Abstract
In 2000, the Guatemalan Ministry of Health initiated a Chagas disease program to control Rhodnius prolixus and Triatoma dimidiata by periodic house spraying with pyrethroid insecticides to characterize infestation patterns and analyze the contribution of programmatic practices to these patterns. Spatial infestation patterns at three time points were identified using the Getis-Ord Gi*(d) test. Logistic regression was used to assess predictors of reinfestation after pyrethroid insecticide administration. Spatial analysis showed high and low clusters of infestation at three time points. After two rounds of spray, 178 communities persistently fell in high infestation clusters. A time lapse between rounds of vector control greater than 6 months was associated with 1.54 (95% confidence interval = 1.07–2.23) times increased odds of reinfestation after first spray, whereas a time lapse of greater than 1 year was associated with 2.66 (95% confidence interval = 1.85–3.83) times increased odds of reinfestation after first spray compared with localities where the time lapse was less than 180 days. The time lapse between rounds of vector control should remain under 1 year. Spatial analysis can guide targeted vector control efforts by enabling tracking of reinfestation hotspots and improved targeting of resources.
Introduction
Chagas disease (CD) is among the most prevalent neglected diseases in Latin America, with approximately 8–10 million people infected and as many as 15,000 deaths per year.1,2 Caused by the protozoan parasite Trypanosoma cruzi, CD is transmitted primarily by vectors of the subfamily Triatominae that infest homes in poor communities throughout this region. In addition, it is currently estimated that CD is attributed with the loss of about 670,000 disability-adjusted life-years per annum globally.3 The two available treatments for CD, Benznidaznole and Nifurtimox, are associated with frequent adverse effects,4 particularly when administered in adults. Therefore, prevention, most often through vector control, remains the most common public health strategy for addressing this disease.
Local and later, national efforts to control CD began as early as the 1940s, although the most widely recognized regional attempt to control the disease commenced in 1991 when Brazil, Argentina, Chile and Paraguay, Uruguay, and Bolivia formed the Southern Cone Initiative (Iniciativa de Salud del Cono Sur [INCOSUR]).5–8 With the support of this regional collaboration and the Pan American Health Organization (PAHO), a majority of INCOSUR member countries effectively strengthened their national vector control programs, improving house spraying and control of blood-borne transmission as well as diagnosis and treatment initiatives. These strategies resulted in the reduction of disease incidence by 60–95% in each country and the certified interruption of disease transmission by Triatoma infestans, the main vector in South America, in several Southern Cone nations including Brazil, Chile, and Uruguay as well as parts of Paraguay and Argentina.2,8
Using the successful Southern Cone Initiative as a model, the Japan International Cooperation Agency (JICA) partnered with the Guatemalan Ministry of Health (MoH), in collaboration with the PAHO, in establishing a National Chagas Disease Control Project in 2000. The project's primary aim was to implement periodic domestic spraying with pyrethroid insecticides (deltamethrin) in specific areas where the disease was believed to be endemic to decrease infestation. In cross-sectional surveys estimating seroprevalence of disease that were conducted by the Universidad del Valle de Guatemala from 2000 to 2001, it was found that some municipalities had an estimated seroprevalence of CD in children under 14 years of age greater than 20%.9,10 With a national prevalence of 5% as of 2007, it is estimated that approximately 730,000 Guatemalans are infected with CD.11,12 The project aims were to eliminate Rhodnius prolixus and bring under control domestic infestation with T. dimidiata, specifically maintaining the prevalence of intradomiciliary infestation below 5%.11,12 These two vectors have distinct ecological characteristics, although they have similar rates of natural infection.13 The former is a nonnative species that the PAHO officially regards as eliminable from Central America, because they can only survive in domestic settings.8 The latter can live in both the natural environment and domestic settings, making it especially difficult to control and impossible to eliminate. Because R. prolixus is considered eliminable and because evidence suggests that it is a more efficient vector, it has become the priority for vector elimination.8,11 In addition to house spraying, other primary emphases of the program included local capacity building, community education, and community participation.14–16
In 2005, JICA's involvement in the project officially ended, but the project continued under local government administration and with informal technical and financial support from JICA. In 2008, the World Health Organization certified Guatemala for the interruption of transmission by R. prolixus. Although a new official second phase of collaboration between JICA and the MoH was launched in 2009, the long-term work of the initiative has been affected by increasingly scarce resources. There has not yet been an evaluation of the vector control program initiated in 2000 across the entire coverage area, although several previous studies in the departments of Zacapa and Jutiapa have showed the efficacy of one round of spraying in reducing domestic infestation with T. dimidata as well as the continued presence of localities with high infestation indices after several rounds of house spraying in the state of Jutiapa.15,17–19 This evaluation allows for a comprehensive assessment of the efficacy of the program, and it provides crucial information for targeting resources and improving spray operations in the future.
Our objective was to appraise the spatial pattern of vector infestation in the areas targeted by the CD control project at three time points: (1) 2000–2002, a baseline period before any spraying was initiated, (2) 2003–2006, when an assessment was conducted after the first round of house spraying with pyrethroid insecticides, and (3) 2005–2007, when an assessment was conducted after the second round of house spraying with pyrethroid insecticides. These analyses aim to offer concrete recommendations to improve vector control interventions to allow for better planning, monitoring, and evaluation of program activities.
