Abstract
Sylvatic populations of Triatoma infestans might be involved in the recolonization of human dwellings. We report here the discoveries of new T. infestans sylvatic foci in the Bolivian Chaco. Eighty-one triatomines were caught, 38 of which were identified as T. infestans. Triatoma sordida and Panstrongylus geniculatus were the other species collected. One T. infestans and one T. sordida were infected with Trypanosoma cruzi TcI; one T. infestans was infected with TcII. These discoveries add to the debate on the geographic distribution of sylvatic T. infestans populations, the geographic origin of the species, and the epidemiological role of these populations.
In South America, Triatoma infestans remains the main vector of Trypanosoma cruzi, the causative agent of Chagas disease. This species has been considered almost exclusively domestic for a long time: despite old reports of occasional findings of T. infestans in sylvatic areas in Argentina, Brazil, and Paraguay,1–5 it was thought that true sylvatic foci were restricted to the Andean valleys of Cochabamba in Bolivia, where sylvatic populations had repeatedly been detected.6–8 This belief led to the hypothesis that the origin and initial domestication of the species occurred in the Bolivian Andes.9,10 Moreover, when the vector control programs started, the possibility of a recolonization of treated areas by sylvatic bugs was mainly discarded. Recently, it has been shown that Andean sylvatic populations in Bolivia are distributed in larger areas than the Cochabamba valleys.11–14 In addition to the Bolivian Andean valleys, dark morphs of T. infestans were reported in 1997 in one sylvatic area of the Bolivian Chaco,15 and recently, other sylvatic foci were discovered in the Argentinean and Paraguayan Chaco and in Chile,16–19 challenging the hypothesis that the species originated in the Bolivian Andes. All of these data show that sylvatic T. infestans populations are much more widespread than previously thought and that their role in the recolonization of treated areas has to be considered.13,20 Indeed, domestic T. infestans persists primarily in the Bolivian Andean valleys and in the Gran Chaco ecoregion, precisely where sylvatic populations have been found.21
Here, we report the discovery of new sylvatic foci in the Bolivian Chaco, highlighting the wide distribution of sylvatic T. infestans in this region and questioning the geographic origin of the species, its domestication process, and the epidemiological role played by sylvatic populations.
The search for triatomines was conducted in October and November 2009, using mouse-baited adhesive traps in 14 forest sites located in 11 different sylvatic areas (Table 1) of the Bolivian Chaco (Figure 1).22 Two search methods were used. In the first method, five sylvatic sites were randomly pre-selected (GC1–GC5), and traps were placed along transects (200–500 m) into the forest, in hollows of live (35.1%) or dead (42.2%) trees, burrows (13.4%), and other locations, such as under woodpiles (9.3%). The second method focused the search on nine forest sites selected according to the information provided by inhabitants (Y01 to Y09), and traps were placed mostly in hollows of live trees (88.2%). Traps were set in the afternoon and inspected the next morning. The triatomines caught were first identified with morphological keys,23 and then sequencing of the rDNA ITS-2 region according to previous descriptions.14,24
Table 1.
Geographical localization of the investigated sylvatic areas in the Bolivian Chaco and triatomine captures
| Number and species of caught triatomines | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sylvatic area | Department | Nearest village | Forest site code | Distance to nearest human habitat (m) | Latitude (S) | Longitude (w) | Altitude (m) | Number of traps | Number positive trap (%) | T. infestans | T. sordida | P. geniculatus | Undetermined species* | Total |
