Abstract
The Health Post of Corte de Pedra is located in a region endemic for American tegumentary leishmaniasis (ATL) in the Brazilian state of Bahia, and it treats 500–1,300 patients annually. To describe temporal changes in the epidemiology of ATL, we reviewed a random sample of 10% of patient charts (N = 1,209) from 1988 to 2008. There was a twofold increase in the number of cases over the 20-year period, with fluctuations in 10-year cycles. Patients were most frequently male, between the ages of 10 and 30 years, and engaged in agricultural labor; 4.3% of patients had mucosal disease, and 2.4% of patients had disseminated disease. Over the study period, the number of disseminated cases increased threefold, the proportion of cases in younger patients and agricultural workers decreased, and the proportion of patients residing in coastal areas increased. ATL is on the rise in Bahia, with a 10-year periodicity and evolving changes in epidemiology and manifestations of disease.
Introduction
American tegumentary leishmaniasis (ATL) is a vector-transmitted infectious disease caused by an intracellular protozoan of the genus Leishmania. Over 350 million people in 88 countries around the world are at risk of Leishmania infection, with an estimated 1.5 million new cases of cutaneous leishmaniasis occurring annually. 1 In Brazil, ATL represents a significant public health problem, with approximately 30,000 new reported cases annually and an estimated annual incidence of 18.5 cases per 100,000 inhabitants. 2 The three major species that cause ATL in Brazil are Leishmania (Viannia) braziliensis, L. (V.) guyanensis, and L. (L.) amazonensis, which are transmitted by the phlebotomine sandflies of the genus Lutzomyia. 3
ATL is endemic to the Brazilian state of Bahia (BA) in northeastern Brazil, and the village of Corte de Pedra is recognized as one of the most important areas of L. braziliensis transmission in Brazil, with a high prevalence of ATL cases. Visceral leishmaniasis does not occur in this area; in addition to L. braziliensis, L. amazonensis was identified in the village of Corte de Pedra, but only L. braziliensis hase been isolated in the last 15 years in this endemic area. Clinical forms of L. braziliensis infection include localized cutaneous leishmaniasis, mucosal leishmaniasis, and disseminated leishmaniasis. More recently, atypical forms of the disease have been described, such as verrucous lesions and multiple nodular lesions in a specific area of the body. 4 For the past 25 years, patients in this endemic region have been followed at the Health Post of Corte de Pedra. Over the course of the past two decades, clinicians have noted an increase in the number of cases of more severe disease (namely mucosal and disseminated leishmaniasis), a decrease in the efficacy of antimonials, and changes in the demographics of the patient population. 4,5
The last epidemiological study in the region performed in 1985 revealed an annual incidence of disease of 8.1 per 1,000 inhabitants and a prevalence of 14.9%. 6 Peak occurrence of ATL was observed in male patients between 10 and 15 years of age. Mucosal disease was documented in 2.7% of patients and was found to be associated with male gender and multiple or large initial skin lesions. Disseminated disease was not described. Moreover, although in the 1980s, fewer than 200 patients were seen annually in the Corte de Pedra clinic, more than 1,000 leishmaniasis patients presented to the clinic in 2009. Therefore, we decided to perform a retrospective chart review of patients seen at the Health Post of Corte de Pedra during the last 21 years to determine if patterns of disease and risk factors for infection have changed and whether there has been a true increase in the frequency of more severe clinical forms.
Methods
Study area
Corte de Pedra is a village located in the endemic southeastern coastal region of Bahia, 280 km from Salvador, the capital of Bahia. Epidemiological and clinical studies have been performed in this area since 1980. The region covers an area of over 8,000 km2, and it is 15–500 m above sea level, with an estimated population of over 240,000 people according to 2007 Brazilian census data. Ecologically, the area is a tropical rainforest, which has been entirely cleared for cultivation of cacao, banana, and manioc, although in some places, the rainforest has regrown. The annual rainfall in the region is between 1,100 and 2,100 mm annually, and ambient temperature ranges between 18°C and 30°C. The Health Post of Corte de Pedra is the reference center for diagnosis and treatment of leishmaniasis for the 14 municipalities within a 38-km radius of the health post, and it has served as the Immunology Service site for clinical research since 1984. The health post has a 200-m2 building with seven outpatient evaluation rooms, a treatment room/holding unit, a surgical suite for biopsy and lesion aspiration, and a clinical laboratory. The health post is staffed by a physician and four trained medical assistants. Medical doctors from the Immunology Service, including three dermatologists, one internist, one pathologist, and one otolaryngologist, work in the health post on a biweekly basis. Although all of the 14 municipalities surrounding the village have health posts and also see patients with leishmaniasis, 90% of the cases of cutaneous leishmaniasis from these municipalities seek medical assistance at the health post of Corte de Pedra.
