Abstract
We report a 47 year-old man from the State of Mato Grosso do Sul, Brazil, with unifocal tibial paracoccidioidomycosis. A radiograph showed an osteolytic lesion on the tibial middle third diaphysis. The diagnosis was confirmed by histopathologic analysis, and treatment with sulfamethoxazole and trimethoprim was started. After three months, the patient showed significant clinical improvement. Ten months after treatment, the patient showed lesion bone healing. This case describes the rare occurrence of an osteolytic lesion caused by endemic Paracoccidioides brasiliensis in Latin America.
Introduction
Paracoccidioidomycosis is a systemic, fungal disease caused by Paracoccidioides brasiliensis. With a limited geographic distribution, this disease affects Latin America; higher incidences are observed in Brazil, Venezuela, Colombia, Ecuador and Argentina. It is acquired through inhalation and spreads by lympho-hematogenous dissemination. Once the fungus has established itself in the body, it can affect any organ or tissue, but most commonly the skin, mucous membranes and lungs. Osteoarticular impairment has also occurred, but unifocal form is rare.1–3 The purpose of this study was to describe an unusual case of unifocal paracoccidioidomycosis located on the middle third of the left tibia, without pulmonary impairment, and to characterize its evolution and treatment.
Case Report
The patient was a white, 47 year-old, rural worker born and presently residing in the State of Mato Grosso do Sul, located in the west central region of Brazil. The patient sought medical attention at the orthopedic care service in April 2007, because of pain in the left leg that had started a month before. During that month, pain worsened and no improvement was achieved by taking analgesics for a week. There were no signs of trauma at the pain focus. The patient was not asthenic, no weight loss was observed, and there were no complaints of fever or cough. The patient had been an alcoholic for 20 years and was a non-smoker.
The patient was in good general health, afebrile and normally colored. Cardiac and pulmonary auscultation were normal. The patient's left leg showed a slight, local volume increase with signs of inflammation and severe pain while palpated in the region of the middle third region. Moderate edema in the left ankle was also observed during physical examination. No significant changes were observed during the remainder of the osteoarticular examination and during the examination of other organs and systems.
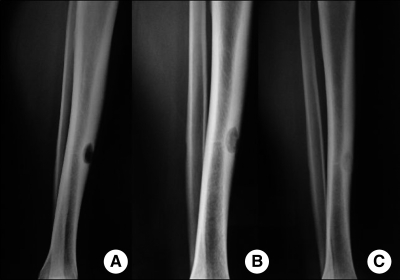
A radiographic study of the left leg showed an oval, osteolytic lesion (length = 22 mm) on the tibial middle third diaphysis and significant periosteal thickening (Figure 1A).
Figure 1.
A, Pre-treatment radiograph of the patient showing an oval osteolytic lesion on the left tibia. B, Radiograph after five months of treatment showing partial bone healing. C, Radiograph after 10 months of treatment showing consolidation of the bone injury.
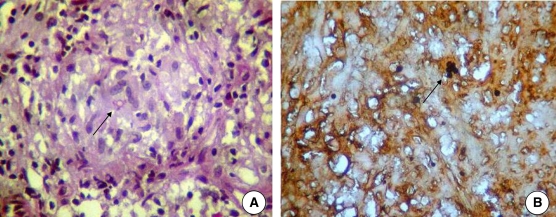
Initial diagnostic hypotheses included osteomyelitis and bone neoplasia. In June 2007, an open biopsy and lesion curettage were performed. Gram bacterioscopy and bacterial culture of the material collected from the injury did not confirm the presence of any pathogenic microorganism. Therefore, the material was not submitted for direct mycologic study and culture for fungi. However, the histopathologic report indicated bone tissue with inflammation composed of lymphocytes, histiocytes, neutrophils and multinucleated giant cells permeated by round and encapsulated structures. Within these structures, double walls and multiple budding, typical fungal structures, were observed and identified as Paracoccidioides brasiliensis by Grocott staining (Figure 2). No malignant formations were observed. Thus, the patient was given a diagnosis of paracoccidioidomycosis osteomyelitis.
