Abstract
As part of a field ecology study of arbovirus and malaria activity in the Amazon Basin, Loreto Department, Peru, we collected mosquitoes landing on humans at a forest site and inside and outside of residences and military barracks at periurban, rural, and village sites. We collected 11 Anopheles spp. from these four sites. An. darlingi, the principal malaria vector in the region, accounted for 98.7% of all Anopheles spp. collected at Puerto Almendra. Peaks in landing activity occurred during the December and April collection periods. However, the percent of sporozoite-positive Anopheles spp. was highest 1–2 months later, when landing activity decreased to approximately 10% of the peak activity periods. At all sites, peak landing activity occurred about 2 hours after sunset. These data provide a better understanding of the taxonomy, population density, and seasonal and habitat distribution of potential malaria vectors within the Amazon Basin region.
Introduction
Malaria is on the increase in South America, reaching epidemic proportions in many countries and reemerging as a significant public health problem in others.1,2 For example, the incidence of malaria in the Department of Loreto, Peru, increased sharply beginning in the early 1990s.3,4 Concomitant with this increase in the 1990s was a change in the predominant causative agent of malaria from Plasmodium vivax to P. falciparum and the appearance of Anopheles darlingi Root as the primary vector, which was associated with these changes and increases of malaria in the Department of Loreto.3,5–7 Although reasons for the changes in malaria transmission have not been clearly established, inadequate healthcare services, delayed diagnostics, poor housing, climate events (i.e., El Niño), inadequate vector control programs, banned use of dichlorodiphenyltrichloroethane (DDT), habitat modifications because of agriculture and deforestation, and reintroduction, reemergence, or expansion of An. darlingi all likely contributed.1,3,6,8,9
Because of its association with malaria throughout South America,10 the bionomics of An. darlingi has been well-studied throughout much of the Amazon Basin. These studies, reviewed by Charlwood,11 show that An. darlingi is exophilic and/or endophilic throughout its range. To examine the roles of An. darlingi and other Anopheles spp. in the resurgence of malaria in the vicinity of Iquitos, Peru, we evaluated the seasonal and daily human landing activity, exophilic and endophilic behavior, and seasonal Plasmodium spp. infection rates for Anopheles spp. collected at a Peruvian military base (periurban), two rural single dwelling residences, Puerto Almendra (rural village), and an adjacent forest site about 300 m from the village. The results reported here expand on the preliminary findings of the work by Turell and others.12
Materials and Methods
Study site.
Mosquitoes were collected at several sites near Iquitos (approximate population is 350,000), Loreto Department, in the Amazon Basin of northeastern Peru, approximately 125 m above sea level and bordered by the Amazon, Itaya, and Nanay Rivers (3°51′S, 73°13′W). The study sites are described in detail in the work by Jones and others,13 and in addition, they include a periurban site (Fort Vargas Guerra, a Peruvian military training base partially surrounded by unmanaged lands and individual houses) in the city of Iquitos, Puerto Almendra (a forested rural village approximately 20 km west–southwest of Iquitos), a dense forest site about 0.3 km from Puerto Almendra, and two single dwelling residences: Casa de Sabino (located on the Nauta highway near the intersection of the dirt road in Quistococha that goes to Llanchama) and Casa de Juan (located on the road to Llanchama just under 1 km from the Nauta Highway and about 4.5 km from Puerto Almendra).
Mosquito collections.
A total of nine adult mosquito collections were made from October of 1996 to September of 1997. Each collection consisted of four to six 24-hour collections at 12-hour intervals over 10–16 days. Human collectors, each wearing a hooded screened jacket to prevent biting on the upper parts of the body, conducted hourly human landing collections for 40 minutes starting on the hour followed by a 20-minute rest break from 0600 to 1800 hours (daytime collection) and from 1800 to 0600 hours (nighttime collection); they used oral aspirators under a human use protocol approved by the Naval Medical Research Center's and the US Army Medical Research Institute of Infectious Diseases' Institutional Review Boards in compliance with all applicable Federal regulations governing the protection of human subjects. Collectors were positioned both inside and approximately 5 m outside each resident-occupied house located adjacent to young forests and at ground level and 10 m in the forest canopy (forest site).
