Abstract
Background
Annual movements of tri-colored bats (Perimyotis subflavus) are poorly understood. While this species has been considered a regional migrant, some evidence suggests that it may undertake annual latitudinal migrations, similar to other long distance North American migratory bat species.
Methodology/Principal Findings
We investigated migration in P. subflavus by conducting stable hydrogen isotope analyses of 184 museum specimen fur samples and comparing these results (δDfur) to published interpolated δD values of collection site growing season precipitation (δDprecip). Results suggest that the male molt period occurred between June 23 and October 16 and 33% of males collected during the presumed non-molt period were south of their location of fur growth. For the same time period, 16% of females were south of their location of fur growth and in general, had not travelled as far as migratory males. There were strong correlations between δDfur from the presumed molt period and both growing season δDprecip (males – r 2 = 0.86; p<0.01; females – r 2 = 0.75; p<0.01), and latitude of collection (males – r 2 = 0.85; p<0.01; females – r 2 = 0.73; p<0.01). Most migrants were collected at the northern (>40°N; males and females) and southern (<35°N; males only) extents of the species' range.
Conclusions/Significance
These results indicate a different pattern of migration for this species than previously documented, suggesting that some P. subflavus engage in annual latitudinal migrations and that migratory tendency varies with latitude and between sexes. We suggest that this species' hibernation ecology makes it particularly susceptible to long winters, making migration from the northern extent of the species' range to more southern hibernacula preferable for some individuals. Fur δD values for some of the northern individuals may indicate an increase in the currently accepted northern range of this species. Sex-biased differences in migration may be the result of differences in reproductive pressures.
Introduction
Many species of North American bats migrate and employ several strategies to do so. Some species are regional migrants, radiating annually from winter hibernation sites to summer sites, then travelling among swarming sites in the autumn [1]–[4]. Bats engaging in this type of migration have been recorded travelling distances of 500 km or more [2], [5] and may move in any direction to hibernacula. Other species are latitudinal migrants, travelling south in the autumn and north in the spring [6]–[8]. There is evidence that one species of latitudinal migrant, the hoary bat (Lasiurus cinereus), may travel >2000 km one way during annual movements [7].
Tri-colored bats (Perimyotis subflavus, formerly included in genus Pipistrellus [9], [10]) are common in eastern North America ranging from Central America in the south to southern Canada in the north [11], [12]. Since the 1980s, the range of this species has expanded considerably, both to the west into New Mexico, Colorado, Wyoming, and South Dakota [13] as well as into the Great Lakes basin [14], [15].
During summer, P. subflavus roosts both in buildings [16] and in foliage [17]–[19]. Females may roost alone or in colonies, while males roost singly [12], [18]. In autumn, P. subflavus engage in swarming behavior, after which they hibernate in caves, abandoned mines and occasionally human-made structures [14], [15], [20]–[25]. There is little information about their movements among summering grounds, swarming sites, and hibernacula [26], but they are currently believed to be a short-distance regional migrant [12], [27], [28].
However, seasonal variation in abundance and sex ratios of P. subflavus has led some authors to speculate that individuals migrate farther distances than previously suspected [26], [29], [30] and that migratory behavior may vary between sexes [31], [32]. Further, recent studies have documented local increases in or an appearance of P. subflavus activity concurrent with increased activity of other latitudinal migrants during the autumn migration time period [33], [34]. Bats are frequently killed by wind turbines [26] and the species most susceptible to this type of mortality tend to engage in long-distance latitudinal migration [26], [35] and rely heavily on trees as roost sites. Within its range, P. subflavus is among the most frequently killed species around wind turbines and may account for up to 25% of total bat mortality [35], a much higher proportion than known regional migrants.
Stable hydrogen isotope analysis is now a common tool used to learn about the origin of migratory animals [36], [37]. There is a latitudinal pattern in the stable hydrogen isotope ratios of precipitation, with precipitation at more northern latitudes being increasingly depleted of deuterium compared to that at more southern latitudes. The stable hydrogen isotope composition of precipitation is recorded in the tissues of local animals. In the case of inert tissues such as fur and claw, stable hydrogen isotope composition is fixed at the time of formation and subsequent analyses of these tissues can provide information about the location of a migrant when the tissue was formed. Several authors have investigated tissue stable hydrogen isotope variability in bats across latitudes [38], and have used this tool to investigate altitudinal migration [39] and to describe annual migration in hoary bats (Lasiurus cinereus) [7].
The purpose of the current study was to investigate continental patterns in the annual movements of P. subflavus using stable hydrogen isotope analyses of fur samples. We predicted that the stable hydrogen isotope values of fur (δDfur) taken from animals collected between June and August, when the annual molt is believed to occur [7], would correlate closely with the latitude of capture [38], as well as the stable hydrogen isotope values of the predicted growing season precipitation (δDprecip) at that location [7], [40]. Further, we predicted that the difference between δDfur and δDprecip at the site of capture would be smallest during summer months (when fur is grown) and greater during winter (when the bats have migrated from the site of fur growth).
Methods
Sample collection and analysis
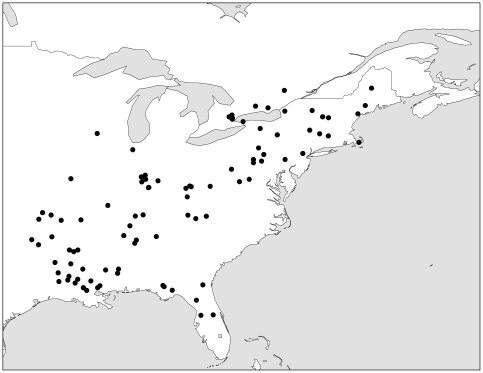
We obtained 184 fur samples taken from the lower dorsal region of P. subflavus study skins from four North American museum collections: Royal Ontario Museum (Toronto, ON), Louisiana State University Museum of Natural Science (Baton Rouge, LA), Harvard University Museum of Comparative Zoology (Cambridge, MA) and Cornell University Museum of Vertebrates (Ithaca, NY). Museum specimens were collected between 1878 and 1986 during all seasons and across most of the species' range (Figure 1; Table S1).
