Abstract
Molecular characterization of Enterocytozoon bieneusi has led to better understanding of microsporidiosis transmission in humans. This study aimed to detect and genotype E. bieneusi in human immunodeficiency virus (HIV)-infected persons. Stool specimens were collected from 463 HIV-infected patients and analyzed for E. bieneusi by polymerase chain reaction (PCR) and DNA sequence analysis of the internal transcribed spacer. E. bieneusi was detected in 77 HIV patients. CD4 cell counts < 200 cells/μL was associated with E. bieneusi infection (P = 0.09). E. bieneusi was significantly associated with weight loss (P < 0.0001), diarrhea (P = 0.006), fever (P < 0.0001), not being married (P < 0.0001), and flush type of toilet (P = 0.0007). Six known genotypes of D, A, IV, CAF2, EbpA, and Peru 8 in 31, 22, 14, 2, 1, and 1 patients, respectively, five novel genotypes of E. bieneusi, and one infection with mixed genotypes were observed in this study. Three of the novel genotypes were genetically distant to the genotypes commonly found in humans.
Introduction
The phylum Microspora represents more than 1,200 species belonging to over 100 genera. Of them, about 14 species have been identified as human pathogens, with Enterocytozoon bieneusi being the most common one. 1 E. bieneusi is one of the major causes of diarrhea in human immunodeficiency virus (HIV)-infected persons, 2,3 and it has been increasingly found in children with diarrhea or malnutrition. 4 It is also a common parasite in animals. 5 The infective form of E. bieneusi is the resistant spore that is commonly found in ditch and other water bodies. 6 Transmission may be through the fecal–oral route, the oral–oral route, inhalation of aerosols, ingestion of food contaminated with fecal material, 6 or contact with pigs. 7
The use of molecular diagnostic techniques in the characterization of E. bieneusi has led to the identification of extensive genetic diversity of this pathogen in humans and animals and better understanding of the role of animals in the transmission of microsporidiosis in human. 1,5,8 There is a dearth of data on genotype diversity of E. bieneusi in developing countries. Although microsporidiosis has been identified in humans in Nigeria by microscopy, 9 there is no molecular characterization of E. bieneusi in Nigeria. This study was conducted to detect and genotype E. bieneusi in HIV-infected persons in Benin City, Nigeria and identify the risk factors associated with the acquisition of microsporidiosis.
Materials
Study population
The study was carried out between August of 2009 and August of 2010 at the University of Benin Teaching Hospital, Benin City, Nigeria—a teaching hospital with a referral status and a center for the US President's Emergency Plan for AIDS Relief (PEPFAR). A total of 463 HIV patient (134 males and 329 females) adults attending the hospital was enrolled in this study. The age of the study participants ranged from 21 to 70 years. Patients on highly active antiretroviral therapy (HAART) or antiparasitic agents and patients with acquired immunodeficiency syndrome (AIDS)-defining conditions were excluded from this study. A structured questionnaire was administered to collect sociodemographic characteristics of the patients. Informed consent was obtained from all study participants. The study was approved by the Ethics Committee of the University of Benin Teaching Hospital, Benin City, Nigeria.
Sample collection
Stool specimens were collected from each patient at the time of study enrolment. No follow-up of study participants was conducted. The fecal specimens were preserved in 2.5% potassium dichromate and stored at 4°C. Aliquots of the stool specimens were shipped to the laboratory at the Centers for Disease Control and Prevention, Atlanta, GA, for E. bieneusi detection and genotyping.
Methods
Detection of E. bieneusi
On arrival at the Centers for Disease Control and Prevention laboratory, the fecal specimens were washed three times with distilled water by centrifugation. DNA was extracted from the specimens using the FastDNA SPIN kit for soil (BIO 101, Carlsbad, CA). To detect E. bieneusi, each DNA was analyzed in duplicate by a nested polymerase chain reaction (PCR) targeting an approximately 392-bp region of the partial 18S rRNA gene, the entire internal transcribed spacer (ITS), and the partial 5.8S rRNA gene. 10 The PCR products were analyzed by agarose gel electrophoresis and visualized under ultraviolet illumination after ethidium bromide staining.