Mosquitoes were placed in humidified coolers at the end of each 12-hour collection period and transported to a central laboratory in Iquitos where they were identified according to the works by Lane,14 Faran,15 Faran and Linthicum,16 and Wilkerson and Sallum.17 A list of mosquito species identified from the collections is provided in the work by Pecor and others,18 and preliminary mosquito bionomic findings are described in the works by Jones and others13 and Turell and others.12 Culicine mosquitoes were pooled (25–40 specimens) according to species and collection period, placed in sterile 1.5-mL cryovials, and maintained on dry ice or at −70°C until assayed for the presence of viruses by plaque assay on Vero cell monolayers.19 Anopheles spp. were similarly pooled (1–10 specimens) according to species and collection period, placed in 1.5-mL cryovials, and assayed by enzyme-linked immunosorbent assay (ELISA) for P. falciparum, P. vivax-210, and P. vivax-247 circumsporozoite antigen.20–22 Voucher specimens were deposited in the Walter Reed Biosystematics Unit, Smithsonian Institution, Washington, DC, where field mosquito identifications were confirmed.18
Animal hosts.
Domestic or peridomestic animals were not observed at Fort Vargas Guerra. However, a large fishpond adjacent to the medical clinic was home to water fowl. Dogs and chickens were the most common peridomestic animals observed at rural residences and the village of Puerto Almendra. Additionally, feral animals (i.e., squirrel monkeys [Saimiri spp.], wooley monkeys [Lagothrix spp.], bandicoots [Nassua spp.], and parrots) were kept as pets at some of the residences in Puerto Almendra. Grasslands with free-ranging water buffalo, cattle, and horses were located approximately 1 km from the forest and village collection sites. Numerous birds, including parrots, were observed at the forest study site near Puerto Almendra. The local population included hunters and gatherers who had virtually hunted much of the wild game, including rodents. Low numbers of animals were reported for forest areas surrounding Puerto Almendra, included rodents (Proechimys spp., Oryzomys spp., and Neacomys spp.), marsupials (Philander spp., Metachirus spp., and Marmosops spp.), and sloths (Choloepus hoffmanni and Bradypus spp.)
Temperature and rainfall.
Rainfall data for Iquitos were provided by the Universidad Nacional de la Amazonía Peruana, and river level measurements for the Amazon River at Iquitos were provided by the Servicio de Hidrografia y Navegacion de la Amazonia de la Marina de Guerra del Peru, Iquitos, Peru. The level of the Amazon River in Iquitos varies by as much as 10 m annually from 108 to 118 m above sea level, and it may affect mosquito populations.3
Statistical analysis.
The total numbers of An. darlingi collected were correlated with the accumulated values of rainfall 15 days before the collection date. The distributions of numbers of mosquitoes and rainfall were tested to normality by the Kolmogorov–Smirnov test and the Spearman coefficient (ρ; α < 0.5), and they were used in the analysis for correlations between relative numbers of mosquitoes and rainfall. The association between mosquito abundance (mean mosquitoes/collection per 24 hours either < 5 or ≥ 5) and concurrent river levels (either < 112 or ≥ 112 m above sea level) were examined using Fisher's exact test. The entomological inoculation rate (EIR) was calculated by multiplying the human landing rate times the infection rate and then adjusting for a monthly rate by multiplying by 30.
Results
Anopheline composition and distribution.
We collected 10,748 Anopheles mosquitoes representing 11 species from three subgenera as they landed on the collectors (Table 1). Overall, three species, An. darlingi, An. benarrochi Gabaldon, Cova Garcia, and Lopez, and An. nuneztovari Gabaldon, accounted for 94.7% of all anopheline mosquitoes collected (Table 1). An. darlingi was the predominant Anopheles spp. collected at the rural residences (66.6%) and the village of Puerto Almendra (98.7%). Despite total mosquito collection rates being 10-fold higher at the forest site than the nearby village of Puerto Almendra,12 300-fold more An. darlingi was collected at Puerto Almendra. Although An. benarrochi, An. nuneztovari, and An. oswaldoi (Peryassu) were rarely collected at Puerto Almendra, these species were routinely collected at rural residences that were bordered by young forests. An. triannulatus (Neiva and Pinto) was collected similarly at both Puerto Almendra and the forest site. Although not commonly collected at the other sites, An. kompi Edwards and members of the An. forattinii Wilkerson and Sallum and An. mediopunctatus (Lutz) complexes (perhaps An. costai Fonseca and Ramos and a species related to An. forattinii; Wilkerson R, personal communication) were the most commonly collected Anopheles spp. at the forested site. Only a few Anopheles mosquitoes were collected at Fort Vargas Guerra (< 10% and < 1% compared with the forest site and the rural or village sites, respectively). However, more An. mattogrossensis Lutz and Neiva were collected at Fort Vargas Guerra than at any of the other sites.