Patients and diagnosis of ATL
Between 1988 and 2008, 12,424 individuals who had lesions thought to be attributable to ATL visited the Corte de Pedra leishmaniasis clinic for evaluation and treatment. All patients were evaluated by a physical examination by a physician, and those patients with suspicion of leishmaniasis received a leishmanial antigen skin test. 7 The soluble Leishmania antigen (0.1 mL) was injected intradermally, and a delayed-type hypersensitivity reaction was observed after 48 hours. A positive skin test was defined as a diameter of induration of 5 mm or greater. The diagnosis of cutaneous leishmaniasis was made based on the presence of a typical skin ulcer associated with a positive delayed-type hypersensitivity reaction to Leishmania antigen or evidence of Leishmania infection by parasite isolation or histopathology. Disseminated leishmaniasis was defined by the presence of 10 or more acneiform, popular, and ulcerated lesions in at least two parts of the body. 5 This distinct clinical, histopathological, and immunological entity from the anergic form of diffuse cutaneous leishmaniasis is characterized by the presence of nodular, non-ulcerated lesions without mucosal involvement. 5,8 Moreover, diffuse cutaneous leishmaniasis is associated with L. amazonensis, whereas disseminated leishmaniasis is predominantly caused by L. braziliensis. 5,8,9 Patients with suspected mucosal lesions were evaluated by an otolaryngologist. Diagnosis of mucosal leishmaniasis was performed based on visualization of a typical mucosal leishmaniasis lesion and a positive Leishmania skin test or evidence of Leishmania infection by parasite isolation or histopathology. Patients diagnosed with leishmaniasis are treated with a 20-day course of intravenous pentavalent antimony (15–20 mg/kg per day) for cutaneous leishmaniasis and a 30-day course for mucosal and disseminated leishmaniasis.
The clinic assigned patient file numbers in consecutive order of registration, maintained a registry in central books, and stored clinic files on site. It was, therefore, possible to estimate the number of missing charts, which clinic staff attempted to locate. In the initial years of the clinic, documentation was less controlled, and the removal of paper charts from the clinic premises was common for the purposes of documentation or medical research; however, at the present time, removal of paper charts from the clinic premises is not permitted for any purpose. The total number of charts estimated to be missing was 220, representing between 0.2% and 7.5% patients per year. Total patient numbers include the number of missing charts as well.
Study design
A random number generator was used to select a 10% random sample of registered leishmaniasis clinic patients' files for abstraction. Patients who did not fulfill the diagnostic criteria for leishmaniasis (19 patients; 1.5% of charts) were excluded from the study. No personally identifiable data beyond the study subjects' clinic file number was recorded. This study was approved by the institutional review boards of the Federal University of Bahia and Weill Cornell Medical College.
Data were entered into an Epi Info database by a medical student, who double-checked 100% of data entry. In addition, four medical doctors reviewed the charts to confirm the diagnosis of cutaneous, mucosal, or disseminated disease.
Descriptive data for the study population are reported. Analyses were performed using Epi Info 3.4.3 (Centers for Disease Control and Prevention, Atlanta, GA) and Stata (version 10, StataCorp, College Station, TX). Based on exploratory data analysis of the time periods of interest ranging from months to groups of years, years were grouped into four categories of 5- to 6-year periods for most analyses. Discrete variables were compared using χ2 test or Fisher exact tests as appropriate. Because of data skewness, continuous variables such as age, lesion size, lesion duration, number of lesions, and year of first presentation were grouped into categories and analyzed by a χ2, Fisher exact, Kruskall–Wallis, or non-parametric Jonckeheere–Terpstra test for trend as appropriate. 10,11 All tests were two-tailed, and significance was defined as P < 0.05.
Results
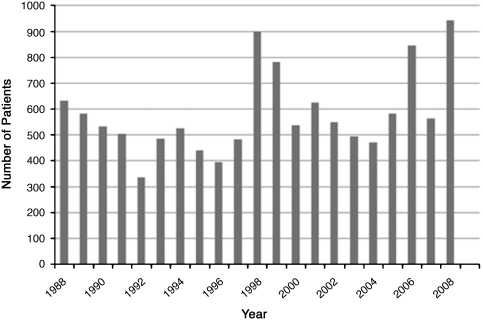
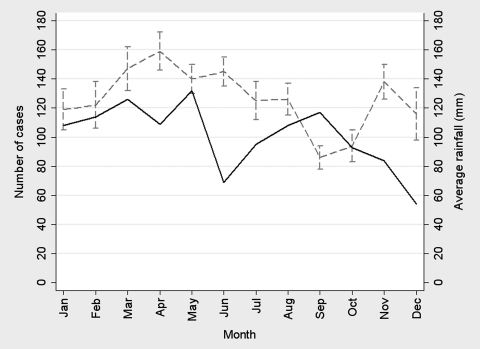
The 1,209 patients included in this study were diagnosed and treated for ATL. Figure 1 depicts the number of Leishmania patients per year from 1988 to 2008. A rise and fall in cases of ATL occurring in 10-year cycles was observed, with peaks in 1988, 1998, and 2008. The median age of the 1,209 patients was 22 years (interquartile range [IQR] = 14–37 years). The greatest number of patients was between 10 and 19 years old (329 patients; 27.2%), whereas patients 50 years and older comprised only 11.2% of the population. The monthly average rainfall and the number of patients per month in Corte de Pedra are shown in Figure 2. A peak in patient presentation was noted in February through April and again in September. When months of presentation were grouped into four seasonal categories, the observed distribution differed from the distribution expected by chance (P < 0.001, χ2 test for goodness of fit); 276 (22.8%) patients presented between December and February, 367 (30.4%) patients presented between March and May, 272 (22.5%) patients presented between June and August, and 294 (24.3%) patients presented between September and November.
Figure 1.
Number of patients presenting to the leishmaniasis clinic in Corte de Pedra from 1988 to 2008.
Figure 2.