Materials and Methods
Study area
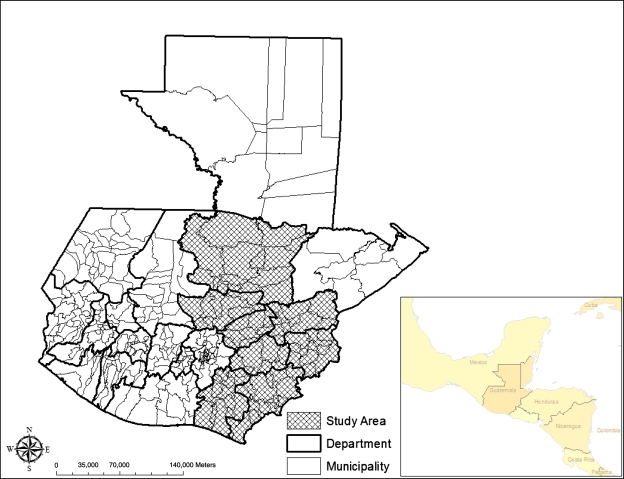
Guatemala is divided into 22 states, commonly referred to as departments, that are subdivided into smaller administrative units called municipalities that contain individual communities called localities. The coverage area of the intervention includes 45% of the departments of Guatemala (Figure 1), a majority of which are located on the eastern side of the country, comprising a population at risk of approximately 4.1 million.11
Figure 1.
Guatemalan National Chagas Disease Control Program coverage area.
Description of spray activities and data on CD
This study used data on domestic infestation from the Guatemalan Chagas Disease Control program as recorded by national and departmental staff of the MoH's Program on Malaria, Dengue and Chagas Disease. The program conducted a baseline survey of vector infestation between 2000 and 2002 in five departments (Zacapa, Jutiapa, Jalapa, Santa Rosa, and Chiquimula) and between 2002 and 2004 in an additional five departments (Alta Verapaz, Baja Verapaz, El Progreso, Quiche, and Huehuetenango). The baseline survey consisted of a search of selected houses with thatched roofs or mud walls distributed throughout the locality. In the majority of localities, between 10 and 20 houses were surveyed, regardless of the total number of houses in the locality. In localities where greater than 5% of houses searched were found to be infested, all houses in the locality with mud walls and/or thatched roofs underwent residual spraying with pyrethroid insecticides. Post-spraying vector survey was performed after both the first and second spray and included search of houses that were previously infested.17
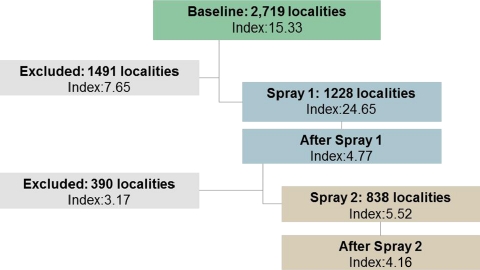
The variables collected by the program included the index of infestation (defined as 100×number of infested houses/number of houses evaluated) observed in each locality,20 the date when spraying occurred, and the date that house evaluation of infestation occurred. Mean indices of infestation at different time points were compared using non-parametric Wilcoxon sign rank tests. Domestic vector infestation was defined by the presence of bugs inside the home or in the peridomestic areas attached to the home. The data were collected for 4,419 localities in 10 departments during the baseline survey, and a subset of these localities was followed-up over the first two rounds of house spraying that occurred between 2002 and 2007. The locality was chosen as the unit of analysis, because no house-level data were available. Localities from two departments, Huehuetenango and Quiche, were excluded, because no geographical coordinates were recorded. The remaining eight departments included 3,987 localities, among which 3,571 had been surveyed at baseline and 2,317 had correct geographical coordinates. It was possible to obtain geographical coordinates for 402 localities based on their name and the information available on the United States Geological Survey's Earth Explorer (http://edcsns17.cr.usgs.gov/EarthExplorer/). These 2,719 localities were then used in the analysis at baseline. However, not all localities were followed up over the course of the intervention because of insufficient resources. Thus, only 1,252 localities were followed up after the first spray, and 950 localities were followed up after the second spray.
We defined reinfestation as having a locality-level index of infestation greater than zero after spray. We then defined the time lapse between spray and evaluation in categories, reflecting the efficacy of the pyrethroid insecticides used by the program as per empirical data available and recommendations made by other CD vector control programs.21,22 As such, we defined these groups as short (time lapse less than 180 days), medium (time lapse of 180–360 days), and long (time lapse of 360 days or more), with the short time lapse group serving as the reference group in our regression models.
In spray 1, 380 (30%) of 1,252 localities were missing information about the time lapse between spray and evaluation, whereas in spray 2, 334 (35%) of 950 localities were missing this information. As such, missing data were imputed using the municipality mean time lapse in days. After this imputation, the first spray was missing time lapse information for only 23 localities, whereas the second spray was missing this time information for 32 localities. In both cases, no time data had been recorded for the municipality. Moreover, 80 localities that were sprayed and evaluated in spray 2 did not have data recorded for spray 1, leaving 1,228 localities that contained all information relevant for inclusion in the regression analysis at spray 1 and 838 localities that contained all relevant information for inclusion in regression analysis at sprays 1 and 2. Finally, after incorporating the season in which spray was administered for the models of reinfestation after spray 1 and reinfestation after spray 2, these models were left with 1,156 localities and 663 localities, respectively.
