaluated the economic value of vaccination from a societal perspective and compared the cost-effectiveness of vaccination in the setting of two different types of available treatments for VL: (1) standard amphotericin B or (2) liposomal amphotericin drug treatments. Figure 1 illustrates the five Markov states within the model:
-
•
Susceptible/Well;
-
•
Immune (natural and vaccine induced);
-
•
Visceral leishmaniasis (VL);
-
•
Post-kala-azar dermal leishmaniasis (PKDL); and
-
•
Death.
Figure 1.
(A) Model Structure and (B) Markov States. Susceptible/Well subtreea death resulting from natural causesb death resulting from drug treatment.
Markov states were mutually exclusive, i.e., an individual could be in only one state at a given time. All individuals entered the model through the “Susceptible” state at age 0. Death could have resulted from treated or untreated VL, standard amphotericin B treatment, or unrelated causes (age-specific crude mortality rate). 2–4,21–23
Those traveling through the “Susceptible and Well” state had the probability of developing VL or remaining uninfected (Figure 1B). Cases could either be symptomatic or asymptomatic. Symptomatic cases were tested for VL infection. These individuals had a probability of seeking treatment if test results were positive. Because the symptoms of VL are non-specific (fever, chills), some people without VL had the possibility of being tested for the disease and subsequently treated because of imperfect test (rk39 dipstick) specificity. 18 Asymptomatic cases were left untreated, were otherwise healthy, and experienced no effects of active infection (i.e., mortality from VL); however, these individuals had a higher probability of developing subsequent PKDL. 2–4 Those in the model who developed PKDL were not treated, because the condition is not directly associated with clinical morbidity. As the maximum duration of active VL reported in the literature is < 2 years, individuals could remain in the “VL” state for only one cycle before continuing on to either the “Death” or “Immune” states. 22–24 Those present in the “Immune” state as a result of vaccination were distinguished from those who had previous infection through a tracker variable. Therefore, only recovered cases that entered the “Immune” state had the probability of developing PKDL up to 2 years after VL infection. 25
Each simulation run sent 1,000 individuals through the model 1,000 times each for the lifetime of the individual, for a total of 1,000,000 individual outcomes. Each simulated individual accrued a distinctive set of costs and utility decrements. For each simulation run, the following equation computed the incremental cost-effectiveness ratio (ICER), or cost per disability-adjusted life year (DALY) avoided, with administration of vaccine:
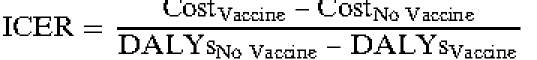 |
For each scenario, vaccination was considered highly cost-effective if the resulting ICER was < $287.94 US dollars (US$), or 13,490.85 Indian rupees (INR) per DALY avoided, which is one times the per capita gross domestic product (GDP) in Bihar, which is substantially lower than the rest of India. 26 ICERs between $287.94 (13,490.85 INR) and $863.81 (40,472.09 INR) per DALY avoided (or between one and three times the per capita GDP), suggested that vaccination was cost-effective; ICER greater than $863.81 (40,472.09 INR) per DALY avoided suggested that vaccination was not cost-effective.
Data inputs and base case scenario
Table 1 shows cost, probability, andDummy 28,29,30 DALY model input values and their corresponding sources. The probability of infection was age-specific and can be seen in Table 1. Currently, many of the leading VL vaccine candidates are polyprotein vaccines and do not pose as high a risk of severe adverse reactions as live attenuated formulations. 36 For this analysis, we therefore assumed that a VL vaccine could be safely administered to young children. All individuals entered the model at age 0 and cycled in the model until they ended up in the “Death” state.
Table 1.
Data inputs for model variables
| Variable | Mean | Lower limit | Upper limit | Distribution type | Reference |
|---|---|---|---|---|---|
| Probability | |||||
| Infection risk | |||||
| 0 to 4 years | 0.034 | 0.017 | 0.065 | Triangular | 27 |
| 5 to 14 years | 0.056 | 0.048 | 0.065 | Triangular | 27 |
| 15 to 29 years | 0.029 | 0.023 | 0.036 | Triangular | 27 |
| 30 to 44 years | 0.030 | 0.024 | 0.038 | Triangular | 27 |
| 45 to 59 years | 0.024 | 0.015 | 0.035 | Triangular | 27 |
| 60 years and up | 0.005 | 0.002 | 0.01 | Triangular | 27 |
| PKDL after clinical infection | – | 0.05 | 0.15 | Uniform | 25 |
| PKDL after subclinical infection | – | 0.15 | 0.2 | Uniform | 25 |
| Subclinical infection | 0.50 | 0.19 | 0.80 | Triangular | 45 |
| Mortality (standard Amphotericin B) | 0.003 | – | – | – | 29 |
| Mortality (after treatment) | 0.10 | – | – | – | 3,4,22,23 |
| Mortality (no treatment) | – | 0.75 | 0.95 | Uniform | 30 |
| Rk39 dipstick sensitivity | 0.9 | 0.85 | 0.99 | Triangular | 2,31–33 |
| Rk39 dipstick specificity | 0.94 | 0.90 | 0.99 | Triangular | 2,31–33 |
| Elevated creatinine | 0.4 | – | – | – | 43,44 |
| Cure rate (standard Amphotericin B) | 0.97 | 0.96 | 0.99 | Triangular | 2,34 |
| Cost (US$) | |||||
| Crocin (500 mg) | 0.05 | – | – | – | Expert opinion |
| Rk39 dipstick test | 1.16 | – | – | – | 35 |
| Lost wages (per day)*† | 7.50 | 3.24 | 11.68 | Triangular | 5 |
| Standard Amphotericin B | |||||
| Hospital accommodations (per day) | – | 3.87 | 4.09 | Uniform | 5 |
| Medical workup† | 68.81 | 67.54 | 69.71 | Triangular | 5 |
| Medicine†‡ | 78.42 | 48.65 | 107.02 | Triangular | 5 |
| Food (per day)† | 1.34 | 1.15 | 1.76 | Triangular | 5 |
| Transportation† | 11.72 | 8.67 | 16.82 | Triangular | 5 |
| Liposomal amphotericin | |||||
| Treatment and care | 386.61 | 278.68 | 483.97 | Triangular | 17 |
Includes lost wages of patient and attendant during hospital stay.