| 1 | Tarija | Caiza | Y01 | 6,850 | 21°50′50.6′′ | 63°14′51.6′′ | 442 | 40 | 4 (10.0) | 1 | 3 | 4 | ||
| 2 | Tarija | La Quinta-Caiza | Caiza GC01 | 850 | 21°49′56.0′′ | 63°33′15.2′′ | 605 | 48 | 0 (0.0) | 0 | ||||
| 3 | Tarija | Cueva de Leon | GC02 | 810 | 21°22′37.0′′ | 63°21′34.8′′ | 347 | 61 | 8 (13.1) | 4 | 4 | 1 | 9 | |
| 4 | Tarija | Taiguati | Y04 | 570 | 21°10′34.0′′ | 63°23′05.5′′ | 471 | 41 | 2 (4.9) | 2 | 2 | |||
| Y02 | 760 | 21°09′25.3′′ | 63°22′30.7′′ | 488 | 20 | 1 (5.0) | 1 | 1 | ||||||
| 5 | Chuquisaca | Machareti | Y03 | 2,840 | 20°49′06.4′′ | 63°19′18.8′′ | 646 | 20 | 0 (0.0) | 0 | ||||
| 6 | Santa Cruz | San Antonio | Y07 | 110 | 20°15′06.8′′ | 62°57′46.5′′ | 581 | 45 | 4 (8.9) | 8 | 2 | 2 | 12 | |
| Y08 | 8,555 | 20°12′02.3′′ | 63°01′21.4′′ | 578 | 49 | 7 (14.3) | 8 | 3 | 11 | |||||
| Y09 | 1,500 | 20°10′15.9′′ | 63°01′22.7′′ | 615 | 60 | 11 (18.3) | 17 | 1 | 13 | 31 | ||||
| 7 | Santa Cruz | 17 de Noviembre | Y05 | 170 | 20°00′48.3′′ | 61°54′11.2′′ | 352 | 22 | 0 (0.0) | 0 | ||||
| 8 | Santa Cruz | Yanahigua | Y06 | 260 | 19°56′09.1′′ | 62°07′35.8′′ | 385 | 15 | 0 (0.) | 0 | ||||
| 9 | Santa Cruz | San Lorenzo | GC03 | 450 | 19°38′54.3′′ | 63°06′46.1′′ | 697 | 63 | 6 (9.5) | 1 | 5 | 1 | 7 | |
| 10 | Santa Cruz | Estancia Basilio | GC04 | 2,200 | 18°06′55.9′′ | 63°12′57.5′′ | 543 | 48 | 0 (0.0) | 0 | ||||
| 11 | Santa Cruz | Sinai | GC04 | 1,200 | 17°56′24.0′′ | 62°65′6.07′′ | 272 | 48 | 3 (6.3) | 1 | 2 | 1 | 4 | |
| Total | 580 | 46 (7.9) | 38 | 17 | 2 | 24 | 81 | |||||||
All the triatomines in this column were nymphs whose species have not been genetically confirmed.
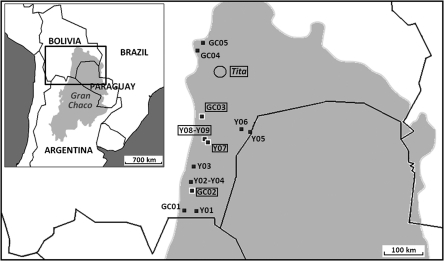
Figure 1.
Forest sites in which the search for sylvatic triatomines was carried out in the Bolivian Chaco (see also Table 1). Sylvatic areas: 1 =Y01; 2 = GC01; 3 = GC02; 4 = Y02 + Y04; 5 = Y03; 6 = Y07 + Y08 + Y09; 7 = Y05; 8 = Y06; 9 = GC03; 10 = GC04; 11 = GC05; Tita = only one with sylvatic Triatoma infestans previously recorded in the Bolivian Chaco.15 Circled names: positives for sylvatic T. infestans.
A total of 580 traps were set. Forty-six were positive for triatomines (7.9%) in nine different forest sites (64%, Table 1). Eighty-one triatomines belonging to T. infestans, Triatoma sordida, and Panstrongylus geniculatus were collected. Only five adults were found: four were morphologically and genetically identified as T. infestans and exhibited the dark phenotype15; one was identified as T. sordida. The other specimens were nymphs and were identified only after sequencing. Complete or partial ITS-2 sequences (386 bp at least) were resolved for 57 specimens. Thirty-eight were characterized as T. infestans: 1) 27 presented the ITS2Hap2 haplotype, previously found in sylvatic T. infestans from the Bolivian Chaco (accession no. HQ333212)14; 2) one, collected in GC03, presented the ITS2Hap1 haplotype (accession no. HQ333211), previously reported as the only Andean haplotype in sylvatic T. infestans from Bolivia14; and 3) 10, for which the resolved sequences were partial, presented 99% or 100% identity with various sequences deposited for T. infestans (accession nos. HQ333214, HQ333212, HQ333211, AY860388, AY860387, AJ582025, AJ582024, AJ576055, AJ576054, AJ576052, AJ576051). Seventeen specimens were characterized as T. sordida (accession no. AJ576063, 99% identity) and two as P. geniculatus (accession no. AJ306543, 99% identity). T. infestans and T. sordida specimens were all collected in hollow trees (Figure 2); the two P. geniculatus were captured in burrows.
Figure 2.
Hollow tree of the Bolivian Chaco, positive for Triatoma infestans. Typical ecotope in which sylvatic triatomines were collected in the current fieldwork.