Figure 2.
Histologic examination of bone lesion of the patient showing Paracoccidioides brasiliensis yeast (arrows). A, Hematoxylin and eosin stained (magnification×400). B, Grocott stained (magnification×400).
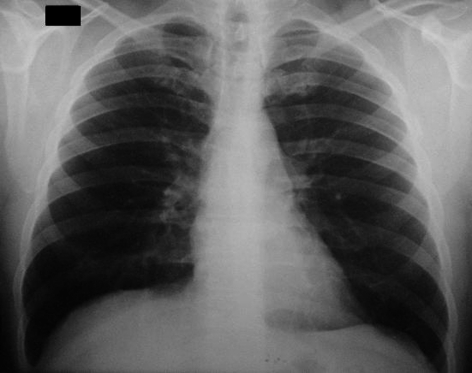
A chest radiograph was normal (Figure 3). In two samples of sputum, fungi or acid-fast bacillus were not found. Serologic testing for paracoccidioidomycosis (agar immunodiffusion) showed negative results.
Figure 3.
Chest radiograph of the patient showing no evidence of pulmonary involvement.
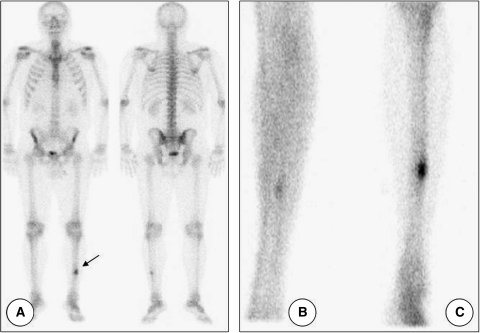
Three-phase bone scintigraphic study with technetium-99m methylene diphosphonate (99mTcMDP) was used to identify lesion foci in other bones. Scintigraphy showed that blood flow in the lower limbs was preserved. The bone metabolic phase showed high focal uptake of the radiopharmaceutical in the middle third of the left tibia (Figure 4). Radiopharmaceutical distributions observed in other bones were physiologically normal.
Figure 4.
Technetium-99m methylene diphosphonate scintigraphy of the patient. A, Physiologic radiopharmaceutical distribution with uptake in the middle third of left tibia (arrow). B, Equilibrium phase. C, Late phase.
The treatment prescribed for paracoccidioidomycosis was 800 mg of sulfamethoxazole and 160 mg of trimethoprim (cotrimoxazole), twice per day. As a general recommendation, the patient was instructed to use crutches when walking.
The patient showed significant clinical improvement after three months of treatment. After five months, there was an improvement in radiologic signs, which showed a clear healing focus (Figure 1B). Complete radiologic healing was achieved 10 months after the aforementioned treatment (Figure 1C). However, to avoid relapses, the patient was recommended to continue to take the medication until June 2009. An ambulatory follow-up was conducted every six months. In April 2011, the patient was asymptomatic.
Discussion
Paracoccidioidomycosis is acquired through inhalation of propagules of the dimorphic fungus Paracoccidioides brasiliensis and results in a primary pulmonary complex either in the lungs or lymph nodes of the hilum. Hematogenous dissemination of the fungus may then occur with the establishment of metastatic foci in any organ. This initial infection is usually asymptomatic and tends to resolve spontaneously, leaving residual lesions that may contain viable fungi for many years (latent or quiescent foci). However, on rare occasions, the primary infection progresses, evolving to a severe acute/subacute disease form (juvenile type).4 Almost 90% of paracoccidioidomycosis cases present as chronic disease (adult type) arising from reactivation of a latent pulmonary or extrapulmonary focus, or from new exposure to the fungi.
In this study, the patient had clinical and epidemiological characteristics commonly observed in chronic adult form paracoccidioidomycosis. These characteristics included being male, a rural worker in a disease-endemic area, and an age of 40–60 years.5,6 In this case, we believe that the patient acquired this infection earlier and a latent bone focus developed by hematogenous dissemination; after a long period of incubation, this focus was reactivated. The patient had always resided in a disease-endemic area and demonstrated no signs of systemic disease, supporting our hypothesis. Bone involvement in paracoccidioidomycosis is usually asymptomatic,7 but may show pain, edema, and feeling of heat in the lesion area,4,6,8 as described in this case.