Reported as a median and interquartile range (IQR).
Standard amphotericin B, medical supplies, and other drugs such as aspirin and antibiotics if necessary.
PKDL = post-kala-azar dermal leishmaniasis.
For the base case scenario, the model used regional costs associated with VL treatment with standard amphotericin B (first line drug for VL in Bihar). 2 Treatment of confirmed cases with standard amphotericin B required a hospital stay of 30 days. 2,37 For example, the cost of standard amphotericin B treatment would be the sum of 30-day hospital accommodations (range: $3.87–4.09×30), food (range: $1.15–1.76×30), and lost wages for the patient and an attendant, assuming a family member accompanied the patient during the hospital stay (range: $3.24–11.68×30), as well as the medical workup (range: $67.54–69.71), medication (range: $48.65–107.02), and transportation (range: $8.67–16.82) costs, which would result in a total treatment cost between $372.66 and $719.45 (17,460.24 and 33,708.39 INRs, respectively). The cost of treatment included transportation costs and lost wages (based on averages from Bihar) for the duration of treatment. 5,38 The base case scenario also used a vaccination cost of $5, as a VL vaccine is not currently marketed, as a conservative estimate of the average cost of incorporating a new vaccine into the Expanded Program on Immunization in this region. 39 Vaccination cost includes the cost of vaccine components, accessories, storage, distribution, labor, and training. 39,40 Higher vaccination costs may reflect the need for additional vaccine storage or transport devices as well as the hiring of additional personnel. A 3% discount rate and an exchange rate of 46.853 rupees to 1 US$ converted costs to 2011 US$. 41,42 Elevated creatinine levels were a possible side effect of receiving standard amphotericin B, and increased hospital stay and treatment duration by 1 week. 43,44
The illness duration was the time between symptom onset and seeking treatment plus the length of inpatient treatment. 2,17,22–24,37 To accommodate for variation in illness duration, a triangular distribution was created using values of 44, 150, and 570 days. 22–24 Data from the WHO provided regional, age-specific crude mortality rates and life expectancies. 21 Disability was measured in DALYs and incorporated age-specific life expectancy and crude mortality rates for India as well as the disability weight assigned to VL by the WHO. 21,45 Individuals who were currently in the VL state acquired a disability weight of 0.243 for the duration that they were in this state; those in the Susceptible/Well, Immune, and PKDL states acquired no disability, whereas in these states, as the Global Burden of Disease gives no disability value for PKDL. 45 A 3% discount rate was applied to convert future DALYs to 2011 values. 46,47
Sensitivity analyses
We also ranged vaccine efficacy (range: 25–75%), minor side effect (headache or local inflammation) probabilities (range: 30–80%), and vaccination costs (range: $5 up to $350). Individuals experiencing vaccine minor side effects were treated with Crocin. To account for varying treatment seeking behavior, additional analyses ranged the probability that cases would seek treatment once diagnosed, from 60% to 90%. 2–4
Although the base case scenarios assumed cases were treated with standard amphotericin B, a secondary analysis assumed that liposomal amphotericin (Ambisome) was used for treatment. 3,17,37 Administration of this drug required a hospital stay of 10 days. Unlike standard amphotericin B, this treatment did not increase an individual's mortality probability.
Results
VL cases averted and PKDL cases avoided
Table 2 shows the effect of varying vaccination costs ($5–$100) and vaccine efficacies (25–75%) on the number of VL and PKDL cases averted, treatment costs averted, DALYs averted, and the ICER (all per 1,000 people vaccinated). These results assume that standard amphotericin B treatment was used and that the probability that leishmaniasis test false positive rate was 5%. Ninety-five percent ranges represent the impact of treatment-seeking behavior and the risk of minor vaccine side effects across the ranges evaluated in the sensitivity analysis. Enhancing the efficacy of the vaccine from 25% to 75% decreased the VL cases by approximately one-half, and increased the VL cases averted by ∼5-fold. The PKDL cases avoided per 1,000 people vaccinated increased by ∼5-fold as vaccine efficacy increased from 25% to 75%. A 75% efficacious vaccine prevented about twice as many VL cases without symptoms as did a 25% efficacious vaccine.
Table 2.