Feces of 43 bugs were microscopically observed, and no flagellates were found. The infection was then determined for 24 bugs (11 T. infestans, 11 T. sordida, and the two P. geniculatus) using the mini-exon multiplex polymerase chain reaction method after DNA extraction from the digestive tracts,25 and the genetic characterization of parasites was completed by sequencing of the glucose-6-phosphate isomerase gene. Only three bugs were positive: two T. infestans (one male and one 4th instar captured in Y08 and GC02, respectively) and one T. sordida (4th instar captured in GC05). The T. infestans male was infected with TcII. The two other bugs were infected with TcI. This report is the first to document the presence of TcII in a sylvatic T. infestans specimen.
Sylvatic T. infestans were captured in three areas (3, 6, and 9) farther south (130–320 km) than Tita, the only area with sylvatic T. infestans previously recorded in the Bolivian Chaco (Figure 1).15 These discoveries, and the recent finding of sylvatic foci in the Argentinean and Paraguayan Chaco,17,19 highlight the wide distribution of sylvatic T. infestans in the Gran Chaco ecoregion. Interestingly, the Y01 and Y08 sites were distant from human habitat (≈8 km). As in the Tita area, this supports the primary occurrence of sylvatic populations in these areas from the Gran Chaco and makes the hypothesis of a secondary colonization of the sylvatic environment by bugs derived from domestic populations unlikely. Different studies have recently shown that the Andean origin of T. infestans was not unequivocally supported and that a Gran Chaco origin could not be rejected.14,26,27 Among the arguments used to support the hypothesis of an Andean origin is the scarcity of T. infestans sylvatic foci in the Gran Chaco; this hypothesis is now weakened given the new foci reported here and those reported in the Argentinean and Paraguayan Chaco.17,19
In this study, several forest sites positive for T. infestans were close to human habitat (< 500 m), supporting the possibility of incursion events of sylvatic specimens into houses. The traditional hypothesis to explain the current distribution of domestic T. infestans in South America puts forward an initial and major vector domiciliation in the Bolivian Andes, followed by a recent passive human-mediated spread.9,10 This hypothesis has been further supported by the genetic similarities between domestic and sylvatic T. infestans populations in the Bolivian Andes.7,28 In fact, the same genetic argument can be applied to the Bolivian Chaco, where ITS-2 and mtCytB haplotypes are shared between domestic and sylvatic T. infestans.14,24 The diversity of ecoregions and ecotopes in which T. infestans sylvatic populations have been found to date and the propensity of this species to diversify feeding sources suggest a long evolutive process that allowed it to acquire a strong adaptive ability and to survive in a great variety of environments. Consequently, domestication could be an opportunity rather than a costly adaptive change. In this way, new human settlements intruding into the sylvatic environment would provide an opportunity for T. infestans to feed on new mammal species (humans, domestic animals) that are more stable (an easy feeding source) than sylvatic mammals. Easy domestication of T. infestans sylvatic populations suggests that their epidemiological role has to be considered carefully because they can infest dwellings after vector control and new human settlements in sylvatic environments.
In the current study, the infection rate of sylvatic T. infestans was low (18.2%) but not negligible. In contrast, in the Andes, sylvatic T. infestans populations have a high infection rate.13 The low infection reported here agrees with previous results in the Gran Chaco.17,29 It might be related to the main ecotope investigated (hollow trees), perhaps occupied by birds (which are not T. cruzi reservoirs). This result might strengthen the hypothesis of an ornithophilic feeding behavior of these populations. Nevertheless, most of the hollow trees in which T. infestans were found during the current fieldwork did not seem to be inhabited by birds. Consequently, other blood sources (lowly infected small mammals, reptiles) and the occurrence of hemolymphagy cannot be discarded. Future studies determining the blood sources of T. infestans in the Gran Chaco will clarify the T. infestans ecology in this region.
Extensive searches for sylvatic T. infestans in the Gran Chaco must be pursued to determine the distribution of sylvatic populations in this ecoregion more accurately. Future genetic studies of these sylvatic populations with Andean populations will help determine the geographical origin and dispersion routes of the species. Other molecular studies comparing the genetic characteristics of sylvatic and domestic bugs are also needed to clarify the epidemiological role of sylvatic populations and to improve our understanding of the domestication processes of T. infestans.
ACKNOWLEDGMENTS
We thank Carlos Chura and Marcelo Claure (IRD) for their valuable help in the field.
Footnotes
Financial support: This study was supported by the ANR (Agence Nationale de la Recherche, France), the IRD (Institut de Recherche pour le Développement, France), the UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR, no. A70596), and the IDRC (International Development Research Centre, Canada, no. 103696-012).