Average rainfall and number of patients per month at Corte de Pedra Leishmaniasis Clinic from 1988 to 2008. Rainfall is depicted by the grey dashed line, and the number of cases is depicted by the black solid line.
Demographic and occupational profiles of ATL patients
Table 1 summarizes the demographic and clinical profiles of the patients according to the clinical forms. Cutaneous leishmaniasis was diagnosed in 1,128 cases (91.9%) compared with 52 cases (4.3%) of mucosal leishmaniasis and 29 cases (2.4%) of disseminated leishmaniasis. Older patients were more frequently affected by mucosal and disseminated disease (P < 0.001, Kruskall–Wallis test) as were agricultural workers (P = 0.012, Fisher exact test). The majority of patients visiting the clinic were male (N = 781; 64.6%). Of the patients with cutaneous disease, the male:female ratio was 1.7:1, whereas the ratios for patients with mucosal and disseminated disease were 2.7:1 and 8.7:1, respectively. The predominance of male patients with mucosal and disseminated lesions was statistically significant (P = 0.004, Fisher exact test).
Table 1.
Demographic and clinical characteristics of patients by clinical form of disease
| Cutaneous | Mucosal | Disseminated | P value | |
|---|---|---|---|---|
| N | 1,128 | 52 | 29 | |
| Age, median years (IQR) | 21 (12–35) | 29.5 (18–52) | 36 (22–44) | 0.0002* |
| Male:female ratio | 1.7:1 | 2.7:1 | 8.7:1 | 0.006† |
| Occupation | 0.012ठ| |||
| Agricultural worker | 519 (46.9%) | 35 (67.3%) | 21 (77.8%) | |
| Student | 218 (19.7%) | 6 (11.5%) | 1 (3.7%) | |
| Domestic worker | 153 (13.8%) | 5 (9.6%) | 1 (3.7%) | |
| Not apply | 162 (14.6%) | 5 (9.6%) | 2 (7.4%) | |
| Others (children < 5 and subjects > 60) | 55 (5.0%) | 1 (1.9%) | 2 (7.4%) | |
| Duration of illness, median days (IQR; N) | 30 (28–60; 1,098) | 90 (30–365; 42) | 60 (30–120; 26) | 0.0001* |
| Median size of largest ulcer, mm2 (IQR; N) | 300 (120–560; 1,062) | 509 (340–900; 14) | 600 (220–900; 17) | 0.024* |
| Median number of cutaneous lesions (IQR; N) | 1 (1–2; 1,126) | 1 (1–3; 16) | ¶ | 0.070* |
| Lesions above the waist (%; N) | 433 (38.9; 1,114) | 23 (53.5; 43) | 27 (96.4; 28)⊥ | < 0.0001‡ |
Kruskall–Wallis test.
χ2 test.
Fisher exact test.
Excluding patients without data on occupation.
Was not calculated, because 10 of 26 patients had too many lesions to count.
One patient had no data on lesion location.
Clinical characteristics and clinical form of disease
Of 52 patients with mucosal leishmaniasis, 43 (83%) patients presented with cutaneous lesions or a history of cutaneous disease with a scar. Concomitant cutaneous and mucosal lesions were documented in 16 (31%) of the patients, whereas 14 patients (48%) with disseminated leishmaniasis had concomitant mucosal lesions. Fifty-nine (5.2%) patients with cutaneous leishmaniasis had a history of cutaneous disease with an associated scar, and only one (3.4%) patient with disseminated disease had an associated scar. Median scar duration for patients with cutaneous disease was 9 years (range = 4 months to 74 years). Median scar duration for patients with mucosal disease was 16 years (range = 1–46 years). The patient with disseminated disease had a scar for 1 year.
Lesion location was examined, with the notion that lesions above the waist suggest peridomiciliary infection or may be caused by factors related to the vectors or isolates of L. braziliensis. Of patients with concomitant cutaneous and mucosal lesions, 11 (68.8%) patients had cutaneous lesions above the waist, whereas only 433 (38.9% of patients with cutaneous disease with information about lesion location) patients had lesions above the waist and 27 (96.4% of patients with disseminated disease with information about lesion location) patients had lesions above the waist (P < 0.001, χ2 test). Disseminated patients were excluded from the remaining analysis, because they have lesions above the waist practically by definition. Patients in the youngest age quartile tended to have more lesions above the waist; 153 patients (48.6% of patients between 0 and 13 years) had lesions above the waist, whereas in other age quartiles, 111 (37.5%) patients between ages 14 and 22 years, 85 (31.8%) patients between ages 23 and 37 years, and 95 (37.5%) patients between ages 38 and 95 years had lesions above the waist (P = 0.001, Jonckeheere–Terpstra test). There was no relationship between sex and lesions above the waist (χ2 P = 0.45).
Relationship between lesion size and age, gender, and clinical forms
Table 2 summarizes the relationship between size of the lesion and demographic characteristics of cutaneous leishmaniasis patients. The area of the largest cutaneous lesion was calculated as an indicator of severity of cutaneous disease. The median area of the largest lesion was 300 mm2 (IQR = 120–500 mm2). Lesion size was found to be significantly associated with disease type, because patients with larger lesions were more likely to have mucosal or disseminated disease (P = 0.024, Kruskall–Wallis test) (Table 1). The largest lesion size was directly related to age (P = 0.002, Jonckeheere–Terpstra test) and illness duration (P < 0.0001, Jonckeheere–Terpstra test). No difference in size of largest lesion with respect to gender was noted. Of note, lesion duration was directly related to age. For example, in patients under 14 years, 183 (29.4%) patients had lesion duration of 0–30 days, whereas 45 (18.3%) patients had lesion duration greater than 60 days. In comparison, 135 (21.7%) patients over 37 years had lesion duration of 0–30 days, whereas 75 (30.5%) patients had lesion duration greater than 60 days (P = 0.0001, Jonckeheere–Terpstra test).