Statistical analysis
We used the Getis-Ord Gi*(d), a local indicator of spatial autocorrelation, to assess if domestic infestation indicated any clustering pattern across the intervention area. This test enables one to delineate clusters of high or low values within a specified radius d of a given location i.23 In this test, we defined the neighborhood as a fixed distance band of 24 km based on methodological and conceptual considerations. Specifically, because the evaluation after first and second rounds of spraying did not follow-up all locations surveyed at baseline, it was necessary to set the distance band for these tests at 24 km to ensure that each index location had at least one neighbor within the specified neighborhood. The minimum number of neighbors at spray 1 was 1 and the maximum number of neighbors at spray 1 was 48, whereas at spray 2, the minimum number of neighbors was 1 and the maximum number of neighbors was 29. This distance would also be sufficient to capture the potential flight range of a vector up to 1,500 m as well as carriage by animals or humans.24
The results were corrected for multiple testing through the false discovery rate (FDR) procedure.25,26 Location of clusters of high and low infestation was compared over time to assess persistence and changes in spatial patterns of vector presence after initiation of control. This comparison allowed us to create three additional variables: (1) high infestation cluster luster at baseline, indicating that a locality had fallen in a high infestation cluster at baseline according to the Getis-Ord Gi*(d), (2) high infestation cluster after first residual insecticide spray, indicating that a locality had fallen in a high cluster after the first round of spraying had been administered., and (3) high infestation cluster after second residual insecticide spray, indicating that a locality had fallen in a high cluster after the second round of spray with pyrethroid insecticides had been administered. High infestation cluster at baseline was meant to capture the effect of differences between localities that existed at baseline before the initiation of house spraying. Finally, categorical variables representing the quarter of the year in which spray was administered were created and included in the models of reinfestation after first residual insecticide spray and reinfestation after second residual insecticide spray. In these models, administration of spray in quarter 2 (April–June), quarter 3 (July–September), and quarter 4 (October–December) were each compared with the reference group, which was quarter 1 (January–March).
We used logistic regression to analyze how the time lapse between spray and evaluation affected infestation after the first and second rounds of spray. The association was evaluated in two ways. First, we used reinfestation as the dependent variable. Second, we used the variables high infestation cluster after first residual insecticide spray and high infestation cluster after second residual insecticide spray as dependent variables. Because we had data at three points in the intervention, we compared baseline and time after first spray as well as first and second sprays. In total, four different logistic regression models were used: (1) model 1 examined the outcome of reinfestation after first residual insecticide spray, (2) model 2 examined the outcome of high infestation cluster after first residual insecticide spray, (3) model 3 examined the outcome of reinfestation after second residual insecticide spray, and (4) model 4 examined the outcome high infestation cluster after second residual insecticide spray. Reinfestation was defined as having a locality-level index of infestation greater than zero. In all models, fit was assessed through examination of the log likelihood. Interaction terms were tested but not statistically significant.
Results
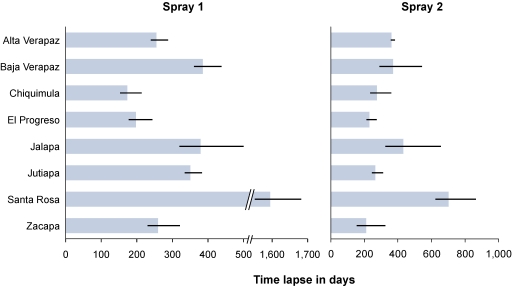
Infestation across the study area significantly decreased after the first round of spraying compared with baseline. The mean index of infestation at baseline was 15.33 and fell to 4.77. However, similar gains were not realized between the first and second sprays; the index of infestation decreased from 4.77 after spray 1 to 4.16 after spray 2, but this decline was not statistically significant. In addition, the difference in mean index of infestation at baseline between those localities that were followed-up in the first round of spray (24.65, N = 1,228) and those localities that were not followed-up (7.64, N = 1,491) was statistically significant (P < 0.01) and suggests the choice of which localities to follow-up in the program may have been targeted to areas of higher infestation. The same was true after the first residual spray, because those localities that were sprayed in the second spray administration had a higher mean index of infestation (5.52, N = 838) than those localities that were not included in this second spray (3.20, N = 390). Figure 2 depicts the progression of localities in the intervention. Finally, the mean number of days between spraying and evaluation differed notably by department for both spray 1 and spray 2 (Figure 3).
Figure 2.
Progression of localities in the Guatemalan National Chagas Disease Program.
Figure 3.
Mean time lapse between spray and evaluation at sprays 1 and 2 by department.