Figure 1. Tri-colored bat (Perimyotis subflavus) collection sites.
Dorsal fur samples from 184 Perimyotis subflavus museum study skins were sampled from individuals collected across most of the species' range. Each black dot represents a collection location; multiple individuals were collected from some locations.
Fur samples were soaked overnight in a 2∶1 solution of chloroform∶methanol [41], rinsed the next day in the same solution and then dried in a fume hood for ≥48 hrs. Complex organic materials contain a fraction of hydrogen that is readily exchangeable with ambient vapor at room temperature. To correct for this uncontrolled exchange, we analyzed all samples alongside five in-house fur standards with known non-exchangeable δD values using a comparative equilibration approach [42]. Standards and samples were weighed into silver capsules (0.175 mg +/− 10 µg) with a 10% rate of duplication. After being weighed, samples and standards were left to equilibrate with laboratory air for a minimum of four days [43]. Samples were pyrolyzed using a High Temperature Conversion Elemental Analyzer (TC/EA) and analyzed using online continuous-flow isotope ratio mass spectrometry (IRMS). Results are expressed as parts per thousand (‰) relative to Vienna Standard Mean Ocean Water (VSMOW). Analytical precision, based on repeated analyses of fur from the same individual bat during each analysis, was less than 2‰ (1 standard deviation). The mean (± standard deviation) difference between duplicates of the same sample was 2±2‰.
Data analysis
In some instances, GPS coordinates were available from individual museum databases for the locations of specimen collection. When this was not the case, coordinates were determined for the centroid of the county of collection using the United States Geological Survey Geographic Names Information System (http://geonames.usgs.gov/domestic/, accessed September 2010). Predicted growing season δDprecip values were determined for the collection locality of each specimen using the geospatial data available from waterisotopes.org [40], [44].
We conducted analyses of males and females separately and first plotted the difference between δDfur and predicted growing season δDprecip at the location of capture (ΔDfur-precip) for individual bats against Julian date. Based on these results, we visually determined the time when bats were at their location of molt as the time period when ΔDfur-precip was least variable (similar to the approach taken by [7]). This definition of the molting timeframe is an estimate based on proxy evidence and we did not directly observe fur replacement in this species. For the remainder of this manuscript, we refer to samples collected during the estimated molt period as having been collected during the “molt period”, and samples collected outside of the estimated molt period has having been collected during the “non-molt period”.
We then regressed the mean δDfur values of bats collected during the molt period from each sampling location (number of bats from each location ranged from one to seven) against both collection latitude [38] and predicted growing season δDprecip values [40], [44], using linear and quadratic regressions for both predictors. We used the equations of the male and female δDfur/δDprecip regression lines to calculate predicted δDfur values for each individual (based on the predicted growing season δDprecip value). In order to determine if there was isotopic evidence of latitudinal movement, and if so, if this behavior was more prevalent in some parts of the species' range than others, we plotted the difference between δDfur and predicted δDfur (ΔDfuractual-predict) of all bats against Julian date and of all bats collected during the non-molt period against latitude.
We calculated the approximate origin of the individual bat that was farthest from its location of fur growth (greatest ΔDfuractual-predict value) by using the equation of the δDfur/δDprecip quadratic regression to calculate the predicted δDprecip value at its presumed location of fur growth. Substantial variation exists in the δDfur values of bats collected from the same location during the molt period (which theoretically should be isotopically identical) [45], [46]. The expectation that one can estimate the δDprecip value at the location of fur growth based on the stable hydrogen isotope composition of one tissue sample is overly simplistic. Accordingly, to account for variation in molt period δDfur values, we referred to the sex-specific range of ΔDfuractual-predict values that we recorded during the molt period and used the equation of the δDfur/δDprecip quadratic regression to calculate the δDprecip value associated with the individual bat's δDfur value ±50% of the ΔDfuractual-predict variation. The results provide conservative estimates of the maximum and minimum δDprecip values at the presumed location of fur growth according to the available data on variation in ΔDfuractual-predict values during the molt period.
Results
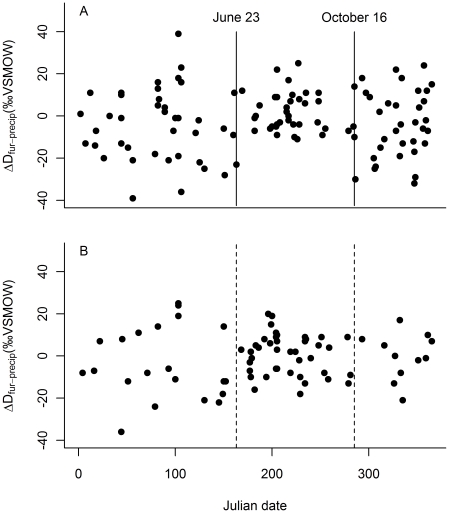
Male ΔDfur-precip values were least variable during the summer months, indicating that male P. subflavus molted between June 23 and October 16 (though a period for which we had no samples means that this end date may have been as early as September 9). For females, there was no clear period of low variability that would indicate a molt and then movement away from the molt location (Figure 2). Hereafter, we refer to the molt period as the time when the bat is at its location of fur replacement and define that time as being between June 23 and October 16. The molt period was indicated isotopically only by male bats and we make the explicit assumption that the female molt timing is identical to that of males.