Cost-effectiveness analysis of vaccination assuming baseline infection risk and the use of amphotericin B
| Vaccination cost | $5 | $30 | $100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine efficacy | 25% | 50% | 75% | 25% | 50% | 75% | 25% | 50% | 75% |
| VL cases (per 1,000 people) | |||||||||
| No vaccination | 805* (804–805) | 805 (804–805) | 805 (805–805) | 805 (804–805) | 805 (804–805) | 805 (804–806) | 805 (804–805) | 805 (804–805) | 805 (804–806) |
| Vaccination | 724 (723–725) | 589 (589–590) | 368 (368–369) | 724 (723–725) | 590 (589–590) | 368 (367–368) | 724 (723–725) | 590 (589–590) | 407 (367–653) |
| Cases averted | 81† (80–82) | 216 (215–216) | 437 (436–437) | 81 (80–81) | 215 (215–216) | 437 (436–438) | 81 (80–81) | 215 (215–216) | 398 (152–438) |
| PKDL cases (per 1,000 people) | |||||||||
| No vaccination | 197 (196–198) | 197 (196–199) | 197 (196–198) | 197 (196–198) | 197 (196–199) | 197 (196–199) | 197 (196–199) | 197 (196–199) | 197 (196–199) |
| Vaccination | 177 (176–178) | 144 (143–145) | 90 (89–91) | 177 (176–178) | 144 (143–145) | 90 (89–90) | 177 (176–179) | 144 (143–145) | 99 (89–160) |
| Cases averted | 20 (19–21) | 53 (52–54) | 107 (107–108) | 20 (19–21) | 53 (52–54) | 108 (107–109) | 20 (19–20) | 53 (25–54) | 98 (37–108) |
| Cost, US$ (per 1,000 people) | |||||||||
| No vaccination | 134,131‡ (107,567–160,835) | 133,937 (107,342–160,679) | 134,033 (107,305–160,721) | 134,029 (107,448–160,713) | 133,926 (107,134–160,785) | 134,077 (107,133–160,846) | 134,054 (107,561–160,669) | 134,034 (107,372–160,737) | 137,027 (107,290–160,891) |
| Vaccination | 117,465 (95,111–139,853) | 101,107 (82,042–120,103) | 78,562 (63,930–93,023) | 136,899 (95,113–164,651) | 126,143 (107,061–145,314) | 103,485 (88,827–118,018) | 191,356 (95,113–234,843) | 196,239 (177,025–215,282) | 179,418 (158,843–207,436) |
| Cost averted | 16,666 (12,254–21,203) | 32,829 (25,301–40,586) | 55,470 (42,966–67,867) | −2,870 (−12,199–12,462) | 7,783 (−170–15,873) | 30,592 (18,028–42,998) | −57,301 (−81,914–12,462) | −62,205 (−69,817–−54,505) | −42,391 (−72,904–−27,125) |
| DALYs (per 1,000 people) | |||||||||
| No vaccination | 8,420 (8,208–8,644) | 8,416 (8,221–8,646) | 8,426 (8,213–8,637) | 8,434 (8,198–8,651) | 8,414 (8,198–8,646) | 8,413 (8,188–8,612) | 8,423 (8,215–8,632) | 8,415 (8,202–8,629) | 8,438 (8,168–8,637) |
| Vaccination | 8,296 (8,085–8,507) | 8,091 (7,994–8,243) | 7,767 (7,677–7,848) | 8,300 (8,093–8,509) | 8,060 (7,888–8,227) | 7,764 (7,679–7,864) | 8,297 (8,096–8,482) | 8,072 (7,918–8,232) | 7,826 (7,675–8,215) |
| DALYs averted | 124 (89–174) | 326 (227–426) | 659 (512–799) | 134 (89–183) | 354 (270–447) | 648 (505–777) | 126 (89–167) | 344 (271–456) | 613 (195–830) |
| ICER | Vaccine§ | Vaccine | Vaccine | 126 (20–461) | Vaccine | Vaccine | 757 (445–1,606) | 190 (120–258) | 65 (33–105) |
Mean (95% range). Interval was not listed where both values were equal to the mean after rounding.
Calculations done from the table values may be slightly off because of rounding.
Negative costs are costly, positive costs are cost saving.
“Vaccine” indicates combinations of vaccination cost and efficacy where vaccination was less costly and more effective than no vaccination under all minor side effect and treatment seeking probability conditions explored.
PKDL = post kala-azar dermal leishmaniasis; DALYs = disability adjusted life years; ICER = incremental cost-effectiveness ratio.
Costs averted and DALYs averted
Table 2 shows how the intervention costs averted per 1,000 people vaccinated varied with vaccination efficacy and cost. Positive values represent savings and negative values represent net costs. Cost savings resulted when vaccination cost was≤$5 regardless of vaccine efficacy; vaccination continued to avert costs when vaccination was≤$30 and at least 50% efficacious. The DALYs averted are also displayed in Table 2. As vaccination efficacy increases from 25% to 75%, the DALYs averted increase by about 5-fold.
Cost-effectiveness of vaccination
As Table 2 shows, vaccination dominated (i.e., saved costs and provided health benefits) over no vaccination for all scenarios when vaccination was ≤ $5, and also when the vaccination cost was ≤ $30 and vaccination was at least 50% efficacious. Vaccination was cost-effective (i.e., ICER value of $863.81 per DALY averted or less) for all considered vaccine scenarios (vaccine cost ≤ $100, vaccine efficacy ≤ 25%).