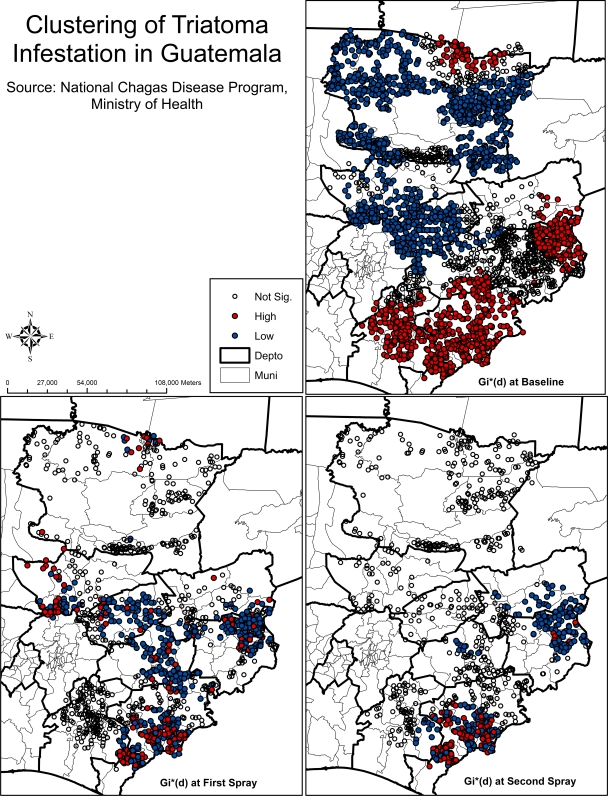
Maps of the results of the Gi*(d) test at baseline and first and second sprays show the presence of high infestation clusters in the departments of Jutiapa, Alta Verapaz, and Chiquimula (Figure 4). After the first spray, the clusters became dispersed throughout six of eight departments, but high infestation cluster areas remained most concentrated in Jutiapa, Alta Verapaz, and El Progreso. Finally, after the second spray, areas of high infestation remained concentrated in Jutiapa, with a large area of low infestation in Chiquimula. A summarized comparison of the clustering pattern over time indicated that 455 localities fell in same category of the Gi*(d) test (not significant, high, or low) at both baseline and first spray and that 632 localities fell in the same clusters at both first and second sprays. Also, 178 localities fell in a high infestation cluster at all three time periods. This finding shows that there are localities that experience persistent infestation and may provide an indication of potential infestation hotspots that should be more intensely surveyed.
Figure 4.
Results of Getis-Ord Gi*(d) statistical test at baseline, spray 1, and spray 2.
Logistic regression showed a strong effect of time between spraying and evaluation on both outcomes for the first spray (Tables 1 and 2). In this case, both a time lapse between spray and evaluation of 180–360 days (odds ratio [OR] = 1.54, 95% confidence interval [CI] = 1.07–2.23, P = 0.022) and a time lapse of greater than 360 days (OR = 2.66, 95% CI = 1.85–3.83, P < 0.001) were associated with statistically significant increased odds of being reinfested compared with localities where this time lapse was less than 180 days. In addition, administering spray in the second quarter had a protective effect on the risk of reinfestation after first spray (OR = 0.68, 95% CI = 0.48–0.97, P = 0.034), although administering spray in the third and fourth quarters had no statistically significant effect. These same independent variables had a more pronounced association with the outcome of falling in a high cluster after first spray. A time lapse between spray and evaluation of 180–360 days had an OR of 3.14 (95% CI = 1.85–5.35, P < 0.001), a time lapse between spray and evaluation of greater than 360 days had an OR of 5.95 (95% CI = 3.57–9.92, P < 0.001) and falling in a high cluster at baseline had an OR of 3.60 (95% CI = 2.61–4.96, P < 0.001). This finding indicates that, although there is an association between baseline characteristics and the odds of being reinfested, the time lapse between spray and evaluation is also an important factor associated with reinfestation.
Table 1.
Relationship between reinfestation after first residual insecticide spray and time lapse during first spray
| Model 1 (N = 1,156) | OR | 95% CI | P value |
|---|---|---|---|
| Time lapse 180–360 days | 1.54 | 1.07–2.23 | 0.022 |
| Time lapse > 360 days | 2.66 | 1.85–3.83 | < 0.001 |
| High cluster at baseline | 1.17 | 0.90–1.53 | 0.220 |
| Quarter 2 vs. quarter 1 | 0.68 | 0.48–0.97 | 0.034 |
| Quarter 3 vs. quarter 1 | 1.07 | 0.75–1.53 | 0.696 |
| Quarter 4 vs. quarter 1 | 0.77 | 0.51–1.17 | 0.229 |
Quarter 1 = January to March; quarter 2 = April to June; quarter 3 = July to September; quarter 4 = October to December.
Table 2.