Figure 2. Molt period estimation.
The difference between δDfur and δDprecip (ΔDfur-precip) for males (panel A) was smallest between June 23 and October 16, as indicated by two vertical lines representing the estimated molt period (n = 111). There was no clear trend in female ΔDfur-precip values across seasons (n = 73) (panel B), so the male estimated molt period was applied to females (dashed vertical lines).
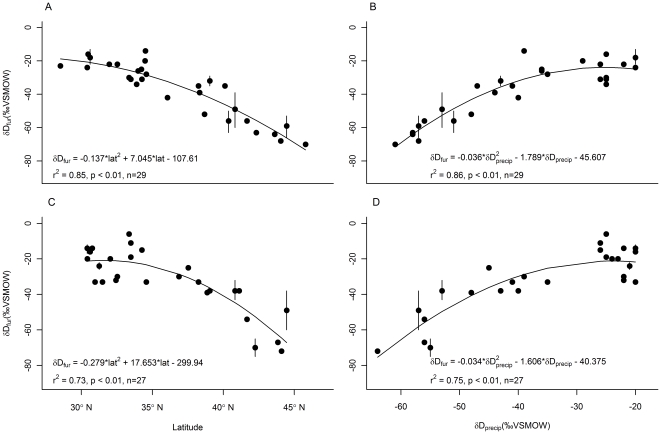
For each molt period δDfur/latitude and δDfur/δDprecip regression, the quadratic model was better than simple linear regression (indicated by higher adjusted r 2), and these are the relationships that we report. Male δDfur values from the molt period correlated significantly with latitude of collection (r 2 = 0.85, F = 73.30, df = 28, p<0.01) and predicted growing season δDprecip at the collection site (r 2 = 0.86, F = 84.8, df = 28 p<0.01). Female molt period δDfur values also correlated significantly with both variables (latitude – r 2 = 0.73, F = 35.4, df = 26, p<0.01; δDprecip – r 2 = 0.75, F = 39.3, df = 26, p<0.01) (Figure 3). Because latitude and δDprecip were almost equally effective at predicting molt period δDfur values, we followed the advice of [47] and used the relationship between molt period δDfur values and predicted growing season δDprecip to generate predicted δDfur values for all bats.
Figure 3. Regressions between molt period δDfur and growing season δDprecip or latitude at the collection sites.
The δDfur values (site mean ± standard deviation) of males collected during the molt period varied significantly with latitude of capture (panel A) and predicted growing season δDprecip at the location of capture (panel B). The same was true of female molt period δDfur values and latitude (panel C) and δDprecip (panel D). n = 1 to 7 individuals per site. Predicted growing season δDprecip values were obtained from waterisotopes.org [40], [44].
Our initial plot of ΔDfur-precip against Julian date (used to estimate molt period) implicitly assumed a linear relationship between molt period δDfur values and predicted local growing season δDprecip. Our data suggest that a quadratic curve better describes this relationship for both male and female bats. To correct for this, we used the sex-specific quadratic regression equations to calculate the predicted δDfur value for each bat based on the predicted growing season δDprecip value at its location of capture [40], [44]. We re-did the initial plot to show ΔDfuractual-predict against Julian date for both male and female bats (Figure 4).
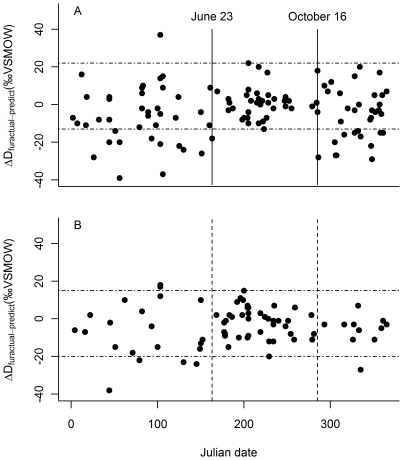
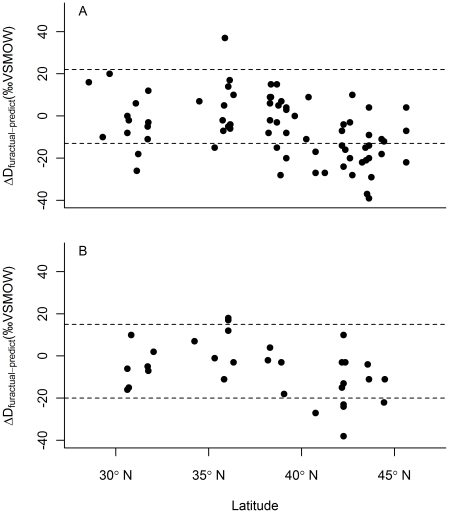
Figure 4. Identification of latitudinal migrants.
The range of variation in molt period ΔDfuractual-predict values (where “molt period” refers to the estimated molt period shown in Figure 2) is indicated with horizontal dashed lines. Individuals with ΔDfuractual-predict values below the horizontal band likely did not grow their fur at their collection site, and are assumed to have migrated from a more northern location. Thirty-three percent of all males (n = 111) (panel A) and 16% of all females (n = 73) (panel B) collected during the non-molt period appeared to be south of their location of fur growth. One non-molt period male and two females appeared to be north of their location of fur growth.