Nucleotide sequencing
Positive PCR products were purified and sequenced in both directions using the Big Dye Terminator Cycle Sequencing Kit on an ABI 3130 automated sequencer (Applied Biosystem, Foster City, CA). The accuracy of nucleotide sequences was confirmed by sequencing of two independent PCR products. The sequences obtained were aligned with reference sequences using the program ClustalX (http://www.clustal.org/). The extent of genetic diversity and evolutionary relationships among the Enterocytozoon genotypes was assessed by a neighbor-joining analysis of the aligned sequences using the program Treecon (http://bioinformatics.psb.ugent.be/software/details/3). Bootstrap analysis was used to assess the reliability of grouping using 1,000 pseudoreplicates. The established nomenclature system was used in naming E. bieneusi genotypes. 11
Statistical analysis
The frequency data were compared using the χ2 test, and odd ratios (ORs) were calculated for each potential risk factor using the software INSTAT (GraphPad Software Inc., La Jolla, CA).
Results
E. bieneusi was detected in 77 (16.6%) of 463 patients enrolled in this study. A CD4 cell count less than 200 cells/μL was marginally associated with the occurrence of microsporidiosis (OR = 1.8; 95% confidence interval [CI] = 0.9, 3.6; P = 0.089). E. bieneusi infection was significantly associated with weight loss (P < 0.0001), diarrhea (P = 0.006), and fever (P < 0.0001). The age group of 41–50 years (P = 0.021), being unmarried (P < 0.0001), having a high school level of education (P = 0.024), using a stream/river as a source of water (P = 0.011), and having a flush type of toilet (P = 0.0007) were associated with higher E. bieneusi infection rates (Table 1). HIV patients that are traders had the least prevalence of E. bieneusi infection compared with patients with other occupations (P < 0.0001). In contrast, gender (P = 0.984) and animal contact (P = 0.907) had no significant effect on the prevalence of E. bieneusi infections.
Table 1.
Risk factors in the occurrence of E. bieneusi in HIV patients
| Risk factor | No. tested | No. with infection | Infection rates (%) | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Male | 134 | 21 | 15.6 | 0.95 | 0.55, 1.64 | 0.984 |
| Female | 329 | 56 | 17.02 | 1.044 | 0.609, 1.792 | |
| CD4 count | ||||||
| < 200 cells/μL | 54 | 14 | 25.9 | 1.88 | 0.97, 3.66 | 0.089 |
| ≥ 200 cells/μL | 409 | 64 | 15.65 | 0.53 | 0.273, 1.030 | |
| Clinical manifestation | ||||||
| Weight loss | 128 | 63 | 44.22 | 20.677 | 11.088, 38.560 | < 0.0001* |
| Without weight loss | 335 | 15 | 4.48 | 0.048 | 0.026, 0.090 | < 0.0001* |
| Diarrhea | 98 | 26 | 26.53 | 2.174 | 1.272, 3.715 | 0.006* |
| Without diarrhea | 365 | 52 | 4.25 | 0.460 | 0.269, 0.786 | 0.006* |
| Fever | 43 | 21 | 48.84 | 6.079 | 3.142, 11.763 | < 0.0001* |
| Without fever | 420 | 57 | 13.57 | 0.165 | 0.085, 0.318 | < 0.0001* |
| Age (years) | 0.021* | |||||
| 21–30 | 194 | 31 | 15.9 | |||
| 31–40 | 159 | 25 | 15.7 | |||
| 41–50 | 61 | 18 | 29.5 | |||
| 51–60 | 40 | 2 | 5.0 | |||
| ≥ 61 | 09 | 1 | 11.1 | |||
| Level of education | 0.024* | |||||