Table 2.
Relationship between area of largest cutaneous lesion and characteristics of cutaneous leishmaniasis patients
| Largest lesion size (mm3) | P value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 126 mm2 | Percent | 127–300 mm2 | Percent | 301–600 mm2 | Percent | > 601 mm2 | Percent | ||
| Age (years) | |||||||||
| 0–13 | 95 | 34.5 | 87 | 31.2 | 71 | 25.0 | 51 | 20.0 | 0.002 |
| 14–22 | 64 | 23.3 | 73 | 26.2 | 75 | 26.4 | 73 | 28.6 | |
| 23–37 | 60 | 21.8 | 54 | 19.4 | 73 | 25.7 | 70 | 27.5 | |
| 38–95 | 56 | 20.4 | 65 | 23.3 | 65 | 22.9 | 61 | 23.9 | |
| Sex | |||||||||
| Female | 103 | 37.5 | 81 | 29.0 | 106 | 37.3 | 95 | 37.3 | 0.098 |
| Male | 172 | 62.5 | 198 | 71.0 | 178 | 62.7 | 160 | 62.7 | |
| Duration of illness (days) | |||||||||
| 0–30 | 171 | 63.6 | 159 | 58.0 | 141 | 50.7 | 110 | 43.5 | |
| 31–60 | 66 | 24.5 | 72 | 26.3 | 85 | 30.6 | 74 | 29.2 | < 0.0001 |
| > 60 | 32 | 11.9 | 43 | 15.7 | 52 | 18.7 | 69 | 27.3 | |
χ2 test.
Relationship between number of lesions and age and sex for patients with localized cutaneous leishmaniasis
Table 3 summarizes the number of lesions in 1,128 patients with localized cutaneous leishmaniasis and its relationship with age and sex. Overall, 777 (68.9%) patients had one lesion, 198 (17.6%) patients had two lesions, 77 (6.8%) patients had three lesions, and 74 (6.5%) patients had four or more lesions. There was a statistically significant relationship between age and number of lesions, with younger patients presenting with more lesions (P = 0.027, Jonckeheere–Terpstra test). However, there was no association between the number of cutaneous lesions and sex (P = 0.28, χ2 test).
Table 3.
Relationship between number of lesions, age, and sex in patients with localized cutaneous leishmaniasis
| One lesion | Percent | Two lesions | Percent | Three lesions | Percent | Four+ lesions* | Percent | P value† | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||
| 0–13 | 199 | 25.6 | 57 | 28.8 | 27 | 35.1 | 27 | 36.5 | 0.027 |
| 14–22 | 211 | 27.2 | 52 | 26.3 | 19 | 24.7 | 13 | 17.6 | |
| 23–37 | 176 | 22.7 | 51 | 25.8 | 20 | 26.0 | 18 | 24.3 | |
| 38–95 | 191 | 24.6 | 38 | 19.2 | 11 | 14.3 | 16 | 21.6 | |
| Sex | |||||||||
| Female | 296 | 38.1 | 68 | 34.3 | 22 | 28.6 | 24 | 32.4 | 0.28‡ |
| Male | 481 | 61.9 | 130 | 65.7 | 55 | 71.4 | 50 | 67.6 |
Excluding disseminated patients.
Jonckeheere–Terpstra test.
χ2 P value.
The number of cutaneous lesions was also directly associated with lesion duration (Jonckeheere–Terpstra P = 0.0096). For example, among patients with only one lesion, 430 (56.6%) patients had lesion duration of 0–30 days, 200 (26.3%) patients had lesion duration of 31–60 days, and 130 (17.1%) patients had lesion duration greater than 60 days. In comparison, of patients with four or more lesions, 29 (40.8%) patients had lesion duration of 0–30 days, 27 (38.0%) patients had lesion duration of 31–60 days, and 15 (21.1%) patients had lesion duration greater than 60 days. The number of cutaneous lesions was also associated with more severe clinical disease forms, because patients with mucosal disease tended to have more lesions, although this finding was not statistically significant (Kruskall–Wallis P = 0.070) (Table 1).
Changes in demographic and clinical characteristics over time
Table 4 summarizes the changes in demographic and clinical characteristics over time. An analysis of these data over 21 years of the study revealed a decrease in the proportion of patients in the youngest age quartile of 0–13 years (P = 0.059, Jonckeheere–Terpstra test). No significant differences in the proportion of cases with respect to sex were observed over time. Also notable was the fact that the proportion of patients involved in agricultural work decreased over the study period compared with those patients not engaged in agricultural work (students, domestic workers, young children, and others), although this finding was not statistically significant (P = 0.060, Jonckeheere–Terpstra test).
Table 4.