During the molt period, the difference between maximum and minimum ΔDfuractual-predict values was 35‰ for both males and females. During the non-molt period, the difference was 76‰ for males and 53‰ for females. Twenty-four of 73 males (32.9%) sampled during the non-molt period had ΔDfuractual-predict values that were more negative than any observed during the molt period, indicating that these individuals were captured south of their location of fur growth. One individual (1.4%) showed evidence of northward movement. Five of 32 females (15.6%) sampled during the non-molt period had ΔDfuractual-predict values indicative of a more northern location of fur growth, although in general, these values were not as negative as those observed among the male migrants. Two females captured during the non-molt period (6.3%) had ΔDfuractual-predict values that may have indicated a slight northward movement.
For male bats, non-molt period ΔDfuractual-predict values were most negative at high (>40°) and low (<35°) latitudes and closest to the range of molt period values at mid-latitudes (Figure 5), suggesting that high and low latitude individuals engage in more substantial southern migrations than do mid-latitude males. The majority of the females with non-molt period ΔDfuractual-predict values indicating they were south of their location of fur growth were captured at the northern end of the species' range.
Figure 5. Latitudinal differences in migratory tendency.
ΔDfuractual-predict values from the non-molt period were more negative than values from the molt period at both northern and southern latitudes for males (n = 73) (panel A) and only at northern latitudes for females (n = 32) (panel B), indicating southern migration from the latitude of fur growth by these individuals. The range of ΔDfuractual-predict values from the molt period is indicated by horizontal dashed lines.
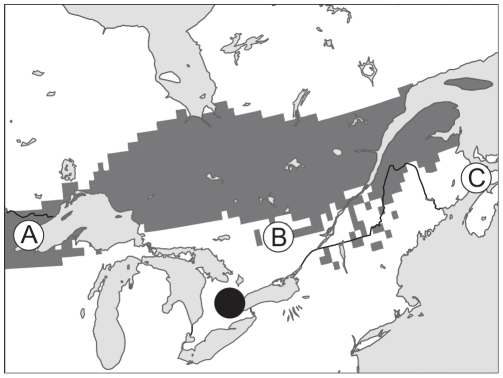
The bat that was most distinct in isotopic composition from the precipitation at its location of capture was a male collected from southwestern Ontario (43.62 decimal degrees N, −80.13 decimal degrees W). This bat also was one of two that had the most negative δDfur composition (−93‰) of all bats sampled, indicating the most northern point of fur growth/origin. Based on an extrapolation of the relationship that we established between molt period δDfur values and predicted growing season δDprecip at the location of capture, we predict that this individual bat grew its fur at a location with a mean predicted growing season δDprecip composition between −63‰ and −74‰ (Figure 6).
Figure 6. Estimated origin of the farthest migrant.
The bat with the δDfuractual-predict value suggesting that it had migrated the farthest from its latitude of fur growth also had a δDfur value indicating that it was from the most northern location (collection site indicated by black circle). It likely originated at a location where the mean growing season δDprecip composition was between −63 and −74‰ (the area shaded in grey). The lettered points indicate the existing most northern records of Perimyotis subflavus (A = [60]; B = [2]; C = [65]). Predicted growing season δDprecip values were obtained from waterisotopes.org [40], [44].
Discussion
Our results indicate a fundamentally different picture of the annual migratory ecology of P. subflavus than has previously been assumed. Perimyotis subflavus visit swarming sites in autumn and hibernate during the winter [12], sharing many characteristics with other species that have been documented to engage in a regional radiation pattern of migration, moving from central hibernacula to summer maternity colonies (e.g., little brown bats (Myotis lucifugus) [1], [2]). However, the δDfur composition of many of the bats collected during the non-molt period suggested that some individuals moved south of their region of fur growth and very few moved north. If P. subflavus only engages in regional, radiating migratory behavior typical of other species of bats that hibernate in caves, then we would expect to see evidence that equal numbers of bats migrate north and south during the non-molt period. Consequently we conclude that at least some P. subflavus of both sexes engage in the type of latitudinal migration that is more typically associated with hoary bats (Lasiurus cinereus), eastern red bats (Lasiurus borealis), and silver-haired bats (Lasionycteris noctivagans) [6], and that this behavior is more common for males than for females.
Sex-biased migration
Sex-biased migratory behavior is common among bats [28]. Typically, when sex-biases exist, females have a greater tendency to migrate than do males [6], [48]. The mechanisms driving female-biased migration have not been identified, but it has been hypothesized that the elevated energetic demands of pregnancy and lactation lead females to move to habitats where there is higher resource availability or access to roosts with better thermoregulatory properties [28]. Our data are in contrast to this trend, suggesting that males have a greater tendency to make long-distance north-south migrations than do females. One of the few examples of male-biased migration in bats was documented in the West African species Myonycteris torquata [49], and the authors hypothesized that females were limited in their movement by the costs of pregnancy and lactation while males were not similarly constrained and were able to track seasonal food resources. It is possible that temporal differences in reproductive-based energetic limitations may be a factor in causing the sex-biased migratory tendencies for which we see evidence in P. subflavus.
Male and female bats experience reproductive stress at different times of the year [50]. Females ovulate and become pregnant following emergence from hibernation and have increased energetic requirements throughout pregnancy and lactation in the early to mid-summer [51], [52]. Conversely, males experience reproductive costs in late summer during spermatogenesis [53], [54]. Variation in reproductive status has been linked to behavioral changes, such as increased food consumption [55], differential torpor use [56], [57], foraging bout duration [58] and home range size [59]. It is possible that reproductive demands upon emergence from hibernation preclude large-scale northern migration by females, whereas males are not subject to the same limitations.
Effects of latitude on migratory tendency
All of the females and many of the males captured during the non-molt period with fur stable isotope compositions indicating that they were south of their location of fur growth, were collected from the northern part of the P. subflavus range. In some cases, δDfur values from the non-molt period indicated that the fur had been grown at least at the northern extent of the known range for the species [2], [12], [60], [65], and perhaps even farther north than these locations. We suggest that some P. subflavus summering at the extreme northern edge of the range may migrate south (although still remaining in the northern portion of the known species range) to hibernate at sites where winters are shorter and their probability of survival is higher [61].