Relationship between falling in a high infestation cluster after first residual insecticide spray and time lapse during first spray
| Model 2 (N = 1,228) | OR | 95% CI | P value |
|---|---|---|---|
| Time lapse 180–360 days | 3.14 | 1.85–5.35 | 0.000 |
| Time lapse ≥ 360 days | 5.95 | 3.57–9.92 | 0.000 |
| High cluster at baseline | 3.60 | 2.61–4.96 | 0.000 |
In the second spray (Tables 3 and 4), both a medium and long time lapse between spray and evaluation were not significantly associated with the odds of being reinfested after the second residual insecticide administration, whereas having been reinfested after first spray was a statistically significant predictor of subsequent reinfestation after second spray (OR = 2.12, 95% CI = 1.50–3.01, P < 0.001). Quarter in which spray was administered was highly non-significant in the second spray. In contrast, a time lapse of 180–360 days was significantly associated with increased odds of falling in a high cluster after second residual insecticide spray (OR = 1.90, 95% CI = 1.36–2.63) as was having been reinfested after the first spray (OR = 2.56, 95% CI = 1.82–3.59, P < 0.001), whereas a long time lapse was associated with a protective effect on this outcome (OR = 0.52, 95% CI = 0.37–0.72, P < 0.001).
Table 3.
Relationship between reinfestation after second residual insecticide spray and time lapse during second spray
| Model 3 (N = 663) | OR | 95% CI | P value |
|---|---|---|---|
| Time lapse 180–360 days | 1.37 | 0.96–1.58 | 0.084 |
| Time lapse > 360 days | 1.17 | 0.81–1.68 | 0.411 |
| Reinfested after spray 1 | 2.12 | 1.50–3.01 | < 0.001 |
| Quarter 2 vs. quarter 1 | 1.10 | 0.68–1.80 | 0.699 |
| Quarter 3 vs. quarter 1 | 0.80 | 0.477–1.34 | 0.400 |
| Quarter 4 vs. quarter 1 | 1.32 | 0.74–2.33 | 0.346 |
Quarter 1 = January to March; quarter 2 = April to June; quarter 3 = July to September; quarter 4 = October to December.
Table 4.
Relationship between falling in a high infestation cluster after second residual insecticide spray and time lapse during second spray
| Model 4 (N = 838) | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Time lapse 180–360 days | 1.90 | 1.36–2.63 | 0.000 |
| Time lapse ≥ 360 days | 0.52 | 0.37–0.72 | 0.000 |
| Reinfested after spray 1 | 2.56 | 1.82–3.59 | 0.000 |
Discussion
Our analysis indicated that the Chagas Disease Control Program of Guatemala has achieved overall and significant declines in domestic vector infestation after two rounds of house spraying. However, the results show that areas of high infestation have persisted over time, suggesting the presence of hotspots that should be more intensely surveyed. We also showed a significant impact of programmatic practices on reinfestation. Specifically, our results indicate that the time between the rounds of house spraying may have been too long, particularly in the first spray, which contributed to the occurrence of reinfestation in several localities.
There were statistically significant clusters of high infestation at each point in the intervention, and 178 localities remained in high infestation clusters throughout this period of the intervention, which may be relevant when targeting of program resources is necessary. Specifically, the areas of high infestation at baseline were primarily located in Chiquimula, Jutiapa, and Baja Verapaz, with a subset of localities in Chiquimula and Jutiapa persisting as high infestation clusters throughout both the first and second spray.
Our analysis also shows heterogeneity in the time lapse between spraying and evaluation and consequently, the time between rounds of spray, department, and its impact on the efficacy of the first round of pyrethroid insecticide administration. Long time lapses between spraying and evaluation resulted in similarly long time lapses between subsequent rounds of house spraying. We could show that those localities in which greater than 1 year passed between spraying and evaluation after the first round of house spraying had a substantially increased risk of reinfestation after the first spray. These findings are consistent with studies showing that the residual efficacy of deltamethrin insecticide decreases dramatically by 3 months after spray.21
Both prior experience from INCOSUR as well as existing international guidelines for CD vector control programs point to the importance of systematizing the time lapse between rounds of house spraying. In the case of INCOSUR, the guidelines governing the first phase of the vector control program in all participating countries prescribed two consecutive rounds of spraying performed 6 months apart in all houses, regardless of whether they were found by the program to be infested. These first rounds of spraying are considered an attack phase in which the goal is to control infestation to the greatest degree possible. This attack phase is then followed by a sustained surveillance phase in which evaluation is ongoing, and additional house spraying is performed in areas where infestation is found. In addition, the technical guidelines for CD control programming that are provided by the PAHO suggest that the post-spray evaluation should take place 3–6 months after spray, because this time frame is consistent with studies suggesting that the residual effect of pyrethroids tends to be highest during the first month after spray, with efficacy almost completely diminished in 3–6 months after spray is administered or sooner in peridomestic settings.21,22,27,28 The results of this study, thus, confirm that failing to follow-up within 6 months of spray does, in fact, increase the risk of reinfestation and highlight the critical nature of these recommendations.
The effect of this inconsistent time lapse between spray and evaluation in the intervention also has implications for evaluation. Specifically, in areas where the time to post-spray evaluation was less than 6 months, estimates of reinfestation may be masked by a lack of follow-up in the period after the insecticide efficacy diminished. In contrast, in localities where this time to post-spray evaluation far exceeded the period of insecticide efficacy, reinfestation can be measured, although it is difficult to determine whether the bugs were exterminated during the first round of spraying and subsequently returned or whether the insecticide was not effective in fully eliminating bugs from the house. In addition to residual insecticide efficacy, it has been suggested that proximity to untreated foci is a cause of domestic reinfestation in the case of Triatoma vectors.29
Spraying in the second quarter of the year was protective for reinfestation after first spray. This result confirmed previous findings indicating that a maximum reduction in triatomine abundance is achieved when insecticide spray is administered in early April, just as the transient seasonal infestation with T. dimidata begins on the Yucatan peninsula.30,31 In these previous studies, the effectiveness of spray decreased slightly if administered in May or June and dropped to a much lower level during the remaining month of the year. Although this effect of season of spray administration was present in the first spray, season did not impact reinfestation after second spray.