Changes in demographics, clinical characteristics, and geographic distribution of the disease over time
| 1988–1993 | Percent | 1994–1998 | Percent | 1999–2003 | Percent | 2004–2008 | Percent | P value* | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||
| 0–13 | 99 | 32.1 | 79 | 29.2 | 70 | 23.8 | 79 | 23.4 | 0.059 |
| 14–22 | 69 | 22.4 | 57 | 21.0 | 90 | 30.6 | 87 | 25.9 | |
| 23–37 | 73 | 23.7 | 66 | 24.4 | 63 | 21.4 | 85 | 25.5 | |
| 38–95 | 67 | 21.8 | 69 | 25.5 | 70 | 24.3 | 85 | 25.3 | |
| Sex | |||||||||
| Female | 111 | 36.0 | 100 | 36.9 | 97 | 33.0 | 120 | 35.7 | 0.73 |
| Male | 197 | 64.0 | 171 | 63.1 | 197 | 67.0 | 216 | 64.3 | |
| Occupation | |||||||||
| Agriculture | 163 | 53.6 | 128 | 48.3 | 129 | 45.6 | 155 | 46.4 | 0.060 |
| Not agriculture | 141 | 46.4 | 137 | 51.7 | 154 | 54.4 | 179 | 53.6 | |
| Type of disease | |||||||||
| Cutaneous | 275 | 89.3 | 262 | 96.7 | 279 | 94.9 | 312 | 92.9 | 0.21 |
| Mucosal | 28 | 9.1 | 5 | 1.9 | 8 | 2.7 | 11 | 3.3 | |
| Disseminated | 5 | 1.6 | 4 | 1.5 | 7 | 2.4 | 13 | 3.9 | |
| Largest lesion area (mm2) | |||||||||
| 1–126 | 53 | 19.3 | 57 | 20.7 | 78 | 28.4 | 87 | 31.6 | < 0.001 |
| 127–300 | 41 | 14.7 | 68 | 24.4 | 81 | 29.0 | 89 | 31.9 | |
| 301–600 | 98 | 34.5 | 61 | 21.5 | 56 | 19.7 | 69 | 24.3 | |
| 601–770 | 89 | 34.9 | 64 | 25.1 | 53 | 20.8 | 49 | 19.2 | |
| Region | < 0.001 | ||||||||
| I | 176 | 62.9 | 149 | 57.1 | 138 | 49.8 | 153 | 48.7 | |
| II | 46 | 16.4 | 42 | 16.1 | 44 | 15.9 | 33 | 10.5 | |
| III | 58 | 20.7 | 70 | 26.8 | 95 | 34.3 | 128 | 40.8 |
Jonckeheere–Terpstra test.
The frequency of mucosal and disseminated disease varied greatly over time, although these changes were not statistically significant (P = 0.21, Jonckeheere–Terpstra test). Although the percentage of mucosal disease decreased nearly threefold from the beginning to the end of the study, the percentage of cases of disseminated disease doubled over the same period. Over time, the number of patients with larger lesions decreased, and the number of patients with smaller lesions increased (P < 0.001, Jonckeheere–Terpstra test). The percentage of patients with larger lesions (lesion area greater than 600 mm2) fell from 34.9% from 1988 to 1993 to 19.2% from 2004 to 2008. In contrast, the percentage of patients with smaller lesions (lesion area less than 127 mm2) increased from 19.3% from 1988 to 1993 to 31.6% from 2003 to 2008.
Over the course of the study, lesion duration also decreased. For example, from 1988 to 1993, 142 (47.0%) patients had lesion duration of 0–30 days, and 90 (29.8%) patients had lesion duration greater than 60 days. In comparison, from 2004 to 2008, 185 (56.9%) patients had lesion duration of 0–30 days, and 59 (18.2%) patients had lesion duration greater than 60 days (Jonckeheere–Terpstra P = 0.0052).
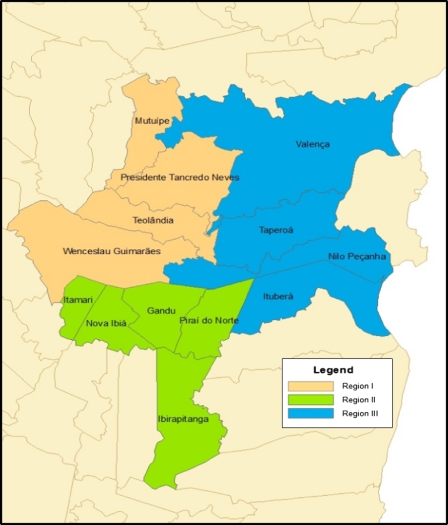
To determine if there was a geographical change in the distribution of cases within the study area over time, the municipalities were grouped into regions based on geographical location for the purposes of analysis. Region I includes the municipalities of Presidente Tancredo Neves, Wenceslau Guimarães, Teolândia, and Mutuipe (average altitude = 198 m). Region II comprises Ibirapitanga, Itamarí, Gandú, and Piraí do Norte (average altitude = 164 m), and Region III includes Valença, Taperoá, Nilo Peçanha, and Ituberá (average altitude = 25 m). Figure 3 shows a map of the study area. Over time, the number of patients from different municipalities also shows a pattern, with an increase in the number of patients residing in coastal areas of lower altitude and a decrease in the number of patients from more inland, higher-altitude areas (P < 0.001, Jonckeheere–Terpstra test) (Table 4).
Figure 3.
Corte de Pedra endemic area. The right side represents the Atlantic Ocean.