Table 1.
Anopheles spp. collected at human landing collections in the vicinity of Iquitos, Peru, from October of 1996 to September of 1997
| Species | Number (%) of adult females collected by site* | ||||
|---|---|---|---|---|---|
| Periurban area† | Rural area | Forest village‡ | Forest | Totals | |
| Anopheles (Nys.) darlingi | 11 (38) | 2,671 (67) | 6,225 (99) | 20 (5) | 8,927 (83) |
| Anopheles (Nys.) benarrochi | 0 (0) | 795 (20) | 7 (< 1) | 2 (< 1) | 804 (8) |
| Anopheles (Nys.) nuneztovari | 0 (0) | 432 (11) | 13 (< 1) | 9 (2) | 454 (4) |
| Anopheles (Ano.) forattinii§ | 0 (0) | 7 (< 1) | 32 (< 1) | 150 (40) | 189 (2) |
| Anopheles (Ste.) kompi | 0 (0) | 5 (< 1) | 1 (< 1) | 163 (43) | 269 (2) |
| Anopheles (Nys.) oswaldoi | 0 (0) | 66 (2) | 7 (< 1) | 18 (5) | 91 (< 1) |
| Anopheles (Nys.) triannulatus | 7 (24) | 25 (< 1) | 7 (< 1) | 23 (6) | 62 (< 1) |
| Anopheles (Ano.) mattogrossensis | 11 (38) | 4 (< 1) | 5 (< 1) | 3 (< 1) | 23 (< 1) |
| Anopheles (Ano.) shannoni | 0 (0) | 2 (< 1) | 7 (< 1) | 9 (2) | 18 (< 1) |
| Anopheles (Ano.) peryassui | 0 (0) | 4 (< 1) | 6 (< 1) | 0 (0) | 10 (< 1) |
| Anopheles (Nys.) rangeli | 0 (0) | 1 (< 1) | 0 (0) | 0 (0) | 1 (< 1) |
| Total | 29 | 4,012 | 6,310 | 397 | 10,748 |
Sum of 168 24-hour collections (40 minutes/hour; indoors and outdoors at all sites except the forest site, where collections were made at ground level and in the canopy [10 m] at each site).
Fort Vargas Guerra.
Village of Puerto Almendra located about 300 m from the forest collection site.
Consists of two species: Anopheles forattinii and a species related to An. mediopunctatus.
Comparison of mosquitoes collected inside and outside of houses.
Paired human collectors collected mosquitoes inside and outside houses at Puerto Almendra for 84 trap days to determine which mosquito species readily entered dwellings. Over a 24-hour period, similar numbers of An. darlingi were collected inside (3,969) and outside (3,955) of houses. Although relatively few An. darlingi (342/7,924) were collected during the daytime (0600–1800 hours), significantly more were collected inside (62.3%; binomial test, P < 0.001) than outside of houses.
Time of day of An. darlingi activity.
A total of 96.2% (8,585/8,927) An. darlingi was collected between 1800 and 0600 hours, whereas only 3.8% (342/8,927) were collected during the daytime (0600–1800 hours). Although 73% of An. darlingi were collected during the evening (1800–2400 hours), only 27% were collected after midnight (2400–0600 hours).
Biting and infection rates among An. darlingi.
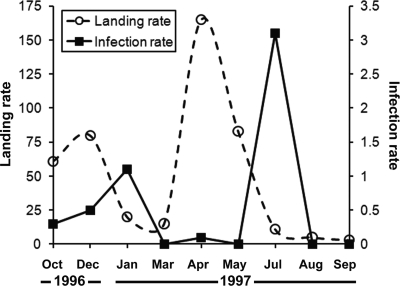
We detected P. falciparum sporozoites (circumsporozoite protein) in 21/6,719 An. darlingi by ELISA (Table 2). At Puerto Almendra, An. darlingi populations fluctuated throughout the seasons, with peak numbers collected during December of 1996 and April of 1997. In contrast, the highest sporozoite infection rates were detected approximately 1–2 months later (January and July of 1997) (Figure 1). In addition to the detection of P. falciparum in 21 An. darlingi, the variant Pv210 of P. vivax was detected from a single unidentified Anopheles (Nyssorhynchus) spp.