Previous research [62] suggests that while winter length is a factor in survivorship of hibernating little brown bats (Myotis lucifugus), a more important factor is the extent to which the bats hibernate in clusters. Hibernating in clusters decreases the amount of energy lost during normothermic bouts within the hibernation period [63] and as long as bats are hibernating in clusters, the impact of varying winter length on survivorship is negligible [62]. However, the hibernation ecology of P. subflavus differs from that of many other species; it is well documented hibernating singly or occasionally in small clusters, but not frequently in large clusters [12], [15], [24], [64]–[67]. We suggest that hibernating singly makes P. subflavus more susceptible to the longer winters at northern hibernacula than clustering species; resulting in an increased probability of survival for individuals who migrate south to hibernate.
Bat residency and the molt period
Interpretation of our results relies heavily on the timing and location of bat fur growth, so it is appropriate to consider molting patterns of P. subflavus. Previous studies of molting patterns of temperate bats suggest that molt usually occurs once annually between mid-June and September [7], [68], [69], similar to the period that we have defined for male P. subflavus. However, it is important to remember that our isotopically estimated molt period does not describe the period of actual hair replacement, but only the period when the bat remains a resident at its location of fur growth; i.e., fur replacement happens at some point during the isotopically defined “molt period”. Currently we know of no data documenting inter-annual consistency in resident bat δDfur values, and P. subflavus philopatry is poorly understood, although there is evidence that females return to the same summer roosting sites each year [16], [70]. It is unclear if the initial date of the molt period (June 23) is actually indicative of the beginning of fur replacement or just the date when all sampled bats had returned to regular summering grounds and hence had fur grown the previous year that was still isotopically indicative of their location of capture.
Our earliest estimate for when bats may have begun to leave their location of fur growth is September 9. This date is a much later start to the autumn migratory movement than has previously been recorded for P. subflavus specifically, and for other North American bat species in general. In Missouri, subadult P. subflavus started arriving at swarming sites on August 5 [29] and studies of other swarming and hibernating species indicate that male bats begin to arrive at swarming sites as early as August 1 [2]. Our stable isotope results may indicate a tendency for bats to only engage in long distance southern migration in mid to late fall, but we think that the late migration date that we detected is more likely an artifact of sampling bias. Logistically, the easiest places to capture and collect a colonial species such as P. subflavus are summer colonies and winter hibernacula. Little is known about the mobility and roosting habits of these bats during the late summer and fall when they engage in swarming behavior, making them challenging to reliably locate. Minimal collection data exists for the specimens used in this study, so we do not know the circumstance in which most were collected. If the late summer bats included in this study were collected from known summer colonies, then they may represent a minority of bats that remained at their summer location into the late summer and early fall and may not be representative of the majority of P. subflavus. A follow-up study using samples collected from bats captured at swarming sites during the fall season may clarify this point.
Variation in female fur isotopic compositions was similar throughout the year, so we could not identify a time period for the female molt. Throughout this study, we have applied the male isotopically defined molt period to both sexes, which is a necessary but potentially problematic approach. Other authors [7], [68], [71] reported that male and female molt patterns may vary in timing, likely as a function of the increased energetic demands faced by females during reproduction. Male Tadarida brasiliensis and Myotis velifer molted before female conspecifics [68] and female Miniopterus schreibersii ceased molting during lactation [71]. Delayed molt timing in females could translate to decreased isotopic migration detectability. If females are still molting as they begin to migrate, then the stable isotope signature of the food and water that they drink at a range of locations will be integrated into their fur. The δDfur values would not be indicative of one location of fur growth, but the average of a range of locations.
Summary - Perimyotis subflavus as a partial and differential latitudinal migrant
We found that the δDfur values of P. subflavus collected during the molt period are a good predictor of both the latitude and the predicted local growing season δDprecip of the collection location and so can be used as an indicator of bat movement away from that location. Stable isotope evidence suggested that some bats of both sexes underwent a southern fall migration during the non-molt period and that this behavior was more prevalent in males than in females. The majority of individuals for which isotope values suggested a latitudinal migration (both sexes) were captured in the northern portion of the species' range. Some non-molt period males from the southern portion of the range also showed evidence of southern migration.
It is important to note that migration is a characteristic of individuals and not of populations or species [28], [72]. Migratory behavior varies greatly among individuals based on a variety of intrinsic and extrinsic factors. Both partial migration (movement of some members of a species, but not all) and differential migration (sex or age cohorts exhibiting different migratory patterns) [73] are common in bat species [28]. Our data suggest that P. subflavus can be described as both a partial migrant and differential migrant, as stable isotope results provide evidence of differences in migratory behavior between sexes and among latitudes. Though some individuals may undertake regional radiation migrations as previously suggested, our evidence suggests that latitudinal migration is a strategy undertaken by a large proportion of individuals.
Supporting Information
Collection and stable isotope data for all specimens. Each bat is identified with a museum-specific abbreviation (CU - Cornell University Museum of Vertebrates, Ithaca, NY; LSUMZ - Louisiana State University Museum of Natural Science, Baton Rouge, LA; MCZ – Harvard University Museum of Comparative Zoology, Cambridge, MA; ROM - Royal Ontario Museum, Toronto, ON) and its associated catalog number. Collection coordinates for each specimen are included in decimal degrees. For some specimens, exact collection coordinates were available. For specimens where exact collection data were not available, coordinates for the centroid of the county of collection were used and reported below (obtained from the United States Geological Survey Geographic Names Information System (http://geonames.usgs.gov/domestic/, accessed September 2010). Predicted growing season δDprecip values were obtained from waterisotopes.org [40], [44]. All stable isotope data are reported in ‰ relative to VSMOW. F = female; M = male.