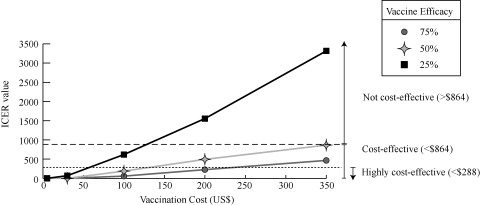
As can be seen in Figure 2, vaccination remained cost-effective across a wide range of costs and efficacies. At 25% vaccine efficacy, vaccination was still cost-effective at a cost of $100 and as indicated by the graph, may continue to be cost-effective at slightly higher price points. When the vaccine was 50% efficacious, vaccination was cost-effective at a cost of ≤ $350, and highly cost-effective when vaccination was ≤ $100. At an efficacy of 75%, vaccination was cost-effective when at a cost of ≤ $350 and highly cost-effective at ≤ $200.
Figure 2.
Impact of vaccination cost and vaccine efficacy on the incremental cost-effectiveness ratio (ICER)* assumes 30% minor vaccine side effect probability, 75% treatment probability, baseline infection risk, and use of amphotericin B for treatment.
Reduced baseline infection risk
Table 3 shows how the VL cases averted, PKDL cases averted, treatment costs averted, DALYs averted, and the ICER varied with different vaccination costs and efficacies when baseline infection risk was decreased by 50%. All other parameters including standard amphotericin B treatment were unchanged from Table 2. Compared with the baseline infection risk scenarios using standard amphotericin B, cost savings again resulted when vaccination cost ≤ $5 regardless of vaccine efficacy. However, with decreased infection risk, cost savings resulted with a vaccination cost of ≤ $30 only when the vaccine was at least 75% efficacious. Vaccination dominated over no vaccination in all situations when vaccination cost was ≤ $5, and also when vaccination was ≤ $30 and ≥ 75% efficacious. Consistent with Table 2, vaccination was cost-effective for all considered scenarios.
Table 3.
Cost-effectiveness analysis of vaccination assuming 50% of baseline infection risk and the use of amphotericin B
| Vaccination cost | $5 | $30 | $100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine efficacy | 25% | 50% | 75% | 25% | 50% | 75% | 25% | 50% | 75% |
| VL cases (per 1,000 people) | |||||||||
| No vaccination | 589* (589–590) | 590 (589–591) | 590 (589–590) | 590 (589–590) | 590 (589–591) | 590 (589–590) | 590 (589–590) | 590 (589–590) | 590 (589–590) |
| Vaccination | 461 (264–493) | 368 (367–369) | 207 (206–208) | 461 (264–494) | 396 (367–494) | 207 (206–207) | 492 (492–493) | 368 (367–368) | 207 (206–207) |
| Cases averted | 129† (96–326) | 222 (221–223) | 383 (382–384) | 129 (96–326) | 194 (96–223) | 383 (382–384) | 97 (97–98) | 222 (221–223) | 383 (382–383) |
| PKDL cases (per 1,000 people) | |||||||||
| No vaccination | 144 (142–145) | 144 (143–145) | 144 (143–145) | 144 (143–145) | 144 (143–145) | 144 (143–145) | 144 (143–145) | 144 (143–145) | 144 (144–145) |
| Vaccination | 113 (64–121) | 90 (89–90) | 50 (50–51) | 113 (65–121) | 97 (89–121) | 50 (50–51) | 120 (120–121) | 90 (89–90) | 50 (50–51) |
| Cases averted | 31 (23–79) | 55 (54–55) | 94 (93–95) | 31 (23–79) | 47 (23–54) | 94 (93–95) | 24 (23–24) | 54 (54–55) | 94 (93–95) |
| Cost, US$ (per 1,000 people) | |||||||||
| No vaccination | 100,314‡ (82,167–123,169) | 102,578 (82,323–122,647) | 102,618 (82,310–122,938) | 100,322 (82,070–122,924) | 102,660 (82,043–123,221) | 102,637 (82,216–123,160) | 102,700 (82,424–123,143) | 102,556 (82,090–122,970) | 102,622 (82,281–123,159) |
| Vaccination | 86,016 (66,052–107,714) | 78,560 (63,883–93,149) | 64,018 (52,207–75,680) | 111,053 (90,979–132,887) | 106,091 (88,931–118,147) | 89,021 (77,367–100,674) | 185,818 (168,704–203,076) | 173,590 (159,215–188,287) | 158,987 (147,382–170,647) |
| Cost averted | 14,298 (8,292–33,813) | 24,018 (17,996–29,655) | 38,600 (29,766–47,555) | −10,731 (−16,925–9,030) | −3,430 (−16,073–5,114) | 13,616 (4,576–22,758) | −83,117 (−86,422–−79,659) | −71,034 (−77,246–−65,107) | −56,365 (−47,447–−65,206) |
| DALYs (per 1,000 people) | |||||||||
| No vaccination | 8,013 (7,873–8,152) | 8,012 (7,868–8,160) | 8,018 (7,870–8,174) | 8,016 (7,885–8,175) | 8,023 (7,866–8,163) | 8,025 (7,881–8,160) | 8,036 (7,915–8,173) | 8,028 (7,895–8,172) | 8,032 (7,902–8,157) |
| Vaccination | 7,876 (7,602–8,043) | 7,763 (7,673–7,871) | 7,578 (7,520–7,637) | 7,878 (7,624–8,027) | 7,802 (7,719–7,931) | 7,574 (7,510–7,617) | 7,922 (7,799–8,061) | 7,768 (7,678–7,852) | 7,578 (7,526–7,632) |
| DALYs averted | 137 (53–407) | 249 (166–329) | 440 (306–555) | 138 (48–394) | 221 (87–350) | 450 (337–548) | 114 (84–135) | 260 (197–329) | 454 (372–537) |
| ICER | Vaccine§ | Vaccine | Vaccine | 156 (61–384) | 9 (Vaccine-45) | Vaccine | 748 (603–1,038) | 285 (198–392) | 129 (88–175) |
Mean (95% range). Interval was not listed where both values were equal to the mean after rounding.