Table 2.
Association between mosquito landing rates and sporozoite infection rates in An. darlingi collected by human volunteers in the vicinity of Iquitos, Peru, from October of 1996 to September of 1997
| Collection period | Landing rate* | Infection rate† | Number tested (number infected) | EIR per month‡ |
|---|---|---|---|---|
| October 23 to November 4, 1996 | 61 | 0.3 | 906 (3) | 5.5 |
| December 3–13, 1996 | 80 | 0.5 | 1,161 (6) | 12.0 |
| January 14–28, 1997 | 20 | 1.1 | 471 (5) | 6.7 |
| February 26 to March 9, 1997 | 15 | 0 | 95 (0) | 0 |
| April 7–14, 1997 | 165 | 0.1 | 3,024 (3) | 5.0 |
| May 20 to June 4, 1997 | 83 | 0 | 885 (0) | 0 |
| July 1–16, 1997 | 11 | 3.1 | 129 (4) | 10.2 |
| August 12–25, 1997 | 5 | 0 | 0 (0) | 0 |
| September 17 to October 1, 1997 | 3 | 0 | 48 (0) | 0 |
Number of An. darlingi captured per 24-hour period per person.
Number of An. darlingi containing P. falciparum sporozoites per 100 tested.
Entomologic inoculation rates = landing rate×infection rate/100×30.
Figure 1.
Number of An. darlingi collected per 24-hour period per collector and P. falciparum sporozoite infection rates (number positive/100 tested) for An. darlingi from Puerto Almendra, Peru, from October of 1996 to September of 1997.
Landing rate and river level.
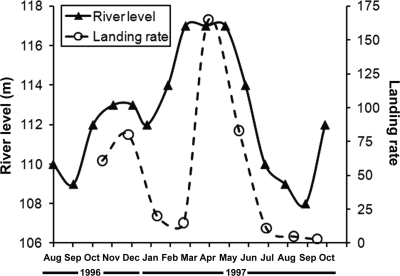
The numbers of An. darlingi collected at Puerto Almendra coincided with the river level, with the increased numbers collected in December and April associated with the rising levels of the Amazon River in addition to other associated rivers and tributary systems (Figure 2).
Figure 2.
Relationship between river level (m above sea level) and landing rates (number of An. darlingi captured per 24-hour period per collector) at Puerto Almendra, Peru, from October of 1996 to September of 1997.
Discussion
An. darlingi was the most frequently collected Anopheles spp. in human landing collections where people resided (98.7%, 66.6%, and 37.9% at Puerto Almendra, two rural residences, and Fort Vargas Guerra, respectively). However, as recently as 1994, this species was not reported from this region.5,6 The rapid increase in the prevalence of this species is believed to be one of the causes for recent increases in malaria cases in this region.3,9 Although An. darlingi readily bites humans and was collected in large numbers at human landing collections, it is not efficiently collected by miniature light traps, and population estimates based on light trap data may be misleading. For example, in an earlier study in Puerto Almendra, > 24-fold more An. darlingi were collected at human landing collections than at dry ice-baited miniature light trap collections conducted concurrently about 10 m apart.12
The principle larval habitat for An. darlingi is among emergent and stationary floating vegetation along streams and river margins in forested areas.23,24 Dispersal of An. darlingi occurs as floating stationary vegetation breaks free from flooded areas, tributaries, and streams and flows downstream. Although larvae of An. darlingi are associated with forest streams and rivers, adults seek open (often deforested and disturbed areas) adjacent to forested sites where people reside for bloodmeals.9,25 This preference is consistent with our results, because human landing collections conducted concurrently at Puerto Almendra (an open area village surrounded by a forest) and a forest site (located about 300 m from the village collection site) resulted in > 300 times as many An. darlingi collected in the village, although > 10 times more mosquitoes (culicines and anophelines) were collected at the forest site.12 Therefore, it may not be the disturbance of the natural environment that allows for the increase in An. darlingi, but rather, this disturbance allows for an increase in the efficiency in capturing this species. Similarly, we collected more An. darlingi at Puerto Almendra (< 0.5 km from the Nanay River) and where there were large numbers of people than from either of the two rural residences located in a disturbed area about 3–4 km from the nearest river. This finding is consistent with the work of Roberts and others26 and may explain the relationship between river levels, human populations, and An. darlingi populations (Figure 2).27