| No formal education | 10 | 0 | 0.0 | |||
| Primary | 55 | 3 | 5.4 | |||
| High school | 357 | 69 | 19.3 | |||
| Tertiary | 41 | 5 | 12.2 | |||
| Marital status | ||||||
| Single | 22 | 19 | 86.4 | 41.00 | 11.76, 142.90 | < 0.0001* |
| Married | 441 | 59 | 13.38 | 0.244 | 0.007, 0.085 | |
| Source of water | 0.011* | |||||
| Treated (tap) water | 23 | 5 | 21.7 | |||
| Borehole | 410 | 65 | 15.8 | |||
| Well/rain | 24 | 3 | 12.5 | |||
| Stream/river | 6 | 4 | 66.6 | |||
| Animal contact | 25 | 4 | 16.0 | 0.93 | 0.31, 2.81 | 0.907 |
| Occupation | < 0.0001* | |||||
| Civil servant | 36 | 12 | 33.3 | |||
| Businessman/woman | 20 | 8 | 40.0 | |||
| Security officer | 2 | 2 | 100.0 | |||
| Artisan | 30 | 8 | 26.6 | |||
| Trader | 361 | 39 | 10.8 | |||
| Farmer | 5 | 3 | 60.0 | |||
| Housewife | 5 | 3 | 60.0 | |||
| Student | 4 | 2 | 50.0 | |||
| Type of toilet | ||||||
| Pit latrine | 220 | 23 | 10.45 | 0.399 | 0.236, 0.676 | |
| Flush | 243 | 55 | 22.6 | 2.50 | 1.48, 4.24 | 0.0007* |
P < 0.05.
A total of 11 E. bieneusi genotypes were observed in this study. They consisted of six known genotypes and five new genotypes. Genotypes D, A, and IV were the most common ones, being observed in 31, 22, and 14 patients in this study, respectively (Table 2). Other genotypes were mostly found in one patient each. One patient was infected concurrently with genotypes D and IV.
Table 2.
E. bieneusi genotypes in HIV patients in the study
| Genotype | Number of cases | Percent of cases |
|---|---|---|
| Known genotypes | ||
| D | 31 | 40.2 |
| A | 22 | 28.5 |
| IV | 14 | 18.1 |
| CAF 2 | 2 | 2.5 |
| Eebp A | 1 | 1.2 |
| Peru 8 | 1 | 1.2 |
| D + IV | 1 | 1.2 |
| Novel genotypes | ||
| Nig1 (A-like) | 1 | 1.2 |
| Nig2 (IV-like) | 1 | 1.2 |
| Nig3 | 1 | 1.2 |
| Nig4 | 1 | 1.2 |
| Nig5 | 1 | 1.2 |
Genotypes A, D, and IV were found mostly in married patients (18, 27, and 13 patients, respectively), traders (13, 16, and 7 patients, respectively), patients with weight loss (17, 28, and 9 patients, respectively), patients using boreholes as a source of water (20, 26, and 11 patients, respectively), and patients with flush-type toilets (18, 25, and 7 patients, respectively).
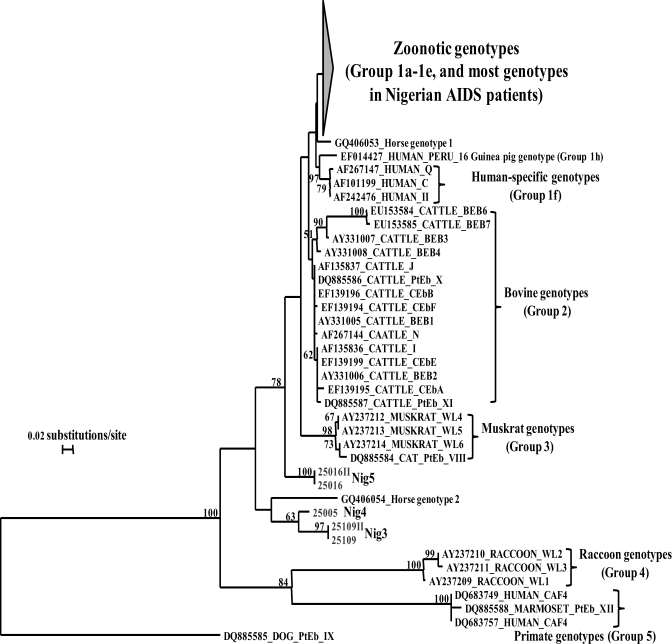
Phylogenetically, most of the study participants were infected with genotypes belonging to the initially described human pathogenic group 10 or the recently renamed Group 1 (Figure 1). 1,12 All six known genotypes and two new genotypes belonged to this group. The latter had one nucleotide difference (G to A substitution) from genotype A or two nucleotide differences (T to C and G to A) from genotype IV, and they were named as Nig1 and Nig2, respectively. The remaining three new genotypes (named as Nig3, Nig4, and Nig5) were genetically different from this group and did not cluster with any of the known E. bieneusi genotype groups (Figure 1). These three divergent new genotypes were recovered from patients with weight loss. Two of the patients with the divergent genotypes had fever, used borehole as source of water, had flush-type toilets, and were civil servants by occupation.