Discussion
The southeastern region of the Brazilian state of Bahia, where the village of Corte de Pedra is located, is one of the most significant endemic areas of L. braziliensis in Central and South America. Studies performed in this area determined the incidence of cutaneous and mucosal leishmaniasis, described new clinical forms of L. braziliensis infection, 4,9,12,13 and established new types of therapies for ATL. 13–15 In the present study, we evaluated the charts of the patients presenting to the Health Post of Corte de Pedra from 1988 to 2008 to characterize the changes in demographic and clinical variables over a 20-year period. Seven main relevant findings were observed over the time period of the study. (1) The number of cases of leishmaniasis doubled. (2) Numbers of annual cases of ATL were found to fluctuate in 10-year cycles. (3) The number of cases of disseminated leishmaniasis increased. (4) Mucosal and disseminated leishmaniasis cases were associated with male sex, older age, and agricultural labor. (5) Mucosal leishmaniasis was associated with cutaneous lesions above the waist. (6) Greater numbers of cutaneous lesions were found in younger patients. (7) Over the course of the 20-year study, the disease spread to coastal areas.
Fluctuations in the number of cases of visceral leishmaniasis have been described and associated with earthquakes, viral infections, and climate events. 16–18 Increased incidence of visceral leishmaniasis has been observed with a periodicity of approximately 10-year intervals, with low incidences coinciding with the occurrence of El Niño and increases after such climatic events. 17,18 Here, for the first time, we described a similar pattern of occurrence of cutaneous leishmaniasis. Additional study may reveal an association between the sandfly vector population and El Niño episodes or other weather patterns.
The seasonal pattern of infection observed may be explained by an increase in the number of phlebotomine sand flies in the study region observed during the rainy season, predominantly from March to April and June and July (unpublished data). This increase in the vector population and by correlation, disease frequency after the rainy season has also been described in studies of visceral leishmaniasis. 19–21 The predominance of patients between 10 and 19 years of age working in agricultural activities suggests that patients are infected though work or play activities. However, the fact that women, small children, and the elderly are also affected indicates that transmission also occurs in peridomiciliary areas.
Male sex and older age were strongly associated with disseminated and mucosal leishmaniasis, which has been previously shown. 5,6,22,23 Agricultural activity was also associated with disseminated and mucosal disease. It is known that host and parasite genetic factors may influence clinical manifestations of ATL. 24,25 L. braziliensis is polymorphic in the region of Corte de Pedra, and there is a relationship between genetic differences in isolates and clinical forms of the disease. 26 Because the vectors found in this area are L. whitmani and L. intermedia, it is unlikely that atypical forms of the disease are associated with differences in vector species. It is more likely that patients engaged in agricultural labor may be exposed more frequently to genetic serovars of L. braziliensis that are associated with mucosal and disseminated disease than those patients infected in the peridomiciliary environment. Occupational exposure may help to explain the association between male sex and older age with severe clinical forms of disease, because agricultural workers tend to be adult males.
We confirmed here that lesions above the waist, greater number of cutaneous lesions, history of previous disease with associated scar, and greater duration of disease were risk factors for mucosal leishmaniasis. 6,22–24 However, we found no association between size of the primary cutaneous lesions and development of mucosal leishmaniasis as previously described. 22 Because of the strong association between lesions above the waist and mucosal disease, patients with cutaneous lesions above the waist should be examined for the presence of mucosal disease.
Largest lesion area and number of cutaneous lesions may be markers of disease severity. We show here that age and duration of illness are also associated with lesion size. Patients younger than 13 years with lesion duration fewer than 30 days tended to have smaller lesions. These findings may be related to the fact that children tended to have significantly shorter lesion duration than older patients. It is likely that parents seek medical attention for children promptly, whereas adults delay doctor visits until they discover that their ulcers are not healing. The observation that younger patients tended to have more lesions than patients in the older age groups may be related to greater exposed skin, because they typically wear short pants and are shirtless for long periods of time during the day. There was no relationship between number of lesions and gender or calendar year. Younger patients also were more likely to have lesions above the waist than domestic and other non-agricultural workers, which is likely explained by their shorter stature. The following reasons may explain why children have a low frequency of mucosal disease despite more frequently having lesions above the waist. First, younger age was associated with short duration of illness and prompt therapy. Second, mucosal disease is highly associated with agricultural activities, likely because of exposure to parasite genotypes associated with mucosal involvement. 25,26
The analyses of changes in demographic and clinical characteristics over a 20-year period revealed interesting data. Although the absolute number of children and women presenting with cutaneous leishmaniasis has increased, the proportion of leishmaniasis cases accounted for by children decreased, and there was no difference in the relative proportion of women with disease over time. The presence of the clinic and improvement in accessibility because of development of the region, educational efforts, and word of mouth likely influenced the rise in patients seen at the clinic over time. However, a significant increasing of cases of leishmaniasis in this endemic area has been reported by the Brazilian Ministry of Health in the past 20 years, 2 and it is unlikely to be secondary to improved disease recognition. The leishmania skin test (LST), histologic analysis, and culture of aspirates have been the main diagnostic tests for leishmaniasis since the health post was created. Only in the past few years has polymerase chain reaction (PCR) been used for diagnosis. Therefore, an improvement in diagnostic tests also does not explain the increase in number of cases. Over the course of the study, we observed a decline in the number of mucosal leishmaniasis cases, although it was not statistically significant. We also observed that lesion duration decreased over the course of the study, and we suspect that the presence and improved accessibility to the clinic and consequently, more prompt treatment of cutaneous disease likely influenced the decline in cases of mucosal leishmaniasis, because prompt therapy for cutaneous disease reduces the risk for future mucosal disease. 2 Moreover, the fact that younger patients present with disease of shorter duration and smaller lesion, suggests that there was improvement in accessibility to earlier diagnosis and treatment.