This work offers additional evidence of the use of spatial analysis for planning and evaluation in an infectious disease control program, specifically in regards to targeting resources to areas with persistent infestation. We show a straightforward approach to assess hotspots or areas of concern in terms of reinfestation after spray in the context of an extensive vector control intervention and highlight potential gains that can be captured through simple alterations to programmatic practices. It has been shown previously that spatial analysis can be used to improve and evaluate CD control programs by optimizing spraying strategies,32,33 monitoring reinfestation,34 and targeting screening for T. cruzi in children35 as well characterizing discordant results of tests for T. cruzi infection.36
In the future, it is critical that the time between spray and evaluation be systematized in accordance with the technical guidelines provided by the PAHO such that houses in need of subsequent sprays be administered insecticide in a timely manner.27 Furthermore, more consistent data should be collected to determine the true infestation status of all localities. It may be advantageous for the program to consider seeking resources for a second national infestation survey in which accurate spatial coordinates for all localities are collected and the sampling of infestation is either exhaustive or performed using a consistent sampling fraction. If performed, such a framework should be maintained in future surveillance and evaluation. This sampling would allow the intervention to gauge existing hotspots as accurately and completely as possible across the coverage area. Although such a survey would require an initial investment of time, personnel, and resources, it would provide new national estimates on which future surveillance, monitoring, and evaluation could be conducted.
This study has several limitations. First, not all localities were followed over time, resulting in a decrease in points that could be included in the analysis over the course of the two sprays and limiting the generalizability of the results. Second, missing spatial coordinates and information about the time lapse between spray and evaluation was another limitation, although we made an effort to overcome this limitation through manual matching of latitude and longitude and imputation of missing time data through use of the municipality average time lapse. Third, the data do not provide information about vector species across all time points and locations, which prevented a separate analysis of reinfestation by each of the two major vectors over the intervention. Although not a limitation to the analysis contained here, such information would have been useful in further illuminating the accomplishments of and challenges to the intervention. Fourth, survey of vector infestation was not performed using a consistent sampling fraction per locality, a fact that may have resulted in underestimation of the true level of infestation, particularly in larger localities where a smaller proportion of homes were sampled.
The significance of having been in a high infestation cluster at baseline on subsequent reinfestation after first spray suggests that there are other factors that may be contributing to the distribution of baseline infestation and in turn, the variation in reinfestation across space after first spray. In addition, we could not show a similar affect for the second time lapse as for the first spray, which again, may indicate the importance of other factors that were not captured in our analysis. Additional investigation into socioeconomic and environmental variables, such as house material type, elevation, and seasonality of spray,30 are warranted to characterize these factors. Also, in this context, the effect of sequential spray strategies on reinfestation seems of interest; however, conducting such an analysis using standard longitudinal methods bares the risk of introducing time-varying confounding. Future studies may use methods, such as marginal structural models, that allow for the estimation of unbiased results in such situations.
In addition to these limitations, it is important to note that the clustering pattern of infestation found using the Getis-Ord Gi*(d) test was not fully consistent with expectations based on a baseline seroprevalence survey in children undertaken by the Universidad del Valle de Guatemala in 1998–2000 before the initiation of spraying.9 Although this inconsistency may seem anomalous given the known relationship of infestation and infection,37 these findings may have been caused by inconsistency in the sampling frames of the seroprevalence study, the school-based study, or the vector infestation data collection, a house by house sampling design, or the insensitive nature of standard methods for bug detection.
In conclusion, two important policy recommendations can be offered based on the results of this study. First, the timing between rounds of house spraying should be systematized in accordance with existing technical guidelines to both increase the potential efficacy of the vector control program and ensure accurate evaluation of the program in the future. Although this timing requires careful consideration of obstacles, such as resource shortages and other acute disease outbreaks, evidence indicates that it would greatly improve the effectiveness of the program. Second, this analysis provides support for a recommendation that the program consider implementing a more extensive monitoring and evaluation system and a suggestion that this system may be based on the use of spatial analysis. By using spatial methods such as those methods used here, the program would be able to track hotspots of reinfestation and optimize targeting of resources to those areas.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Jeff Blossom, Justin O'Hagan, and Kota Yoshioka for their assistance in completing this study as well as Dr. Hugo Alvarez and the staff of the Chagas Disease Control Program at the Ministry of Health of Guatemala for their collaboration and input.
Footnotes
Financial support: This research was supported by the David Rockefeller Center for Latin American Studies at Harvard University as well as the Michael Von Clemm Family Fellowship and the Andrew Spielman Memorial Travel Fellowship of the Harvard School of Public Health.