Calculations done from the table values may be slightly off because of rounding.
Negative costs are costly, positive costs are cost saving.
“Vaccine” indicates combinations of vaccination cost and efficacy where vaccination was less costly and more effective than no vaccination under all minor side effect and treatment seeking probability conditions explored.
VL = visceral leishmaniasis; PKDL = post kala-azar dermal leishmaniasis; DALYs = disability adjusted life years; ICER = incremental cost-effectiveness ratio.
Liposomal amphotericin treatment
Analyses were also conducted assuming the use of liposomal amphotericin treatment, because this drug is another potential alternative to first line pentavalent antimonials and may soon become more widely used in this region. Table 4 presents the cost-effectiveness profile for liposomal amphotericin at baseline infection risk and how this is affected by vaccination cost and vaccine efficacy. As expected, vaccination potential to avert unwanted health outcomes (i.e., VL and PKDL cases, DALYs) in this scenario was comparable to scenarios that included standard amphotericin B. The cost of treatment directly impacted the potential value (e.g., cost savings, cost-effectiveness) of a vaccine. For example, as liposomal amphotericin is on average cheaper than standard amphotericin B, and is without the risk of treatment-associated adverse events that could prolong the length of hospital stay, the potential monetary savings of vaccination was less and often resulted in higher costs than not vaccinating. When the vaccine was 25% efficacious, vaccinating was more costly ($6,831–92,694) than not vaccinating even at a vaccination cost of $5. At a $30 vaccination cost, vaccination only averted costs when it was 75% efficacious, and became costly regardless of vaccine efficacy at a vaccination cost of ≥ $100. Like the comparison of amphotericin B to vaccination, vaccination was dominant (less costly and more effective) over liposomal amphotericin at a cost of ≤ $5 regardless of vaccine efficacy. Vaccination required a higher efficacy (75%) to remain dominant at a $30 vaccination cost and remained highly cost-effective when efficacy was at least 25%; a ≥ $100 vaccination cost required at least 50% efficacy for vaccination to be cost-effective and in some cases was highly cost-effective.
Table 4.
Cost-effectiveness analysis of vaccination assuming baseline infection risk and the use of liposomal amphotericin
| Vaccination cost | $5 | $30 | $100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine efficacy | 25% | 50% | 75% | 25% | 50% | 75% | 25% | 50% | 75% |
| VL cases (per 1,000 people) | |||||||||
| No vaccination | 8058* | 805 (804–805) | 805 (804–805) | 805 (804–805) | 805 (804–806) | 805 | 805 (805–806) | 805 (804–806) | 805 (805–806) |
| Vaccination | 724 | 590 (589–591) | 368 (367–368) | 723 (723–724) | 590 (589–591) | 368 (367–369) | 724 (723–725) | 590 | 368 (367–369) |
| Cases averted | 81† | 215 (214–216) | 437 (437–438) | 81 (80–82) | 215 (215–216) | 437 (436–438) | 81 (80–82) | 215 (214–216) | 437 (436–439) |
| PKDL cases (per 1,000 people) | |||||||||
| No vaccination | 196 | 197 (196–199) | 197 (196–199) | 197 (196–198) | 197 (196–199) | 197 (196–199) | 197 (196–198) | 197 (196–199) | 197 (196–199) |
| Vaccination | 176 (176–177) | 144 (143–145) | 90 (89–91) | 177 (176–178) | 144 (143–145) | 90 (89–90) | 177 (176–179) | 144 (143–145) | 90 (89–90) |
| Cases averted | 19 (19–20) | 53 (52–54) | 107 (107–108) | 20 | 53 (52–54) | 107 (106–108) | 20 (19–21) | 53 (53–54) | 107 (107–109) |
| Cost, US$ (per 1,000 people) | |||||||||
| No vaccination | 97,030‡ (77,476–116,790) | 99,013 (77,529–116,380) | 96,889 (77,390–116,288) | 97,120 (77,722–116,814) | 96,890 (77,713–116,140) | 96,840 (77,341–116,307) | 96,762 (77,203–116,159) | 96,855 (77,489–116,116) | 97,048 (77,822–116,324) |
| Vaccination | 92,343 (74,759–110,098) | 80,878 (64,478–94,179) | 61,110 (49,922–72,254) | 117,463 (99,838–134,912) | 104,281 (89,704–119,194) | 86,032 (74,880–97,229) | 187,361 (169,859–204,909) | 174,248 (159,521–188,807) | 156,099 (145,109–167,025) |
| Cost averted | −4,687 (−6,831–−2,663) | 18,135 (13,013–22,390) | 35,779 (27,350–44,035) | −20,343 (−22,418–−18,072) | −7,391 (−12,006–−2,583) | 10,808 (2,434–19,313) | −90,598 (−92,694–−88,674) | −77,393 (−82,177–−72,551) | −59,051 (−67,305–−50,695) |
| DALYs (per 1,000 people) | |||||||||
| No vaccination | 8,393 (8,167–8,613) | 8,414 (8,171–8,608) | 8,390 (8,171–8,624) | 8,395 (8,183–8,604) | 8,389 (8,154–8,607) | 8,371 (8,166–8,577) | 8,394 (8,182–8,609) | 8,378 (8,166–8,585) | 8,387 (8,166–8,612) |
| Vaccination | 8,273 (8,052–8,458) | 8,060 (7,906–8,185) | 7,747 (7,657–7,831) | 8,265 (8,056–8,479) | 8,045 (7,912–8,176) | 7,739 (7,684–7,807) | 8268 (8,040–8,464) | 8047 (7,907–8,177) | 7,743 (7,667–7,831) |
| DALYs averted | 120 (65–155) | 354 (236–472) | 643 (491–820) | 130 (87–177) | 344 (233–445) | 632 (470–792) | 126 (49–182) | 332 (243–425) | 644 (494–822) |
| ICER | Vaccine§ | Vaccine | Vaccine | 169 (106–249) | 25 (6–51) | Vaccine | 887 (489–2,021) | 246 (172–338) | 97 (62–136) |
Mean (95% range). Interval was not listed where both values were equal to the mean after rounding.