The number of cases in coastal areas (Region III) increased relative to cases in inland, rural areas. Because this change cannot be explained by changes in the population according to census data from 1991, 2001, and 2007, it is also likely that sandfly vectors are adapting to different habitats. In the past, ATL was observed as the result of contact between people and forest environments 27 and later, in agricultural areas that had been previously deforested. 6,28 However, more recently, the disease has been increasingly found in urban areas. 29–31 The fact that the proportion of individuals engaged in agricultural labor decreased over the study period further supports this notion.
In summary, we found that the number of patients presenting to the Corte de Pedra leishmaniasis clinic increased over time but fluctuated in 10-year cycles, which may correspond with El Niño episodes or other meteorological patterns. Additional research regarding this relationship may help develop targeted health interventions regarding prevention of this disease. Although more prompt treatment of leishmaniasis may have helped to reduce the numbers of patients with mucosal disease and larger cutaneous lesions over time, disseminated leishmaniasis is on the rise. Patients residing in coastal areas and people not engaged in agricultural labor are more frequently affected with leishmaniasis than in the past, which suggests that the sandfly vectors are increasingly adapting to peridomiciliary and periurban environments. Lastly, studies evaluating the role of genetic differences in L. braziliensis and its relationship with clinical forms of the disease should be emphasized to better understand its role in the development of different clinical forms of the disease.
ACKNOWLEDGMENTS
The authors thank the dedicated healthcare workers who have tirelessly provided outstanding medical care at the Corte de Pedra field site over the years.
Footnotes
Financial support: This work was supported in part by the Fogarty International Clinical Research Scholars and Fellows (FICRS-F) Program through National Institutes of Health/Fogarty International Center Grant R24 TW007988, National Institutes of Health/National Institute of Allergy and Infectious Disease Grant K24 AI07884, and National Institutes of Health Grant AI-30639.
Authors' addresses: Lara Jirmanus, Department of Family Medicine, Boston University, Boston, MA, E-mail: Lara.Jirmanus@bmc.org. Marshall J. Glesby, Division of Infectious Diseases, Department of Medicine, Weill Cornell Medical College, New York, NY, E-mail: mag2005@med.cornell.edu. Luiz H. Guimarães, Ednaldo Lago, Maria Elisa Rosa, Paulo R. Machado, and Edgar M. Carvalho, Serviço de Imunologia, Hospital Universitário Prof. Edgard Santos, Rua João das Botas, Salvador, Bahia, Brazil, E-mails: luizhenriquesg@ig.com.br, ednaldo-lago@ig.com.br, mariaelisarosa@gmail.com, prlmachado@uol.com.br, and edgar@ufba.br.
Reprint requests: Edgar M. Carvalho, Serviço de Imunologia, Complexo Hospitalar Universitário Prof. Edgard Santos, Universidade Federal da Bahia (UFBA), Rua João das Botas s/n, Canela, 40110-160, BA, Brazil, E-mail: edgar@ufba.br.
References
- 1.World Health Organization . Leishmaniasis. http://www.who.int/leishmaniasis/burden/en Available at. Accessed August 16, 2010. [Google Scholar]
- 2.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Manual de Vigilância da Leishmaniose Tegumentar Americana. 2nd ed. Brasília, Brazil: Editora do Ministério da Saúde; 2007. [Google Scholar]
- 3.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 4.Guimarães LH, Machado PR, Lago EL, Morgan DJ, Schriefer A, Bacellar O, Carvalho EM. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg. 2009;103:712–715. doi: 10.1016/j.trstmh.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Jr, Carvalho EM. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–1834. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- 6.Jones TC, Johnson WD, Jr, Barreto AC, Lago E, Badaro R, Cerf B, Reed SG, Netto EM, Tada MS, Franca TF, Wiese K, Golightly L, Fikrig E, Costa JML, Cuba CC, Marsden PD. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 7.Reed SG, Badaró R, Masur H, Carvalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD, Jr, Jones TC. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–325. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 9.Costa JM, Marsden PD, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, Rosa AC, Cuba CC, Magalhaes AV, Barreto AC. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg. 1986;89:319–323. [PubMed] [Google Scholar]
- 10.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometricka. 1954;41:133–145. [Google Scholar]
- 11.Terpstra TJ. The asymptomatic normality and consistency of Kendall's test against trend, when ties are present in one ranking. Indagationes Mathmaticae. 1952;14:327–333. [Google Scholar]