Authors' addresses: Jennifer Manne and Marcia Castro, Department of Global Health and Population, Harvard School of Public Health, Boston, MA, E-mails: jmanne@post.harvard.edu and mcastro@hsph.harvard.edu. Jun Nakagawa, Department of Community and Global Health, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan, E-mail: junnakagawa@hotmail.com. Yoichi Yamagata, Institute for International Cooperation, Japan International Cooperation Agency, Tokyo, Japan, E-mail: yamagata_yoichi@hotmail.com. Alexander Goehler, Department of Radiology, Massachusetts General Hospital, Boston, MA, E-mail: agoehler@partners.org. John S. Brownstein, Department of Pediatrics, Harvard Medical School, Boston, MA, E-mail: John.Brownstein@childrens.harvard.edu.
References
- 1.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Control of Chagas Disease: Second Report of the WHO Expert Committee. WHO Technical Report Series. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 3.Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gurtler RE. The challenges of Chagas disease—grim outlook or glimmer of hope. PLoS Med. 2007;4:e332. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bern C. Antitrypanosomal therapy for chronic Chagas' disease. N Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 5.Dias JC. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusional Chagas disease. Historical aspects, present situation, and perspectives. Mem Inst Oswaldo Cruz. 2007;102 (Suppl 1):11–18. doi: 10.1590/s0074-02762007005000092. [DOI] [PubMed] [Google Scholar]
- 6.Schofield CJ. 21 years of parasitology. Trends Parasitol. 2005;21:483–485. doi: 10.1016/j.pt.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Gurtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamagata Y, Nakagawa J. Control of Chagas disease. Adv Parasitol. 2006;61:129–165. doi: 10.1016/S0065-308X(05)61004-4. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo NR, Arana BA, Diaz A, Cordon-Rosales C, Klein RE, Powell MR. Seroprevalence of Trypanosoma cruzi infection among school-age children in the endemic area of Guatemala. Am J Trop Med Hyg. 2003;68:678–682. [PubMed] [Google Scholar]
- 10.Paz-Bailey G, Monroy C, Rodas A, Rosales R, Tabaru R, Davies C, Lines J. Incidence of Trypanosoma cruzi infection in two Guatemalan communities. Trans R Soc Trop Med Hyg. 2002;96:48–52. doi: 10.1016/s0035-9203(02)90236-1. [DOI] [PubMed] [Google Scholar]
- 11.King RJ, Cordon-Rosales C, Cox J, Davies CR, Kitron UD. Triatoma dimidiata infestation in Chagas disease endemic regions of Guatemala: comparison of random and targeted cross-sectional surveys. PLoS Negl Trop Dis. 2011;5:e1035. doi: 10.1371/journal.pntd.0001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan American Health Organization . Guatemala. Health in the Americas. Washington, DC: Pan American Health Organization; 2007. pp. 374–393. [Google Scholar]
- 13.Monroy C, Rodas A, Mejia M, Rosales R, Tabaru Y. Epidemiology of Chagas disease in Guatemala: infection rate of Triatoma dimidiata, Triatoma nitida and Rhodnius prolixus (Hemiptera, Reduviidae) with Trypanosoma cruzi and Trypanosoma rangeli (Kinetoplastida, Trypanosomatidae) Mem Inst Oswaldo Cruz. 2003;98:305–310. doi: 10.1590/s0074-02762003000300003. [DOI] [PubMed] [Google Scholar]
- 14.Yamagata Y, Nakagawa J, Shimoda M, Tabaru Y. Management of infectious disease control in a decentralized organization: the case of the Japan-Guatemala Project for Chagas' Disease Control in Guatemala. Technol Develop. 2003;16:47–54. [Google Scholar]
- 15.Hashimoto K, Cordon-Rosales C, Trampe R, Kawabata M. Impact of single and multiple residual sprayings of pyrethroid insecticides against Triatoma dimidiata (Reduviiade; Triatominae), the principal vector of Chagas disease in Jutiapa, Guatemala. Am J Trop Med Hyg. 2006;75:226–230. [PubMed] [Google Scholar]
- 16.Hashimoto K, Kojima M, Nakagawa J, Yamagata Y. Effectiveness of health education through primary school teachers: activities of Japan overseas cooperation volunteers in the control of Chagas' disease vectors in Guatemala. Technol Develop. 2005;18:71–76. [Google Scholar]
- 17.Nakagawa J, Cordon-Rosales C, Juarez J, Itzep C, Nonami T. Impact of residual spraying on Rhodnius prolixus and Triatoma dimidiata in the department of Zacapa in Guatemala. Mem Inst Oswaldo Cruz. 2003;98:277–281. doi: 10.1590/s0074-02762003000200019. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa J, Hashimoto K, Cordon-Rosales C, Abraham Juarez J, Trampe R, Marroquin Marroquin L. The impact of vector control on Triatoma dimidiata in the Guatemalan department of Jutiapa. Ann Trop Med Parasitol. 2003;97:288–297. doi: 10.1179/000349803235001895. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa J, Juarez J, Nakatsuji K, Akiyama T, Hernandez G, Macal R, Flores C, Ortiz M, Marroquin L, Bamba T, Wakai S. Geographical characterization of the triatomine infestations in north-central Guatemala. Ann Trop Med Parasitol. 2005;99:307–315. doi: 10.1179/136485905X29684. [DOI] [PubMed] [Google Scholar]