Authors' addresses: Etienne Waleckx, Stéphanie Depickère, Renata Salas, Claudia Aliaga, Marcelo Monje, Hiber Calle, Rosio Buitrago, François Noireau, and Simone Frédérique Brenière, Institut de Recherche pour le Développement (IRD), La Paz, Bolivia, E-mails: etienne.waleckx@ird.fr, stephanie.depickere@gmail.com, renabacci2@hotmail.com, aliagaclau@yahoo.es, e_marcelo_monje_salazar@hotmail.com, raul_lupita@hotmail.com, rosiob2002@yahoo.com, francois.noireau@ird.fr, and frederique.breniere@ird.fr.
References
- 1.Mazza S. Comprobaciones de Triatoma platensis, Eutriatoma oswaldoi, Panstrongylus seai y Psammolestes coreodes en la Provincia de Santiago del Estero, todos ellos sin infestación y de Eutriatoma sordida con infestación por S. cruzi. Otros datos sobre infestación esquizotripanósica natural silvestre de Triatoma infestans. Prensa Med Argent. 1943;30:1–23. [Google Scholar]
- 2.Velasquez CJ, González G. Aspectos de la enfermedad de Chagas en Paraguay. Rev Goiana Med. 1959;5:357–373. [Google Scholar]
- 3.Barretto MP, Siqueira AF, Corrêa FM. Estudos sôbre reservatórios e vetores silvestres do Trypanosoma cruzi. I. Encontro do Triatoma infestans em ecótopos silvestres. Rev Inst Med Trop Sao Paulo. 1963;5:289–293. [PubMed] [Google Scholar]
- 4.Bejarano JF. Estado selvático de T. infestans y otros aspectos a tener en cuenta para la eliminación de la enfermedad de Chagas. Jorn Entomoepidemiol Arg. 1967;3:171–196. [Google Scholar]
- 5.Cichero JA, Gimenez AL, Martinez A. Estudio de los vectores de la enfermedad de Chagas en ambientes silvestres, peridomésticos y domésticos. Chagas. 1984;1:33–37. [Google Scholar]
- 6.Torrico RA. Hallazgo de Eratyrus mucronatus, infestación natural de vinchucas de cerro y Eutriatoma sordida en Cochabamba. An Lab Central Cochabamba. 1946;1:19–23. [Google Scholar]
- 7.Dujardin JP, Tibayrenc M, Venegas E, Maldonado L, Desjeux P, Ayala FJ. Isozyme evidence of lack of speciation between wild and domestic Triatoma infestans (Heteroptera, Reduviidae) in Bolivia. J Med Entomol. 1987;24:40–45. doi: 10.1093/jmedent/24.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Bermudez H, Balderrama F, Torrico F. Identification and characterization of sylvatic foci of Triatoma infestans in central Bolivia. Am J Trop Med Hyg. 1993;49((Suppl)):371. [Google Scholar]
- 9.Usinger RL, Wygodzinsky P, Ryckman RE. The biosystematics of Triatominae. Annu Rev Entomol. 1966;11:309–330. doi: 10.1146/annurev.en.11.010166.001521. [DOI] [PubMed] [Google Scholar]
- 10.Schofield CJ. Biosystematics of the Triatominae. Service MW, ed. Biosystematics of Haematophagous Insects. Oxford, UK: Clarendon Press; 1988. pp. 284–312. [Google Scholar]
- 11.Noireau F, Cortez MR, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MR, Emperaire L, Piccinali RV, Gurtler RE, Torrico F, Jansen AM, Noireau F. Sylvatic Triatoma infestans (Reduviidae, Triatominae) in the Andean valleys of Bolivia. Acta Trop. 2007;102:47–54. doi: 10.1016/j.actatropica.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Buitrago R, Waleckx E, Bosseno MF, Zoveda F, Vidaurre P, Salas R, Mamani E, Noireau F, Brenière SF. First report of widespread wild populations of Triatoma infestans (Reduviidae, Triatominae) in the valleys of La Paz, Bolivia. Am J Trop Med Hyg. 2010;82:574–579. doi: 10.4269/ajtmh.2010.09-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waleckx E, Salas R, Huamán N, Buitrago R, Bosseno MF, Aliaga C, Barnabé C, Rodriguez R, Zoveda F, Monje M, Baune M, Quisberth S, Villena E, Kengne P, Noireau F, Brenière SF. New insights on the Chagas disease main vector Triatoma infestans (Reduviidae, Triatominae) brought by the genetic analysis of Bolivian sylvatic populations. Infect Genet Evol. 2011;11:1045–1057. doi: 10.1016/j.meegid.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Noireau F, Flores R, Gutierrez T, Dujardin JP. Detection of sylvatic dark morphs of Triatoma infestans in the Bolivian Chaco. Mem Inst Oswaldo Cruz. 1997;92:583–584. doi: 10.1590/s0074-02761997000500003. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Segura JA, Garcia A, Hidalgo J, Galuppo S, Cattan PE. First finding of Chagas disease vectors associated with wild bushes in the Metropolitan Region of Chile. Rev Med Chil. 2006;134:1230–1236. doi: 10.4067/s0034-98872006001000003. [DOI] [PubMed] [Google Scholar]