- 12.Morgan DJ, Guimaraes LH, Machado PR, D'Oliveira A, Jr, Almeida RP, Lago EL, Faria DR, Tafuri WL, Dutra WO, Carvalho EM. Cutaneous leishmaniasis during pregnancy: exuberant lesions and potential fetal complications. Clin Infect Dis. 2007;45:478–482. doi: 10.1086/520017. [DOI] [PubMed] [Google Scholar]
- 13.Almeida RP, Brito J, Machado PL, Jesus AR, Schriefer A, Guimarães LH, Carvalho EM. Successful treatment of refractory cutaneous leishmaniasis with GM-CSF and antimonial. Am J Trop Med Hyg. 2005;73:79–81. [PubMed] [Google Scholar]
- 14.Machado PRL, Lessa H, Lessa M, Guimarães LH, Bang H, Ho JL, Carvalho EM. Oral pentoxifylline combined with pentavalent antimony: a randomized trialfor mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 15.Machado PR, Ampuero J, Guimarães LH, Villasboas L, Rocha AT, Schriefer A, Souza RS, Talhari A, Penna G, Carvalho EM. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dye C, Wolpert DM. Earthakes, influenza and cycles of Indian kala-azar. Trans R Soc Trop Med Hyg. 1988;82:843–850. doi: 10.1016/0035-9203(88)90013-2. [DOI] [PubMed] [Google Scholar]
- 17.Franke CR, Ziller M, Staubach C, Latif M. Impact of the El Niño/Southern Oscillation on visceral leishmaniasis, Brazil. Emerg Infect Dis. 2002;8:914–917. doi: 10.3201/eid0809.010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebêlo JM. El Niño episodes and temporal distribution of kala azar on São Luís island, Maranhão State, Brazil. Cad Saude Publica. 2008;24:1713–1714. doi: 10.1590/s0102-311x2008000700026. [DOI] [PubMed] [Google Scholar]
- 19.Sherlock IA. Ecological interactions of visceral leishmaniasis in the State of Bahia, Brazil. Mem Inst Oswaldo Cruz. 1996;91:671–683. doi: 10.1590/s0074-02761996000600003. [DOI] [PubMed] [Google Scholar]
- 20.França-Silva JC, Barata RA, Costa RT, Monteiro EM, Machado-Coelho GL, Vieira EP, Prata A, Mayrink W, Nascimento E, Fortes-Dias CL, da Silva JC, Dias ES. Importance of Lutzomyia longipalpis in the dynamics of transmission of canine visceral leishmaniasis in the endemic area of Porteirinha Municipality, Minas Gerais, Brazil. Vet Parasitol. 2005;131:213–220. doi: 10.1016/j.vetpar.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Chaniotis BN, Neely JM, Correa MA, Tesh RB, Johnson KM. Natural population dynamics of phlebotomine sandflies in Panama. J Med Entomol. 1971;8:339–352. doi: 10.1093/jmedent/8.4.339. [DOI] [PubMed] [Google Scholar]
- 22.Llanos-Cuentas EA, Marsden PD, Cuba CC, Barreto AC, Campos M. Possible risk factors in development of mucosal lesions in leishmaniasis. Lancet. 1984;2:295. doi: 10.1016/s0140-6736(84)90346-5. [DOI] [PubMed] [Google Scholar]
- 23.Machado-Coelho GL, Caiaffa WT, Genaro O, Magalhaes PA, Mayrink W. Risk factors for mucosal manifestation of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2005;99:55–61. doi: 10.1016/j.trstmh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Weigle K, Saravia NG. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 25.Schriefer A, Guimarães LH, Machado PR, Lessa M, Lessa HA, Lago E, Ritt G, Góes-Neto A, Schriefer AL, Riley LW, Carvalho EM. Geographic clustering of leishmaniasis in northeastern Brazil. Emerg Infect Dis. 2009;15:871–876. doi: 10.3201/eid1506.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schriefer A, Schriefer AL, Góes-Neto A, Guimarães LH, Carvalho LP, Almeida RP, Machado PR, Lessa HA, de Jesus AR, Riley LW, Carvalho EM. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montoya J, Jaramillo C, Palma G, Gomez T, Segura I, Travi B. Report of an epidemic outbreak of tegumentary leishmaniasis in a coffee-growing area of Colombia. Mem Inst Oswaldo Cruz. 1990;85:119–121. doi: 10.1590/s0074-02761990000100022. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JF, Molyneux DH, Birley MH. Deforestation: effects on vector-borne disease. Parasitology. 1993;106:S55–S75. doi: 10.1017/s0031182000086121. [DOI] [PubMed] [Google Scholar]
- 29.Rangel EF, Acevedo AC, Andrade CA, Souza NA, Wermelinger ED. Studies on sandfly fauna (Diptera: Pscyodidae) in a foci of cutaneous leishmaniasis in Mesquita, Rio de Janeiro State, Brazil. Mem Inst Oswaldo Cruz. 1990;85:39–45. doi: 10.1590/s0074-02761990000100006. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira-Neto MP, Pirmez C, Rangel E, Schubach A, Grimaldi JRG. An outbreak of American cutaneous leishmaniasis (Leishmania braziliensis braziliensis) in a periurban area of Rio de Janeiro City, Brazil: clinical and epidemiological studies. Mem Inst Oswaldo Cruz. 1988;83:427–435. doi: 10.1590/s0074-02761988000400006. [DOI] [PubMed] [Google Scholar]
- 31.Passos VM, Falcão AL, Marzochi MCA, Gontijo CM, Dias ES, Barbosa-Santos EG, Guerra HL, Katz N. Epidemiological aspects of American cutaneous leishmaniasis in a periurban area of the metropolitan region of Belo Horizonte, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1993;88:103–110. doi: 10.1590/s0074-02761993000100016. [DOI] [PubMed] [Google Scholar]