- 20.Schofield CJ. Global Collaboration for the Development of Pesticides for Public Health (GCDPP): Field Testing and Evaluation of Insecticides for Indoor Residual Spraying Against Vectors of Chagas Disease. WHO Pesticide Evaluation Scheme: World Health Organization Communicable Disease Control, Prevention and Eradication. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 21.Rojas de Arias A, Lehane MJ, Schofield CJ, Fournet A. Comparative evaluation of pyrethroid insecticide formulations against Triatoma infestans (Klug): residual efficacy on four substrates. Mem Inst Oswaldo Cruz. 2003;98:975–980. doi: 10.1590/s0074-02762003000700020. [DOI] [PubMed] [Google Scholar]
- 22.Gurtler RE, Canale DM, Spillmann C, Stariolo R, Salomon OD, Blanco S, Segura EL. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- 23.Getis A, Ord JK. Spatial Analysis: Modelling in a GIS Environment. New York: Wiley; 1996. Local spatial statistics: an overview. Batty PLaM, ed. [Google Scholar]
- 24.Gurevitz JM, Ceballos LA, Kitron U, Gurtler RE. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J Med Entomol. 2006;43:143–150. doi: 10.1603/0022-2585(2006)043[0143:fiotih]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro MC, Singer BH. Controlling the false discovery rate: a new application to account for multiple and dependent tests in local statistics of spatial association. Geogr Anal. 2006;38:180. [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 27.Ministerio de Salud Publica y Asistencia Social Republica de El Salvador . Norma Tecnica de Prevencion y Control de la Enfermedad de Chagas. El Salvador: Ministerio de Salud Publica y Asistencia Social Republica de El Salvador; 2007. [Google Scholar]
- 28.Dumonteil E, Ruiz-Pina H, Rodriguez-Felix E, Barrera-Perez M, Ramirez-Sierra MJ, Rabinovich JE, Menu F. Re-infestation of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- 29.Schofield CJ. Global Collaboration for Development of Pesticides for Public Health (GCDPP): Challenges of Chagas Disease Vector Control in Central America. WHO Pesticide Evaluation Scheme: World Health Organization Communicable Disease Control, Prevention and Eradication. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 30.Barbu C, Dumonteil E, Gourbiere S. Optimization of control strategies for non-domiciliated Triatoma dimidiata, Chagas disease vector in the Yucatan Peninsula, Mexico. PLoS Negl Trop Dis. 2009;3:e416. doi: 10.1371/journal.pntd.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferral J, Chavez-Nunez L, Euan-Garcia M, Ramirez-Sierra MJ, Najera-Vazquez MR, Dumonteil E. Comparative field trial of alternative vector control strategies for non-domiciliated Triatoma dimidiata. Am J Trop Med Hyg. 2010;82:60–66. doi: 10.4269/ajtmh.2010.09-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy MZ, Malaga Chavez FS, Cornejo Del Carpio JG, Vilhena DA, McKenzie FE, Plotkin JB. Rational spatio-temporal strategies for controlling a Chagas disease vector in urban environments. J R Soc Interface. 2010;7:1061–1070. doi: 10.1098/rsif.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbu C, Dumonteil E, Gourbiere S. Evaluation of spatially targeted strategies to control non-domiciliated Triatoma dimidiata vector of Chagas disease. PLoS Negl Trop Dis. 2011;5:e1045. doi: 10.1371/journal.pntd.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Prokopec GM, Cecere MC, Canale DM, Gurtler RE, Kitron U. Spatiotemporal patterns of reinfestation by Triatoma guasayana (Hemiptera: Reduviidae) in a rural community of northwestern Argentina. J Med Entomol. 2005;42:571–581. doi: 10.1093/jmedent/42.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy MZ, Kawai V, Bowman NM, Waller LA, Cabrera L, Pinedo-Cancino VV, Seitz AE, Steurer FJ, Cornejo Del Carpio JG, Cordova-Benzaquen E, Maguire JH, Gilman RH, Bern C. Targeted screening strategies to detect Trypanosoma cruzi infection in children. PLoS Negl Trop Dis. 2007;1:e103. doi: 10.1371/journal.pntd.0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy MZ, Bowman NM, Kawai V, Plotkin JB, Waller LA, Cabrera L, Steurer F, Seitz AE, Pinedo-Cancino VV, Cornejo del Carpio JG, Cordova Benzaquen E, McKenzie FE, Maguire JH, Gilman RH, Bern C. Spatial patterns in discordant diagnostic test results for Chagas disease: links to transmission hotspots. Clin Infect Dis. 2009;48:1104–1106. doi: 10.1086/597464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piesman J, Sherlock IA, Mota E, Todd CW, Hoff R, Weller TH. Association between household triatomine density and incidence of Trypanosoma cruzi infection during a nine-year study in Castro Alves, Bahia, Brazil. Am J Trop Med Hyg. 1985;34:866–869. doi: 10.4269/ajtmh.1985.34.866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.