We found that 96.2% of landing activity took place during the hours of darkness (1800–0600 hours), whereas only 3.8% occurred during the daytime (0600–1800 hours). At all sites sampled, peak landing activity occurred about 2 hours after sunset, with 73% of all An. darlingi collected between 1800 and 2400 hours. This finding is consistent with the observations of Roberts and others28 in Brazil and the data previously reported in the work by Turell and others12 for the Peruvian Amazon Basin. Nearly all of the daytime human landing activity for An. darlingi occurred when seasonal populations peaked during April. Although similar numbers of An. darlingi were collected indoors and outdoors during the hours of darkness, significantly more (62.3%) were collected indoors than outdoors during the daytime collections (Table 3). This result may be because of the relative darkness inside houses when resting mosquitoes were disturbed as people moved about and indicates that some of the An. darlingi were using households as daytime resting locations. This finding has implication for indoor residual spraying (IRS). Although only a relative small percentage of the An. darlingi seemed to be resting during the daylight hours in these houses, IRS might actually be effective in killing those individuals that rested on the wall, even if only briefly, during the evening hours.
Table 3.
Percentage (number) of An. darlingi captured by human collectors indoors and outdoors at various locations in the vicinity of Iquitos, Peru, from October of 1996 to September of 1997
| Location | Daytime | Nighttime | Total |
|---|---|---|---|
| Casa de Juan | 63 (197)* | 56 (1,608) | 56.6 (1,805) |
| Casa de Sabino | 43 (7) | 48 (245) | 47.6 (252) |
| Puerto Almendra | 62 (138) | 48 (5,729) | 48.2 (5,867) |
| Total | 62 (342) | 50 (7,582) | 50.1 (7,924) |
Percentage of An. darlingi captured indoors (number captured) at landing collections.
An. darlingi was the only species observed with P. falciparum sporozoites, confirming it as a primary vector of malaria in this region. Peak sporozoite infection rates occurred 1–2 months after peak populations of An. darlingi. This finding may be because of increased longevity of adult mosquitoes after the rainy season (because of lower temperatures) and increased numbers of asymptomatic and symptomatic malaria cases during the rainy season that precedes the receding river levels (Figure 2).3 The lower temperatures observed during the dry season may have little effect on the extrinsic development of Plasmodium spp. as temperatures fall within the optimal range of development.3 However, a more important measure of risk of infection with malaria is the EIR, which accounts for both the number of mosquitoes attempting to bite and the infection rate in these mosquitoes.29 In our study, the EIR remained elevated, even after mosquito populations began to fall, indicating the need for continued mosquito control efforts, even as natural populations were declining.
In summary, the reintroduction or expansion of An. darlingi in urban and rural areas of growth and increased human populations combined with inadequate healthcare services, delayed diagnostics, poor housing (unscreened doors and windows), climate events (i.e., El Niño), inadequate vector control programs, discontinuance of the use of DDT, and habitat modifications (i.e., logging, agriculture, and urban expansion) have greatly contributed to the rapid increase and expansion of malaria in many parts of the Amazon Basin, including Iquitos and the surrounding area.1,3,6,8,9
ACKNOWLEDGMENTS
The technical support of Mark Wooster, Karla Block, Alfonso Gozalo, Helvio Astete, and the Naval Medical Unit 6, Peru, is deeply appreciated. The authors thank the Peruvian Ministry of Health, Direccion Regional de Loreto, for their assistance; Richard C. Wilkerson and E. L. Peyton, Walter Reed Army Institute of Research, for providing taxonomic assistance; Arthur Anderson (US Army Medical Research Institute of Infectious Diseases [USAMRIID]) for providing guidance on obtaining the Human Use Protocol; Martha Harris (USAMRIID) for testing the mosquitoes for sporozoites; Kathy Kenyon (USAMRIID) for expert editorial assistance; Lorraine Farinick (USAMRIID) for the illustrations; and George Korch (USAMRIID) for his support and helpful suggestions.