Figure 1.
Phylogenetic relationship among known E. bieneusi genotypes and new genotypes identified in AIDS patients in Nigeria as indicated by a neighbor-joining analysis of the ITS sequence, based on genetic distances calculated by the Kimura 2-parameter model. Bootstrap values greater than 50% from 1,000 replicates are shown on nodes. The group terminology for the clusters are based on Sulaiman et al., 2003 and Thellier and Breton, 2008. Each sequence from GenBank is identified by the accession number, host origin, and the original genotype designation. Genotypes in these studies are designated as Nig1- to Nig5.
Discussion
Microsporidian parasites are increasingly implicated as opportunistic pathogens in HIV patients and other immunosuppressed persons. 8 Little, however, is known about the routes of transmission and risk factors involved in the acquisition of infection. 1 Because E. bieneusi is also commonly found in domestic and wild mammals, 10,13 it was suggested that zoonotic transmission could be potentially important in the transmission of microsporidiosis. 1,10 Indeed, at least one case of zoonotically transmitted E. bieneusi infection has been reported. 14 In this study, however, contact with animals was not identified as a risk factor in E. bieneusi infection, although two of three major genotypes found in this study, D and IV, are common in domestic animals and wild mammals in many areas. 5,11 In a previous study conducted in Peru, contact with duck and/or chicken droppings was associated with an increased risk of microsporidiosis. 15
The 16.6% infection rate of microsporidiosis reported in this study is higher than the 7.3% infection rate reported in northern Nigeria. 9 The difference could be partially because of the fact that light microscopy was used in the study by Omalu and others, 9 whereas PCR was used in this study. Molecular diagnosis with species-specific PCR is now commonly used in identification of microsporidian spp. 16 Light microscopy did not detect any microsporidia in stool specimens of HIV patients in a recent study conducted in the current study location. 17
Microsporidiosis is usually associated with immunocompromised individuals. 6
Indeed, CD4 counts < 200 cells/μL were a risk factor for acquiring microsporidial infection. (OR = 1.8, 95% CI = 0.9, 3.6). However, this factor was not significant (P = 0.089). A CD4 count of < 50 or < 100 cells/μL was associated with microsporidiosis among HIV-positive patients in previous studies. 9,15 The finding that microsporidiosis was associated with weight loss and diarrhea was previously reported. 8,9 To our knowledge, this report is the first associating fever with E. bieneusi infection.
The prevalence of microsporidial infection is higher in HIV-positive patients with a high school level of education. These individuals are more likely to have non-vocational jobs that may expose them to bodies of water and animals, which are known sources of microsporidial infections. 18,19 HIV patients that used streams and rivers as sources of water had significantly (P = 0.011) higher prevalence of microsporidial infections. Bodies of water are known sources of microsporidial infection. 6,14,19 These bodies of water are used as sources for drinking and domestic use, thereby increasing the chances of E. bieneusi infection. Surprisingly, animal contact was not a risk factor for microsporidiosis in this study. In contrast, being unmarried and using a flush-type toilet were significant risk factors for microsporidial infections in this study. The reason for the higher prevalence among HIV patients that used a flush type of toilet is unclear. However, it may be connected with the fact that the water used for flush toilets in our locality is not treated. Also, it may be connected to the unhygienic habit of not washing hands after using the toilet.