Calculations done from the table values may be slightly off because of rounding.
Negative costs are costly, positive costs are cost saving.
“Vaccine” indicates combinations of vaccination cost and efficacy where vaccination was less costly and more effective than no vaccination under all minor side effect and treatment seeking probability conditions explored.
VL = visceral leishmaniasis; PKDL = post kala-azar dermal leishmaniasis; DALYs = disability adjusted life years; ICER = incremental cost-effectiveness ratio.
Discussion
A vaccine preventing VL could have considerable impact on morbidity and mortality, especially when factoring in the lack of widespread access to safe, affordable drug treatment. Such a vaccine could be very cost-effective and in some cases net cost-savings under a wide range of scenarios (e.g., even when vaccination cost, which includes storage, distribution, and administration, is relatively high). Even a modestly efficacious vaccine (e.g., as low as 25%) may provide substantial economic benefit. Although limited data exists regarding incidence of VL in Bihar, we internally validated our model by calculating the expected number of VL cases without vaccination using age-specific incidence and crude mortality rates and compared them to our results. Using this method, the number of expected VL cases was always within three cases per 1,000 of the cases calculated by the model, with the amount of expected cases always being higher than what was observed in the model.
Our model showed that VL treatment type and cost can affect the value of the vaccine. The recently released liposomal formulation has shown promise to overcome many of the limitations associated with standard amphotericin B treatment. 3,48 Because the liposomal formulation is less costly than standard amphotericin B, vaccination is more cost-effective in a setting where standard amphotericin B is used than when liposomal amphotericin treatment is used. In the past, liposomal amphotericin has been too expensive for most to afford. 3 However, a recent negotiation by the WHO for preferential pricing of liposomal amphotericin in India has substantially reduced the cost of this drug and may eventually allow it to replace standard amphotericin B as the standard of care. 17,48 The continued reduction in treatment costs will likely place further emphasis on the importance of thoughtful vaccine pricing by manufacturers.
The introduction of a vaccine may be of exceptional value in locations such as Bihar, where drug resistance to first line pentavalent antimonials has appeared as a result of repeated and long-term use. It has been suggested that resistance could also develop to other leishmanial drug treatments as they are introduced and used over time. This process may also be perpetuated by inappropriate or unmonitored drug use. 49 In light of the growing use of alternatives to first line treatment options, the potential value of a vaccine in locations without current reports of drug resistance may also increase with time and widespread use.
In the absence of a current VL vaccine, IRS with DDT remains the primary method of disease prevention. 10,50 Many IRS studies have demonstrated a decrease in vector density and infection incidence after short-term follow-up. 11 However, other studies reflected little or no change in infection rates and even have suggested that infection rates may not be as dependent upon vector density as previously believed. 10 As with malaria, public health officials question the sustainability of a long-term IRS program. Insecticide-treated bednets are a possible alternative, but bring their own challenges. 50
Although a number of VL vaccine candidates are in various stages of development, no candidate has yet demonstrated efficacy in the field. 13 Previous candidates using antigens such as the Leishmania homologue for the receptor of activated C-kinase have been unsuccessful at preventing infection in animal models. Vaccine candidate LEISH-F1 has completed phase I trials in India and shown potential to be either protective or therapeutic against VL infection. 13,14,18 As with many parasitic and protozoan vaccines under development, even if sterilizing immunity is unattainable, a reduction in infection intensity may prevent a majority of severe outcomes.
Bihar, India, certainly is not the only region that could benefit from a VL vaccine. Vaccination could have a substantial health and economic impact in neighboring endemic regions such as the state of West Bengal, as well as Nepal and Bangladesh, and may be a highly cost-effective addition to the cross-country TDR Visceral Leishmaniasis Elimination initiative. Vaccine could also prevent the spread of disease by a secondary mechanism. As humans may be the only animal reservoir for leishmaniasis in this region, PKDL cases, which result following treated VL infection or (more commonly) untreated asymptomatic VL infection, could contribute substantially to the persistence of disease. 7 Vaccination would reduce both symptomatic and asymptomatic VL cases, including asymptomatic cases that would not seek treatment and could result in PKDL, and thereby would reduce the human reservoir and further facilitate VL reduction in the Indian subcontinent.
Limitations
All computer models incorporate simplifying assumptions and cannot represent all possible outcomes of VL and vaccine introduction. Additionally, no model can account for the population's vast socio-demographic and health status diversity. Our model aimed to be conservative about the economic benefits of a VL vaccine. For example, it does not account for the effect of human immunodeficiency virus (HIV) on Leishmania infection or treatment. Although the prevalence of HIV in Bihar is not as high as in other parts of India, HIV may be an important consideration in other endemic areas. 51 Although model assumptions and data inputs drew from expert consultation and an extensive review of the literature, the sources may vary in quality, and input values may not hold under all conditions.