(DOC)
Acknowledgments
We thank Dr. Burton Lim (Royal Ontario Museum); Dr. Mark Hafner (Louisiana State University Museum of Natural Science); Judith Chupasko (Harvard University Museum of Comparative Zoology); and Charles Dardia (Cornell University Museum of Vertebrates) for donating fur samples. We thank Li Huang and Kim Law for their assistance in the laboratory; and Dr. Gabor Sass and Johnston Miller for their assistance in mapping these data. Data used to create the map in Figures 1 and 6 were obtained from www.naturalearthdata.com. Comments from three anonymous reviewers significantly improved the quality of this manuscript. This is Western's Laboratory for Stable Isotope Science Contribution #271.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this research was provided by the Natural Sciences and Engineering Research Council (MBF, FJL, LPM, EEF; Discovery grant #s 3331-2010 (MBF); 3890-2009 (FJL)), The Canadian Foundation for Innovation (FJL; infrastructure grant # 2732), and Bat Conservation International (EEF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Davis WH, Hitchcock HB. Biology and migration of the bat, Myotis lucifugus, in New England. J Mammal. 1965;46:296–313. [Google Scholar]
- 2.Fenton MB. Summer activity of Myotis lucifugus (Chiroptera: Vespertilionidae) at hibernacula in Ontario and Quebec. Can J Zool. 1969;47:597–602. [Google Scholar]
- 3.Rivers NM, Butlin RK, Altringham JD. Autumn swarming behavior of Natterer's bats in the UK: Population size, catchment area and dispersal. Biol Conserv. 2006;127:215–226. [Google Scholar]
- 4.Rodrigues L, Palmeirim JM. Migratory behavior of the Schreiber's bat: when, where and why do cave bats migrate in a Mediterranean region? J Zool. 2007;274:116–125. [Google Scholar]
- 5.Dubois JE, Monson KM. Recent distribution records of the little brown bat, Myotis lucifugus, in Manitoba and Northwestern Ontario. Can Field Nat. 2007;121:57–61. [Google Scholar]
- 6.Cryan PM. Seasonal distribution of migratory tree bats (Lasiurus and Lasionycteris) in North America. J Mammal. 2003;84:579–593. [Google Scholar]
- 7.Cryan PM, Bogan MA, Rye RO, Landis GP, Kester CL. Stable hydrogen isotope analysis of bat hair as evidence for seasonal molt and long-distance migration. J Mammal. 2004;85:995–1001. [Google Scholar]
- 8.Findley JS, Jones C. Seasonal distribution of the hoary bat. J Mammal. 1964;45:461–470. [Google Scholar]
- 9.Menu H. Révision du statut de Pipistrellus subflavus (F. Cuvier, 1832). Proposition d'un taxon générique nouveau: Perimyotis nov. gen. Mammalia. 1984;48:409–416. [Google Scholar]
- 10.Hoofer SR, Van Den Bussche RA, Horáček I. Generic status of American Pipistrelles (Vespertilionidae) with description of a new genus. J Mammal. 2006;87:981–992. [Google Scholar]
- 11.Barbour RW, Davis WH. Bats of America. Kentucky: The University Press of Kentucky; 1969. 286 [Google Scholar]
- 12.Fujita MS, Kunz TH. Pipistrellus subflavus. . Mammalian Species. 1984;228:1–6. [Google Scholar]
- 13.Geluso K, Mollhagen TR, Tigner JM, Bogan MA. Westward expansion of the eastern pipistrelle (Pipistrellus subflavus) in the United States, including new records from New Mexico, South Dakota, and Texas. West N Am Naturalist. 2005;65:405–409. [Google Scholar]
- 14.Kurta A, Winhold L, Whitaker JO, Jr, Foster R. Range expansion and changing abundance of the Eastern pipistrelle (Chiroptera: Vespertilionidae) in the central Great Lakes region. Am Midl Nat. 2007;157:404–411. [Google Scholar]
- 15.Slider RM, Kurta A. Surge tunnels in quarries as potential hibernacula for bats. Northeast Nat. 2011;18:378–381. [Google Scholar]
- 16.Allen AA. Banding bats. J Mammal. 1921;2:53–57. [Google Scholar]
- 17.Poissant JA, Broders HG, Quinn GM. Use of lichen as a roosting substrate by Perimyotis subflavus, the Tricolored bat, in Nova Scotia. Écoscience. 2010;17:372–378. [Google Scholar]
- 18.Perry RW, Thill RE. Tree roosting by male and female Eastern pipistrelles in a forested landscape. J Mammal. 2007;88:974–981. [Google Scholar]
- 19.Veilleux JP, Whitaker JO, Jr, Veilleux SL. Tree-roosting ecology of reproductive female eastern pipistrelles, Pipistrellus subflavus, in Indiana. J Mammal. 2003;84:1068–1075. [Google Scholar]
- 20.Davis WH. Awakening patterns in the bats Myotis lucifugus and Pipistrellus subflavus. . J Mammal. 1964;45:645–647. [Google Scholar]
- 21.Goehring HH. Pipistrellus subflavus obscures, Myotis keenii, and Eptesicus fuscus fuscus hibernating in a storm sewer in Central Minnesota. J Mammal. 1954;35:434–436. [Google Scholar]
- 22.Griffin DR. Notes on the life histories of New England cave bats. J Mammal. 1940;21:181–187. [Google Scholar]
- 23.Kurta A, Teramino JA. A novel hibernaculum and noteworthy records of the Indiana bat and Eastern pipistrelle. Am Midl Nat. 1994;132:410–413. [Google Scholar]
- 24.Sandel JK, Benatar GR, Burke KM, Walker CW, Lacher TE, Jr, et al. Use and selection of winter hibernacula by the eastern pipistrelle (Pipistrellus subflavus) in Texas. J Mammal. 2001;82:173–178. [Google Scholar]
- 25.Trombulak SC, Higuera PE, DesMeule M. Population trends of wintering bats in Vermont. Northeast Nat. 2001;8:51–62. [Google Scholar]
- 26.Cryan PM, Barclay RMR. Causes of bat fatalities at wind turbines: hypotheses and predictions. J Mammal. 2009;90:1330–1340. [Google Scholar]
- 27.Bisson I, Safi K, Holland RA. Evidence for repeated independent evolution of migration in the largest family of bats. PLoS ONE. 2009;4:1–6. doi: 10.1371/journal.pone.0007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming TH, Eby P. Ecology of bat migration. In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: University of Chicago Press; 2003. pp. 156–208. [Google Scholar]
- 29.LaVal RK, LaVal ML. Ecological studies and management of Missouri bats with emphasis on cave-dwelling species. 1980. Terrestrial Series #8, Missouri Department of Conservation.