A total of 11 E. bieneusi genotypes were detected in this study. Of the six known genotypes, three have been reported from studies in other African countries. 20,21 Genotypes A (28.5%) and CAF2 (2.5%) were slightly lower in prevalence than the genotypes reported in Cameroon (40.0%) 18 and Gabon (6.6%), 19 whereas genotype D (40.2%) had a higher prevalence than the genotype (15.0%) observed in Cameroon. 18 Both genotypes D and IV have a broad host and geographic range.
Two (Nig1 and Nig2; accession numbers JN997477 and JN997478) of five new genotypes in this study are related to genotypes A and IV and other genotypes commonly found in humans, the so-called Group 1 E. bieneusi. Three of the new genotypes, Nig3 (JN997479), Nig4 (JN997480), and Nig5 (JN997481), are placed outside the group in phylogenetic analysis together with other host-adapted E. bieneusi genotypes. Previously, the only E. bieneusi genotype in humans outside Group 1 was CAF4, which was found in a few cases in Gabon and Cameroon. 20 Thus, unusual E. bieneusi genotypes can be human pathogens in some areas. Additional studies are required to identify the source of these and other genotypes of E. bieneusi in HIV-infected patients in Nigeria.
ACKNOWLEDGMENTS
The authors thank the management of University of Benin Teaching Hospital for providing specimens used in this study and Centers for Diseases Control and Prevention, Atlanta, Georgia, for supporting the molecular analysis of the stool samples.
Disclaimer: The findings and conclusions in this report are the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Frederick O. Akinbo, Department of Medical Laboratory Science, School of Basic Sciences, University of Benin, Benin City, Nigeria, E-mail: fgbengang@yahoo.com. Christopher E. Okaka, Department of Animal and Environmental Biology, Faculty of Life Sciences, University of Benin, Benin City, Nigeria, E-mail: drceokaka@yahoo.com. Richard Omoregie, School of Medical Laboratory Sciences, University of Benin Teaching Hospital, Benin City, Nigeria, E-mail: richyomos@yahoo.com. Theressa Dearen, Eucaris Torres Leon, and Lihua Xiao, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Diseases Control and Prevention, Atlanta, GA, E-mails: tdearen@cdc.gov, hrv6@cdc.gov, and lxiao@cdc.gov.
References
- 1.Thellier M, Breton J. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite. 2008;15:349–358. doi: 10.1051/parasite/2008153349. [DOI] [PubMed] [Google Scholar]
- 2.Raccurt CP, Fouche B, Agnamey P, Menotti J, Chouaki T, Totet A, Pape JW. Presence of Enterocytozoon bieneusi associated with intestinal coccidia in patients with chronic diarrhea visiting an HIV center in Haiti. Am J Trop Med Hyg. 2008;79:579–580. [PubMed] [Google Scholar]
- 3.Saksirisampant W, Prownebon J, Saksirisampant P, Mungthin M, Siripatanapipong S, Leelayoova S. Intestinal parasitic infections: prevalences in HIV/AIDS patients in a Thai AIDS-care centre. Ann Trop Med Parasitol. 2009;103:573–581. doi: 10.1179/000349809X12502035776072. [DOI] [PubMed] [Google Scholar]
- 4.Mor SM, Tumwine JK, Naumova EN, Ndeezi G, Tzipori S. Microsporidiosis and malnutrition in children with persistent diarrhea, Uganda. Emerg Infect Dis. 2009;15:49–52. doi: 10.3201/eid1501.071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santin M, Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. 2011;90:363–371. doi: 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin Microbiol Rev. 2005;18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinder H, Thomschke A, Dengjel B, Gothe R, Loscher T, Zahler M. Close genotypic relationship between Enerocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol. 2000;86:185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh K, Weiss LM. Molecular diagnostic tests for microsporidia. Interdiscip Perspect Infect Dis. 2009;2009:(926521) doi: 10.1155/2009/926521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omalu ICJ, Yako AB, Duhlinska DD, Anyanwu GI, Pam VA, Inyama PU. First detection of intestinal microsporidia in northern Nigeria. Online J Health Allied Sci. 2005;4:1–5. [Google Scholar]