In general, osteoarticular involvement is associated with the lungs and spreads to bones by lympho-hematogenous dissemination from a primary focus located in the lung.7,9 However, the chest radiograph of the patient in this study was considered normal by the attending physician and two independent radiologists. Paracoccidioidomycosis pulmonary involvement has the typical appearance of bilateral, symmetrical, perihilar, interstitial thickening. Chest tomography can identify imperceptible lesions in plain radiography, but it was not performed in the patient of this study.9 Few cases describing bone involvement in unifocal chronic form paracoccidioidomycosis have been published. (Table 1).1–3,8,10–12
Table 1.
Case reports of unifocal osteoarticular paracoccidioidomycosis
| Authors (year), reference | Bone/articulation | Sex | Age (years) | Diagnostic test | Treatment | Outcome, follow-up time |
|---|---|---|---|---|---|---|
| Krivoy and others (1978)11 | Skull | F | 27 | HP | Sulfamethoxypyridazine, surgery | Unknown |
| Barrios and others (1984)10 | Femur | M | 13 | HP | Cotrimoxazole, surgery | Bone remodeling, 1 year |
| Barrios and others (1984)10 | Metatarsus | F | 57 | HP | Cotrimoxazole, surgery | Graft incorporation, 1 year |
| Silvestre and others (1997)2 | Knee | F | 46 | HP | Ketoconazole followed by cotrimoxazole | Asymptomatic, unknown |
| David and others (1997)3 | Femur | M | 31 | HP; negative serologic result | Sulfadiazine | Fracture consolidation, 3 years |
| Lambertucci and others (2002)12 | Metatarsus | M | 20 | HP | Cotrimoxazole | Asymptomatic, 6 months |
| Cassone and others (2005)8 | Patella | M | 75 | HP; serologic titer 1:256 | Itraconazole | Bone remodeling, 4 years |
| Picado and others (2006)1 | Femur | F | 22 | Serologic titer 1:4 | Cotrimoxazole | Bone remodeling, 2 years |
HP = histopathologic analysis.
Bones that are most affected by the disease are those of the chest wall,7 including ribs, sternum, scapula, and acromion. This finding may be explained by the common routine of performing a chest radiography when paracoccidioidomycosis is suspected. Although uncommon, lesions in the tibia have been reported.13
The most common identified radiographic feature is characterized by distinct outlined lytic lesions with no marginal sclerosis and with little or no periosteal reaction.7,10,13 Although there is general consensus regarding the description of injuries, there are no pathognomonic features of the disease. Therefore, it is imperative to make differential diagnoses, mainly neoplasia and bone metastases, in addition to bacterial osteomyelitis, histiocytosis, tuberculosis, and lymphoproliferative disorders.7,14
The recognized standard for paracoccidioidomycosis diagnosis is identification of fungal elements characteristic of P. brasiliensis by direct mycologic examination of sputum or other clinical specimen (scrapings of lesions, lymph node aspirate), or examination of organ biopsy fragments supposedly affected9. Therefore, diagnosis can be made by histologic evaluation with Grocott stain, which shows round elements, 3–40 μm in diameter, with thick birefringent walls. These elements reproduce by single or multiple buds, which are connected to the parent cell by a narrow cytoplasmic bridge, giving a ship's well aspect, pathognomonic of P. brasiliensis.15
In the case presented, definitive diagnosis was made by histopathologic examination of a bone biopsy sample and not by a mycologic examination. The sample was not subjected to direct mycologic examination and culture for fungi because it was not a routine procedure for the initial hypothesis (osteomyelitis and neoplasm). Another possible diagnostic procedure used could have been fine-needle aspiration puncture biopsy,16 which has demonstrated good performance and is less invasive than a bone biopsy.