- 30.Ferrara FJ, Leberg PL. Influence of investigator disturbance and temporal variation on surveys of bats roosting under bridges. Wildlife Soc B. 2005;33:1113–1122. [Google Scholar]
- 31.Davis WH. Disproportionate sex ratios in hibernating bats. J Mammal. 1959;40:16–19. [Google Scholar]
- 32.Jones J, Pagels J. Notes on a population of Pipistrellus subflavus in southern Louisiana. J Mammal. 1968;49:134–139. [PubMed] [Google Scholar]
- 33.Dzal Y, Hooton LA, Clare EL, Fenton MB. Bat activity and genetic diversity at Long Point, Ontario, an important bird stopover site. Acta Chiropterol. 2009;11:307–315. [Google Scholar]
- 34.Reynolds DS. Monitoring the potential impact of a wind development site on bats in the northeast. J Wildlife Manage. 2006;70:1219–1227. [Google Scholar]
- 35.Arnett EB, Brown WK, Erickson WP, Fiedler JK, Hamilton BL, et al. Patterns of bat fatalities at wind energy facilities in North America. J Wildlife Manage. 2008;72:61–78. [Google Scholar]
- 36.Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- 37.West JB, Bowen GJ, Cerling TE, Ehleringer JR. Stable isotopes as one of nature's ecological recorders. Trends Ecol Evol. 2006;21:408–414. doi: 10.1016/j.tree.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Britzke ER, Loeb SC, Hobson KA, Romanek CS, Vonhof MJ. Using hydrogen isotopes to assign origins of bats in the eastern United States. J Mammal. 2009;90:743–751. [Google Scholar]
- 39.Fraser KC, McKinnon EA, Diamond AW. Migration, diet or molt? Interpreting stable-hydrogen isotope values in Neotropical bats. Biotropica. 2010;42:1–6. [Google Scholar]
- 40.Bowen GJ, Wassenaar LI, Hobson KA. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia. 2005;143:337–348. doi: 10.1007/s00442-004-1813-y. [DOI] [PubMed] [Google Scholar]
- 41.Paritte JM, Kelly JF. Effect of cleaning regime on stable-isotope ratios of feathers in Japanese Quail (Coturnix japonica). The Auk. 2009;126:165–174. [Google Scholar]
- 42.Wassenaar LI, Hobson KA. Comparative equilibration and online techniques for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot Environ Healt S. 2003;39:211–217. doi: 10.1080/1025601031000096781. [DOI] [PubMed] [Google Scholar]
- 43.Bowen GJ, Chesson L, Nielson K, Cerling TE, Ehleringer JR. Treatment methods for the determination of δ2H and δ18O of hair keratin by continuous-flow isotope-ratio mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2371–2378. doi: 10.1002/rcm.2069. [DOI] [PubMed] [Google Scholar]
- 44.Bowen GJ. Gridded maps of the isotopic composition of meteoric precipitation. 2010. http://www.waterisotopes.org.