- 10.Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, Xiao L. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. 2003;69:4495–4501. doi: 10.1128/AEM.69.8.4495-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santin M, Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J Eukaryot Microbiol. 2009;56:34–38. doi: 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 12.Henriques-Gil N, Haro M, Izquierdo F, Fenoy S, del Aguila C. Phylogenetic approach to the variability of the microsporidian Enterocytozoon bieneusi and its implications for inter- and intrahost transmission. Appl Environ Microbiol. 2010;76:3333–3342. doi: 10.1128/AEM.03026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulaiman IM, Fayer R, Yang C, Santin M, Matos O, Xiao L. Molecular characterization of Enterocytozoon bieneusi in cattle indicates that only some isolates have zoonotic potential. Parasitol Res. 2004;92:328–334. doi: 10.1007/s00436-003-1049-5. [DOI] [PubMed] [Google Scholar]
- 14.Cama VA, Pearson J, Cabrera L, Pacheco L, Gilman R, Meyer S, Ortega Y, Xiao L. Transmission of Enterocytozoon bieneusi between a child and guinea pigs. J Clin Microbiol. 2007;45:2708–2710. doi: 10.1128/JCM.00725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bern C, Kawai V, Vargas D, Rabke-Verani J, Williamson J, Chavez-Valdez R, Xiao L, Sulaiman I, Vivar A, Ticona E, Navincopa M, Cama V, Moura H, Secor WE, Visvesvara G, Gilman RH. The epidemiology of intestinal icrosporidiosis in patients with HIV/AIDS in Lima, Peru. J Infect Dis. 2005;191:1658–1664. doi: 10.1086/429674. [DOI] [PubMed] [Google Scholar]
- 16.Sing A, Tybus K, Heesemann J. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus seronegative liver-transplant recipient with severe chronic diarrhea. J Clin Microbiol. 2001;39:2371–2372. doi: 10.1128/JCM.39.6.2371-2372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinbo FO, Okaka CE, Machado RLD, Omoregie R, Onunu AB. Cryptosporidiosis among HIV-infected patients with diarrhea in Edo State, Midwestern Nigeria. Malays J Microbiol. 2010;6:99–101. [Google Scholar]
- 18.Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop. 2001;78:89–102. doi: 10.1016/s0001-706x(00)00178-9. [DOI] [PubMed] [Google Scholar]
- 19.Slodkowiez-Kowalska A, Graczyk TK, Tamang L, Jedrzejewski S, Nowosad A, Zduniak P, Solarezvk P, Girouard AS, Majewska AC. Microsporidium species known to infect humans are present in aquatic birds: implications for transmission via water? Appl Environ Microbiol. 2006;72:4540–4544. doi: 10.1128/AEM.02503-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton J, Bart-Delabesse E, Biligui S, Carbone A, Seiller X, Okome-Nkoumou M, Nzamba C, Kombila M, Accoceberry I, Thellier M. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. J Clin Microbiol. 2007;45:2580–2589. doi: 10.1128/JCM.02554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espern A, Morio F, Miegeville M, Illa H, Abdoulaye M, Meyssonnier V, Adehossi E, Lejeune A, Dac Cam P, Besse B, Francoise Gay-Andrieu F. Molecular study of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon intestinalis among human immunodeficiency virus-infected patients from two geographical areas: Niamey, Niger, and Hanoi, Vietnam. J Clin Microbiol. 2007;45:2999–3002. doi: 10.1128/JCM.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]