Conclusions
A VL vaccine could be highly cost-effective (and in many cases economically dominant) under a wide range of conditions. In fact, even a modestly efficacious vaccine could provide substantial value, especially if appropriately priced. This information may be helpful for vaccine developers and manufacturers, policymakers, and other decision makers interested in VL control. Our findings strongly support the continued research and development of a VL vaccine and call for further research into the optimal strategy for vaccine introduction.
Disclaimer: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial support: This study was supported by the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 5U54GM088491-02.
Authors' addresses: Bruce Y. Lee, Kristina M. Bacon, Mirat Shah, Sara Beth Kitchen, Diana L. Connor, and Rachel B. Slayton, Public Health Computational and Operations Research (PHICOR), University of Pittsburgh, Pittsburgh, PA, E-mails: BYL1@pitt.edu, kmb148@pitt.edu, shah.mirat@medstudent.pitt.edu, sbkitchen@verizon.net, dlc56@pitt.edu, and rrb16@pitt.edu.
References
- 1.Hotez PJ, Ferris MT. The antipoverty vaccines. Vaccine. 2006;24:5787–5799. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Vanlerberghe V, Diap G, Guerin PJ, Meheus F, Gerstl S, Van der Stuyft P, Boelaert M. Drug policy for visceral leishmaniasis: a cost-effectiveness analysis. Trop Med Int Health. 2007;12:274–283. doi: 10.1111/j.1365-3156.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 3.Berman JD, Badaro R, Thakur CP, Wasunna KM, Behbehani K, Davidson R, Kuzoe F, Pang L, Weerasuriya K, Bryceson AD. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull World Health Organ. 1998;76:25–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahluwalia IB, Bern C, Costa C, Akter T, Chowdhury R, Ali M, Alam D, Kenah E, Amann J, Islam M, Wagatsuma Y, Haque R, Breiman RF, Maguire JH. Visceral leishmaniasis: consequences of a neglected disease in a Bangladeshi community. Am J Trop Med Hyg. 2003;69:624–628. [PubMed] [Google Scholar]
- 5.Meheus F, Boelaert M, Baltussen R, Sundar S. Costs of patient management of visceral leishmaniasis in Muzaffarpur, Bihar, India. Trop Med Int Health. 2006;11:1715–1724. doi: 10.1111/j.1365-3156.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- 6.Joshi A, Prasittisuk C, Bhatia R, Hashim G, Jorge A, Banjara M, Kroeger A. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–111. [PubMed] [Google Scholar]
- 7.World Health Organization . WHO Special Programme for Research and Training in Tropical Diseases (TDR): research to support the elimination of visceral leishmaniasis. Geneva: WHO; 2007. [Google Scholar]
- 8.Berman J. ABLE: a new and improved amphotericin B for visceral leishmaniasis? Am J Trop Med Hyg. 2009;80:689–690. [PubMed] [Google Scholar]
- 9.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Kesari S, Kumar AJ, Dinesh DS, Ranjan A, Prasad M, Sinha NK, Kumar R, Das P. Vector density and the control of kala-azar in Bihar, India. Mem Inst Oswaldo Cruz. 2009;104:1019–1022. doi: 10.1590/s0074-02762009000700014. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Kesari S, Dinesh DS, Tiwari AK, Kumar AJ, Kumar R, Singh VP, Das P. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): an initiative towards total elimination targeting 2015 (Series-1) J Vector Borne Dis. 2009;46:225–229. [PubMed] [Google Scholar]
- 12.Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. Kathmandu Univ Med J. 2006;4:488–496. [PubMed] [Google Scholar]
- 13.Kedzierski L. Leishmaniasis vaccine: where are we today? J Glob Infect Dis. 2010;2:177–185. doi: 10.4103/0974-777X.62881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagill R, Kaur S. Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol. 2011;11:1464–1488. doi: 10.1016/j.intimp.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Piazza FM. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 17.Olliaro P, Darley S, Laxminarayan R, Sundar S. Cost-effectiveness projections of single and combination therapies for visceral leishmaniasis in Bihar, India. Trop Med Int Health. 2009;14:918–925. doi: 10.1111/j.1365-3156.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 18.Open-Label Safety Study of Three-Antigen Leishmania Polyprotein with Adjuvant MPL-SE in Healthy Adults in India. 2008. http://www.ClinicalTrials.gov Available at. Accessed June 5, 2010. [Google Scholar]
- 19.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010;28:2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . Global Health Observatory: World Health Organization. Geneva: WHO; 2008. [Google Scholar]
- 22.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2:e313. doi: 10.1371/journal.pntd.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahluwalia IB, Bern C, Wagatsuma Y, Costa C, Chowdhury R, Ali M, Amann J, Haque R, Breiman R, Maguire JH. Visceral leishmaniasis: consequences to women in a Bangladeshi community. J Womens Health (Larchmt) 2004;13:360–364. doi: 10.1089/154099904323087024. [DOI] [PubMed] [Google Scholar]
- 24.Bern C, Joshi AB, Jha SN, Das ML, Hightower A, Thakur GD, Bista MB. Factors associated with visceral leishmaniasis in Nepal: bed-net use is strongly protective. Am J Trop Med Hyg. 2000;63:184–188. doi: 10.4269/ajtmh.2000.63.184. [DOI] [PubMed] [Google Scholar]