- 45.Langin KM, Reudink MW, Marra PP, Norris ER, Kyser TK, et al. Hydrogen isotopic variation in migratory bird tissues of known origin: implications for geographic assignment. Oecologia. 2007;152:449–457. doi: 10.1007/s00442-007-0669-3. [DOI] [PubMed] [Google Scholar]
- 46.Torres-Dowdall J, Farmer AH, Bucher EH, Rye RO, Landis G. Variation in isotopic composition of shorebird feathers: implications for determining molting grounds. Waterbirds. 2009;32:300–310. [Google Scholar]
- 47.Wunder MB, Norris DR. Analysis and design for isotope-based studies of migratory animals. In: Hobson KA, Wassenaar LI, editors. Tracking animal migration with stable isotopes. London: Elsevier Academic Press; 2008. pp. 1–20. [Google Scholar]
- 48.Ibáñez C, Guillén A, Agirre-Mendi PT, Juste J, Schreur G, et al. Sexual segregation in Iberian Noctule bats. J Mammal. 2009;90:235–243. [Google Scholar]
- 49.Thomas DW. The annual migrations of three species of West African fruit bats (Chiroptera: Pteropodidae). Can J Zool. 1983;61:2266–2272. [Google Scholar]
- 50.Speakman JR, Thomas DW. Physiological ecology and energetics of bats. In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: University of Chicago Press; 2003. pp. 430–478. [Google Scholar]
- 51.Anthony ELP, Kunz TH. Feeding strategies of the little brown bat, Myotis lucifugus, in Southern New Hampshire. Ecology. 1977;58:775–586. [Google Scholar]
- 52.Kurta A, Bell GP, Nagy KA, Kunz TH. Energetics of pregnancy and lactation in free ranging little brown bats (Myotis lucifugus). Physiol Zool. 1989;62:804–818. [Google Scholar]
- 53.Encarnação JA, Dietz M, Kierdorf U. Reproductive condition and activity pattern of male Daubenton's bats (Myotis daubentonii) in the summer habitat. Mamm Biol. 2004;69:163–172. [Google Scholar]
- 54.Encarnação JA, Kierdorf U, Wolters V. Seasonal variation in nocturnal activity of male Daubenton's bats, Myotis daubentonii (Chiroptera: Vespertilionidae). Folia Zool. 2006;55:237–246. [Google Scholar]
- 55.Kunz TH, Whitaker JO, Jr, Wadanoli MD. Dietary energetic of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia. 1995;101:407–415. doi: 10.1007/BF00329419. [DOI] [PubMed] [Google Scholar]
- 56.Cryan PM, Wolf BO. Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. J Exp Biol. 2003;206:3381–3390. doi: 10.1242/jeb.00574. [DOI] [PubMed] [Google Scholar]
- 57.Dietz M, Kalko EKV. Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton's bats (Myotis daubentonii). . J Comp Physiol B. 2006;176:223–231. doi: 10.1007/s00360-005-0043-x. [DOI] [PubMed] [Google Scholar]
- 58.Barclay RMR. The effect of reproductive condition on the foraging behavior of female hoary bats, Lasiurus cinereus. . Behav Ecol Sociobiol. 1989;24:31–37. [Google Scholar]
- 59.Henry M, Thomas DW, Vaudry R, Carrier M. Foraging distances and home range of pregnant and lactating little brown bats (Myotis lucifugus). J Mammal. 2002;83:767–774. [Google Scholar]
- 60.Knowles B. Bat hibernacula on Lake Superior's North Shore, Minnesota. Can Field Nat. 1992;106:252–254. [Google Scholar]
- 61.Humphries MM, Thomas DW, Speakman JR. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–316. doi: 10.1038/nature00828. [DOI] [PubMed] [Google Scholar]
- 62.Boyles JG, Brack V., Jr Modeling survival rates of hibernating mammals with individual-based models of energy expenditure. J Mammal. 2009;90:9–16. [Google Scholar]
- 63.Boyles JG, Storm JJ, Brack V., Jr Thermal benefits of clustering during hibernation: a field test of competing hypotheses on Myotis sodalis. . Funct Ecol. 2008;22:632–636. [Google Scholar]
- 64.Briggler JT, Prather JW. Use and selection of caves by the Eastern pipistrelle bat (Pipistrellus subflavus). Am Midl Nat. 2003;149:406–412. [Google Scholar]
- 65.Broders HG, McAlpine DF, Forbes GJ. Status of the Eastern Pipistrelle (Pipistrellus subflavus) (Chiroptera: Vespertilionidae) in New Brunswick. Northeast Nat. 2001;8:331–336. [Google Scholar]
- 66.Jones C, Suttkus RD. Colony structure and organization of Pipistrellus subflavus in Southern Louisiana. J Mammal. 1973;54:962–968. [PubMed] [Google Scholar]
- 67.McNab BK. The behavior of temperate cave bats in a subtropical environment. Ecology. 1974;55:943–958. [Google Scholar]
- 68.Constantine DG. Color variation and molt in Tadarida brasiliensis and Myotis velifer. . J Mammal. 1957;38:461–466. [Google Scholar]
- 69.Tiunov MP, Makarikova TA. Seasonal molting in Myotis petax (Chiroptera) in the Russian Far East. Acta Chiropterol. 2007;9:538–541. [Google Scholar]
- 70.Veilleux JP, Veilleux SL. Intra-annual and interannual fidelity to summer roost areas by female Eastern Pipistrelles, Pipistrellus subflavus. . Am Midl Nat. 2004;152:196–200. [Google Scholar]
- 71.Dwyer PD. Seasonal changes in pelage of Miniopterus schreibersi blepotis (Chiroptera) in north-eastern New South Wales. Aust J Zool. 1963;11:290–300. [Google Scholar]
- 72.Dingle H. Migration: The biology of life on the move. New York, Oxford: Oxford University Press; 1996. 474 [Google Scholar]
- 73.Hobson KA, Norris DR. Animal migration: A context for using new techniques and approaches. In: Hobson KA, Wassenaar LI, editors. Tracking animal migration with stable isotopes. London: Elsevier Academic Press; 2008. pp. 1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collection and stable isotope data for all specimens. Each bat is identified with a museum-specific abbreviation (CU - Cornell University Museum of Vertebrates, Ithaca, NY; LSUMZ - Louisiana State University Museum of Natural Science, Baton Rouge, LA; MCZ – Harvard University Museum of Comparative Zoology, Cambridge, MA; ROM - Royal Ontario Museum, Toronto, ON) and its associated catalog number. Collection coordinates for each specimen are included in decimal degrees. For some specimens, exact collection coordinates were available. For specimens where exact collection data were not available, coordinates for the centroid of the county of collection were used and reported below (obtained from the United States Geological Survey Geographic Names Information System (http://geonames.usgs.gov/domestic/, accessed September 2010). Predicted growing season δDprecip values were obtained from waterisotopes.org [40], [44]. All stable isotope data are reported in ‰ relative to VSMOW. F = female; M = male.
(DOC)