- 17.Ceballos LA, Piccinali RV, Berkunsky I, Kitron U, Gurtler RE. First finding of melanic sylvatic Triatoma infestans (Hemiptera: Reduviidae) colonies in the Argentine Chaco. J Med Entomol. 2009;46:1195–1202. doi: 10.1603/033.046.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Torres-Perez F, Segovia V, Garcia A, Correa JP, Moreno L, Arroyo P, Cattan PE. Sylvatic foci of the Chagas disease vector Triatoma infestans in Chile: description of a new focus and challenges for control programs. Mem Inst Oswaldo Cruz. 2010;105:633–641. doi: 10.1590/s0074-02762010000500006. [DOI] [PubMed] [Google Scholar]
- 19.Rolón M, Vega MC, Román F, Gómez A, Rojas de Arias A. First report of colonies of sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Paraguayan Chaco, using a trained dog. PLoS Negl Trop Dis. 2011;5:e1026. doi: 10.1371/journal.pntd.0001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noireau F. Wild Triatoma infestans, a potential threat that needs to be monitored. Mem Inst Oswaldo Cruz. 2009;104:60–64. doi: 10.1590/s0074-02762009000900010. [DOI] [PubMed] [Google Scholar]
- 21.Cortez MR, Monteiro FA, Noireau F. New insights on the spread of Triatoma infestans from Bolivia—implications for Chagas disease emergence in the southern cone. Infect Genet Evol. 2010;10:350–353. doi: 10.1016/j.meegid.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Noireau F, Flores R, Vargas F. Trapping sylvatic Triatominae (Reduviidae) in hollow trees. Trans R Soc Trop Med Hyg. 1999;93:13–14. doi: 10.1016/s0035-9203(99)90161-x. [DOI] [PubMed] [Google Scholar]
- 23.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chaga's disease. Bull Am Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 24.Quisberth S, Waleckx E, Monje M, Chang B, Noireau F, Brenière SF. “Andean” and “non-Andean” ITS-2 and mtCytB haplotypes of T. infestans are observed in the Gran Chaco (Bolivia): population genetics and the origin of reinfestation. Infect Genet Evol. 2011;11:1006–1014. doi: 10.1016/j.meegid.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes O, Santos SS, Cupolillo E, Mendonca B, Derre R, Junqueira AC, Santos LC, Sturm NR, Naiff RD, Barret TV, Campbell DA, Coura JR. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 2001;95:97–99. doi: 10.1016/s0035-9203(01)90350-5. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Perez F, Acuna-Retamar M, Cook JA, Bacigalupo A, Garcia A, Cattan PE. Statistical phylogeography of Chagas disease vector Triatoma infestans: testing biogeographic hypotheses of dispersal. Infect Genet Evol. 2011;11:167–174. doi: 10.1016/j.meegid.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Piccinali RV, Marcet PL, Ceballos LA, Gürtler RE, Dotson EM. Genetic variability, phylogenetic relationships and gene flow in Triatoma infestans dark morphs from the Argentinean Chaco. Infect Genet Evol. 2011;11:895–903. doi: 10.1016/j.meegid.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro FA, Perez R, Panzera F, Dujardin JP, Galvao C, Rocha D, Noireau F, Schofield CJ, Beard CB. Mitochondrial DNA variation of Triatoma infestans populations and its implication on the specific status of T. melanosoma. Mem Inst Oswaldo Cruz. 1999;94:229–238. doi: 10.1590/s0074-02761999000700037. [DOI] [PubMed] [Google Scholar]
- 29.Noireau F, Flores R, Gutierrez T, Abad-Franch F, Flores E, Vargas F. Natural ecotopes of Triatoma infestans dark morph and other sylvatic triatomines in the Bolivian Chaco. Trans R Soc Trop Med Hyg. 2000;94:23–27. doi: 10.1016/s0035-9203(00)90426-7. [DOI] [PubMed] [Google Scholar]