- 25.Salotra P, Singh R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J Med Res. 2006;123:295–310. [PubMed] [Google Scholar]
- 26.Ministry of Finance Government of India . Economics Survey 2009–2010. 2010. http://indiabudget.nic.in/es2009-10/esmain.htm Available at. Accessed June 3, 2010. [Google Scholar]
- 27.Singh VP, Ranjan A, Topno RK, Verma RB, Siddique NA, Ravidas VN, Kumar N, Pandey K, Das P. Estimation of under-reporting of visceral leishmaniasis cases in Bihar, India. Am J Trop Med Hyg. 2010;82:9–11. doi: 10.4269/ajtmh.2010.09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil EA, Zijlstra EE, Kager PA, El Hassan AM. Epidemiology and clinical manifestations of Leishmania donovani infection in two villages in an endemic area in eastern Sudan. Trop Med Int Health. 2002;7:35–44. doi: 10.1046/j.1365-3156.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 29.Thakur CP, Singh RK, Hassan SM, Kumar R, Narain S, Kumar A. Amphotericin B deoxycholate treatment of visceral leishmaniasis with newer modes of administration and precautions: a study of 938 cases. Trans R Soc Trop Med Hyg. 1999;93:319–323. doi: 10.1016/s0035-9203(99)90037-8. [DOI] [PubMed] [Google Scholar]
- 30.Piscopo TV, Mallia Azzopardi C. Leishmaniasis. Postgrad Med J. 83. 2007:649–657. doi: 10.1136/pgmj.2006.047340corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg. 2009;80:929–934. [PubMed] [Google Scholar]
- 32.Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O'Keeffe C, Davidson RN. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. Am J Trop Med Hyg. 2006;74:76–80. [PubMed] [Google Scholar]
- 33.Sundar S, Singh RK, Maurya R, Kumar B, Chhabra A, Singh V, Rai M. Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test. Trans R Soc Trop Med Hyg. 2006;100:533–537. doi: 10.1016/j.trstmh.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Sundar S, Chatterjee M. Visceral leishmaniasis—current therapeutic modalities. Indian J Med Res. 2006;123:345–352. [PubMed] [Google Scholar]
- 35.Sundar S, Maurya R, Singh RK, Bharti K, Chakravarty J, Parekh A, Rai M, Kumar K, Murray HW. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-K39 antibody. J Clin Microbiol. 2006;44:251–253. doi: 10.1128/JCM.44.1.251-253.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4:5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- 37.Maltezou HC. Drug resistance in visceral leishmaniasis. J Biomed Biotechnol. 2010;2010:(617521) doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnoff R, Desai J, Desjeux P, Mittal A, Topno R, Siddiqui NA, Pandey A, Sur D, Das P. Visceral Leishmaniasis Supplement: The Economic Impact of Visceral Leishmaniasis on Rural Households in One Endemic District of Bihar, India. San Francisco, CA: Institute for OneWorld Health; 2010. [DOI] [PubMed] [Google Scholar]
- 39.Brenzel L, Wolfson LJ, Fox-Rushby J, Miller M, Halsey NA. In: Vaccine-preventable diseases. Disease Control Priorities in Developing Countries. Second edition. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Washington, DC: World Bank, Chap; 2006. p. 20. [PubMed] [Google Scholar]
- 40.Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, Brown ST, Wiringa AE, Burke DS. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28:5292–5300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PACIFIC Exchange Rate Service. Today's Exchange Rates: Thursday, June 3, 2010. Vancouver: Bank of Canada; 2010. [Google Scholar]
- 42.Shepard DS. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. J Ment Health Policy Econ. Vol. 2. New York: Oxford University Press; 1999. pp. 91–92. [Google Scholar]
- 43.Sundar S, Mehta H, Suresh AV, Singh SP, Rai M, Murray HW. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004;38:377–383. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- 44.Singh UK, Prasad R, Jaiswal BP, Singh PK, Thakur CP. Amphotericin B therapy in children with visceral leishmaniasis: daily vs. alternate day, a randomized trial. J Trop Pediatr. 2010;47:749–751. doi: 10.1093/tropej/fmp132. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization . Global Burden of Disease 2004 Update: Disability Weights for Diseases and Conditions. Geneva: World Health Organization; 2004. [Google Scholar]
- 46.World Health Organization . Health statistics and health information systems: Disability weights, discounting and age weighting of DALYs. 2010. http://www.who.int/healthinfo/global_burden_disease/daly_disability_weight/en/index.html Available at. Accessed May 25, 2010. [Google Scholar]
- 47.Lopez AD, Ezzati M, Jamison DT, Murray CJL. Global Burden of Disease and Risk Factors. Washington. DC: The World Bank and Oxford University Press; 2006. [PubMed] [Google Scholar]
- 48.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 49.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, Rijal S, Das P, Rowland M, Sundar S, Coosemans M, Boelaert M, Davies CR. Effect of village-wide use of long-lasting insecticidal nets on visceral leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Negl Trop Dis. 2010;4:e587. doi: 10.1371/journal.pntd.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathur P, Samantaray JC, Vajpayee M, Samanta P. Visceral leishmaniasis/human immunodeficiency virus co-infection in India: the focus of two epidemics. J Med Microbiol. 2006;55:919–922. doi: 10.1099/jmm.0.46574-0. [DOI] [PubMed] [Google Scholar]