Serologic analysis performed by using immunodiffusion is another useful method for establishing the correct diagnosis and adequate therapeutic control; its sensitivity ranges from 85% to 100%. Patients with more localized forms of paracoccidioidomycosis may have negative serologic results,9 as did the patient described in our study. Another explanation for the negative result in the immunodiffusion test could be use of antigen obtained from P. brasiliensis from another region of the country. The antigen used in our laboratory is from São Paulo, located in southeastern Brazil. Regional differences in strains of Paracoccidioides brasiliensis have been described, including one study that reported that serum with negative results from a patient in west central Brazil showed positive results when antigens from local strains were used.17
Molecular methods for diagnosis of paracoccidioidomycosis by polymerase chain reaction are not yet available for routine care. However, one study showed that a nested polymerase chain reaction for detection of P. brasiliensis in tissue samples from skin and mucous membranes is feasible and can be used as an auxiliary tool in paracoccidioidomycosis diagnosis.18 Bone scintigraphy is an excellent test for screening other involved foci. Scintigraphy has been used in unifocal bone paracoccidioidomycosis to rule out asymptomatic lesions not identified by conventional radiography.8,19
The treatment currently recommended for paracoccidioidomycosis is itraconazole. However, cotrimoxazole has been widely used in Brazil because of its high efficacy and it is freely available from the public health services. However, treatment with this drug combination might take longer.9
When the appropriate treatment has been selected, clinical and radiological signs proceeded in a favorable direction. Fibrosis and osteogenesis were observed after antifungal agent administration and the characteristics of injuries gradually slowed. However, because relapses cannot be excluded, ambulatory follow-up should be annually performed for early recurrence detection.9 In conclusion, in disease-endemic regions, paracoccidioidomycosis must be included in the differential diagnosis of osteolytic lesions, even in the absence of positive pulmonary radiologic findings.
Footnotes
Authors' addresses: Bruna C. Castro, Mauricio A. Pompílio, and Anamaria M. M. Paniago, Faculdade de Medicina, Hospital Universitário de Mato Grosso do Sul, MS, Avenida Senador Filinto Müller, 1, 79080-190, Campo Grande, MS, Brazil, E-mails: brunacorreac@hotmail.com, mapompilio@yahoo.com.br, and anapaniago@yahoo.com.br. Danilo N. Odashiro and Maçanori Odashiro, Laboratório de Anatomia Patológica, Rua Rui Barbosa, 3716, 79.002-362, Campo Grande, MS, Brazil, E-mails: daniloodashiro@globo.com and macanoriodashiro@gmail.com. Adalberto Arão-Filho, Sonimed Campo Grande, Rua Dr Arthur Jorge, 1202, 79002-450, Campo Grande, MS, Brazil, E-mail: adalbertoarao@yahoo.com.br.
References
- 1.Picado CH, Garcia FL, Marcondes CR. Late outcome of Paracoccidioides brasiliensis isolated infection on the hip. Acta Ortop Bras. 2006;24:97–99. [Google Scholar]
- 2.Silvestre MT, Ferreira MS, Borges AS, Rocha A, De Souza GM, Nishioka SA. Monoartrite de joelho como manifestação isolada de paracoccidioidomicose. Rev Soc Bras Med Trop. 1997;30:393–395. doi: 10.1590/s0037-86821997000500008. [DOI] [PubMed] [Google Scholar]
- 3.David A, Telóken MA, Dalmina V, Oliveira GK, Oliveira RK. Bone paracoccidioidomycosis: report of a case. Rev Bras Ortop. 1997;32:254–256. [Google Scholar]