Disclaimer: The views of the authors do not necessarily reflect the position of the Department of Defense, the Department of the Army, the Department of the Navy, or the naval service at large.
Footnotes
Financial support: This study was supported in part by Work Unit Number 62787A 870 U 8517 of the US Navy.
Authors' addresses: Drew D. Reinbold-Wasson, Michael R. Sardelis, James W. Jones, Terry A. Klein, and Michael J. Turell, Virology Division, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, E-mails: Drew.reinbold@us.army.mil, msardelis@ncmi.detrick.army.mil, mosquitohombre@hotmail.com, Terry.klein@us.army.mil, and michael.turell@amedd.army.mil. Douglas M. Watts, Roberto Fernandez, and Faustino Carbajal, US Naval Medical Research Unit 6, Lima, Peru, E-mails: dowatts@utmb.edu, Roberto.Fernandez@med.navy.mil, and faustinoce@hotmail.com. James E. Pecor, Department of Entomology, Walter Reed Army Institute of Research, Washington, DC, E-mail: PECORJ@si.edu. Carlos Calampa, Director de la Región de Salud de Loreto, Ministry of Health, Iquitos, Peru, E-mail: ccalampa@hotmail.com.
Reprint requests: Michael J. Turell, US Army Medical Research Institute of Infectious Diseases, 1425 Porter St., Fort Detrick, MD 21702-5011, E-mail: michael.turell@amedd.army.mil.
References
- 1.Roberts DR, Laughlin LL, Hsheih P, Legters LJ. DDT, global strategies, and a malaria control crisis in South America. Emerg Infect Dis. 1997;3:295–302. doi: 10.3201/eid0303.970305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowell G, Munayco CV, Escalante AA, McKenzie FE. The spatial and temporal patterns of falciparum and vivax malaria in Perú: 1994–2006. Malar J. 2009;8:142. doi: 10.1186/1475-2875-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aramburu Guarda J, Ramal Asayag C, Witzig R. Malaria reemergence in the Peruvian Amazon region. Emerg Infect Dis. 1999;5:209–215. doi: 10.3201/eid0502.990204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roper MH, Torres RS, Goicochea CG, Andersen EM, Guarda JS, Calampa C, Hightower AW, Magill AJ. The epidemiology of malaria in an epidemic area of the Peruvian Amazon. Am J Trop Med Hyg. 2000;62:247–256. doi: 10.4269/ajtmh.2000.62.247. [DOI] [PubMed] [Google Scholar]
- 5.Need JT, Rogers EJ, Phillips IA, Falcon R, Fernandez R, Carbajal F, Quintana J. Mosquitoes (Diptera: Culicidae) captured in the Iquitos area of Peru. J Med Entomol. 1993;30:634–638. doi: 10.1093/jmedent/30.3.634. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez R, Carbajal F, Quintana J, Chauca H, Watts DM. Presencia del A. (N) darlingi (Diptera: Culicidae), alrededores de la ciudad de Iquitos, Loreto-Peru. Bol Soc Peruana Enfermades Infecciosas Trop. 1996;5:10–20. [Google Scholar]
- 7.Schoeler GB, Flores-Mendoza C, Fernández R, Davila JR, Zyzak M. Geographical distribution of Anopheles darlingi in the Amazon Basin region of Peru. J Am Mosq Control Assoc. 2003;19:286–296. [PubMed] [Google Scholar]
- 8.Gagnon AS, Smoyer-Tomic KE, Bush AB. The El Niño southern oscillation and malaria epidemics in South America. Int J Biometeorol. 2002;46:81–89. doi: 10.1007/s00484-001-0119-6. [DOI] [PubMed] [Google Scholar]
- 9.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 10.Lourenço-de-Oliveira R, Guimarães AE, Arlé M, da Silva TF, Castro MG, Motta MA, Deane LM. Anopheline species, some of their habits and relation to malaria in endemic areas of Rondonia State, Amazon Region of Brazil. Mem Inst Oswaldo Cruz. 1989;84:501–514. doi: 10.1590/s0074-02761989000400008. [DOI] [PubMed] [Google Scholar]