- 4.Nogueira MGS Andrade GM, Tonelli E. Clinical evolution of paracoccidioidomycosis in 38 children and teenagers. Mycopathologia. 2006;161:73–81. doi: 10.1007/s11046-005-3653-7. [DOI] [PubMed] [Google Scholar]
- 5.Blotta MH, Mamoni RL, Oliveira SJ, Nouer SA, Papaiordanou PM, Goveia A, Camargo ZP. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am J Trop Med Hyg. 1999;61:390–394. doi: 10.4269/ajtmh.1999.61.390. [DOI] [PubMed] [Google Scholar]
- 6.Paniago AM, Aguiar JI, Aguiar ES, Da Cunha RV, Pereira GR, Londero AT, Wanke B. Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul. Rev Soc Bras Med Trop. 2003;36:455–459. doi: 10.1590/s0037-86822003000400004. [DOI] [PubMed] [Google Scholar]
- 7.Costa MA, Carvalho TN, Araújo Jr CR, Borba AO, Veloso GA, Teixeira KS. Extra-pulmonary manifestations of paracoccidioidomycosis. Radiol Bras. 2005;38:45–52. [Google Scholar]
- 8.Cassone AE, Mello W, Jr, Alvarenga M, Moreira MP. Patellar paracoccidioidomycosis. Case report. Rev Bras Ortop. 2005;40:288–294. [Google Scholar]
- 9.Shikanai-Yasuda MA, Telles Filho FQ, Mendes RP, Colombo AL, Moretti ML. Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop. 2006;39:297–310. doi: 10.1590/s0037-86822006000300017. [DOI] [PubMed] [Google Scholar]
- 10.Barrios JC, Taniguchi W, Simoneti CA, Andrade AM, Rocha EF. South American blastomycosis with isolated bone affection. Report on two cases. Rev Bras Ortop. 1984;19:117–123. [Google Scholar]
- 11.Krivoy S, Belfort EA, Mondolfi A, Walzer I, Essenfeld E, Landaeta T. Paracoccidioidomycosis of the skull. Case report. J Neurosurg. 1978;49:429–433. doi: 10.3171/jns.1978.49.3.0429. [DOI] [PubMed] [Google Scholar]
- 12.Lambertucci JR, Botelho JS, Melo FH. Osteomyelitis by Paracoccidioides brasiliensis. Rev Soc Bras Med Trop. 2002;35:271–272. doi: 10.1590/s0037-86822002000300015. [DOI] [PubMed] [Google Scholar]
- 13.Nanni L, Pereira IC, Marussi VH. A review on bone paracoccidioidomycosis. Rev Imagem. 2004;26:121–124. [Google Scholar]
- 14.Nogueira SA, Guedes AL, Wanke B, Capella S, Rodrigues K, Abreu TF, Morais JC, Lambert JS. Osteomyelitis caused by Paracoccidioides brasiliensis in a child from the metropolitan area of Rio de Janeiro. J Trop Pediatr. 2001;47:311–315. doi: 10.1093/tropej/47.5.311. [DOI] [PubMed] [Google Scholar]
- 15.Wanke B, Londero AT. In: Topley and Wilson's Microbiology and Microbial Infections. 9th edition. Collier L, Balows A, Sussman M, editors. London: Arnold; 1998. pp. 395–407. (Paracoccidioides brasiliensis). [Google Scholar]
- 16.Borgia G, Reynaud L, Cerini R, Ciampi R, Schioppa O, Dello Russo M, Gentile I, Piazza M. A case of paracoccidioidomycosis: experience with long-term therapy. Infection. 2000;28:119–120. doi: 10.1007/s150100050060. [DOI] [PubMed] [Google Scholar]
- 17.Batista J, Jr, de Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJ, Hahn RC. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses. 2010;53:176–180. doi: 10.1111/j.1439-0507.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 18.Bialek R, Ibricevic A, Aepinus C, Najvar LK, Fothergill AW, Knobloch J, Graybill JR. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J Clin Microbiol. 2000;38:2940–2942. doi: 10.1128/jcm.38.8.2940-2942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaga LY, Benard G, Hironaka FH, Castro LG, Funari MG, De Castro CC, Guertzenstein C, Watanabe T, Buchpiguel C, Cerri GG, Shikanai-Yasuda MA. The role of gallium-67 scan in defining the extent of disease in an endemic deep mycosis, paracoccidioidomycosis: a predominantly multifocal disease. Eur J Nucl Med Mol Imaging. 2003;30:888–894. doi: 10.1007/s00259-003-1172-7. [DOI] [PubMed] [Google Scholar]