- 11.Charlwood JD. Biological variation in Anopheles darlingi root. Mem Inst Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- 12.Turell MJ, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, Pecor JE, Klein TA. Seasonal distribution, biology, and human attraction patterns of mosquitoes (Diptera: Culicidae) in a rural village and adjacent forested area near Iquitos, Peru. J Med Entomol. 2008;45:1165–1172. doi: 10.1603/0022-2585(2008)45[1165:sdbaha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Jones JW, Turell MJ, Sardelis MR, Watts DM, Coleman RE, Fernandez R, Carbajal F, Pecor JE, Calampa C, Klein TA. Seasonal distribution, biology, and human attraction patterns of culicine mosquitoes (Diptera: Culicidae) in a forest near Puerto Almendra, Iquitos, Peru. J Med Entomol. 2004;41:349–360. doi: 10.1603/0022-2585-41.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Lane J. Neotropical Culicidae. Vol. 2. Sao Paulo, Brazil: University of Sao Paulo; 1953. [Google Scholar]
- 15.Faran ME. Mosquito studies (Diptera: Culicidae) XXXIV. A revision of the Albimanus section of the subgenus Nyssorhynchus of Anopheles. Contrib Am Entomol Inst (Ann Arbor) 1980;15:1–215. [Google Scholar]
- 16.Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq Syst. 1981;13:1–81. [Google Scholar]
- 17.Wilkerson RC, Sallum MA. Anopheles (Anopheles) forattinii a new species in series Arribalzagia (Diptera-Culicidae) J Med Entomol. 1999;36:345–354. doi: 10.1093/jmedent/36.3.345. [DOI] [PubMed] [Google Scholar]
- 18.Pecor JE, Jones J, Turell MJ, Fernandez R, Carbajal F, O'Guinn M, Sardelis M, Watts D, Zyzak M, Calampa C, Klein TA. Annotated checklist of the mosquito species encountered during arboviral studies in Iquitos, Peru (Diptera: Culicidae) J Am Mosq Control Assoc. 2000;16:210–218. [PubMed] [Google Scholar]
- 19.Turell MJ, O'Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi CA, Ludwig V, Mangiafico JA, Kondig J, Wasieloski LP, Jr, Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA. Isolation of viruses from mosquitoes collected in the Amazon Basin region of Peru. J Med Entomol. 2005;42:891–898. doi: 10.1603/0022-2585(2005)042[0891:IOVFMD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Wirtz RA, Charoenvit Y, Burkot TR, Esser KM, Beaudoin RL, Collins WE, Andre RG. Evaluation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med Vet Entomol. 1991;5:17–22. doi: 10.1111/j.1365-2915.1991.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 22.Wirtz RA, Sattabonkgot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. J Med Entomol. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- 23.Manguin S, Roberts DR, Andre RG, Rejmankova E, Hakre S. Characterization of Anopheles darlingi (Diptera: Culicidae) larval habitats in Belize, Central America. J Med Entomol. 1996;33:205–211. doi: 10.1093/jmedent/33.2.205. [DOI] [PubMed] [Google Scholar]
- 24.Achee NL, Grieco JP, Masuoka P, Andre RG, Roberts DR, Thomas J, Briceno I, King R, Rejmankova E. Use of remote sensing and geographic information systems to predict locations of Anopheles darlingi-positive breeding sites within the Sibun River in Belize, Central America. J Med Entomol. 2006;43:382–392. doi: 10.1603/0022-2585(2006)043[0382:uorsag]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts DR, Manguin S, Rejmankova E, Andre R, Harbach RE, Vanzie E, Hakre S, Polanco J. Spatial distribution of adult Anopheles darlingi and Anopheles albimanus in relation to riparian habitats in Belize, Central America. J Vector Ecol. 2002;27:21–30. [PubMed] [Google Scholar]
- 27.Rozendaal JA. Relations between Anopheles darlingi breeding habitats, rainfall, river level and malaria transmission rates in the rain forest of Suriname. Med Vet Entomol. 1992;6:16–22. doi: 10.1111/j.1365-2915.1992.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 28.Roberts DR, Alecrim WD, Tavares AM, Radke MG. The house-frequenting, host-seeking and resting behavior of Anopheles darlingi in southeastern Amazonas, Brazil. J Am Mosq Control Assoc. 1987;3:433–441. [PubMed] [Google Scholar]
- 29.Galardo AKR, Arruda M, Couta AARD, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am J Trop Med Hyg. 2007;76:461–469. [PubMed] [Google Scholar]