Short abstract
Skeletal muscle remodeling is a critical component of an organism's response to environmental changes. Exercise causes structural changes in muscle and can induce phase shifts in circadian rhythms, fluctuations in physiology and behavior with a period of around 24 hours that are maintained by a core clock mechanism. Both exercise-induced remodeling and circadian rhythms rely on the transcriptional regulation of key genes.
Abstract
Background
Skeletal muscle remodeling is a critical component of an organism's response to environmental changes. Exercise causes structural changes in muscle and can induce phase shifts in circadian rhythms, fluctuations in physiology and behavior with a period of around 24 hours that are maintained by a core clock mechanism. Both exercise-induced remodeling and circadian rhythms rely on the transcriptional regulation of key genes.
Results
We used DNA microarrays to determine the effects of resistance exercise (RE) on gene regulation in biopsy samples of human quadriceps muscle obtained 6 and 18 hours after an acute bout of isotonic exercise with one leg. We also profiled diurnal gene regulation at the same time points (2000 and 0800 hours) in the non-exercised leg. Comparison of our results with published circadian gene profiles in mice identified 44 putative genes that were regulated in a circadian fashion. We then used quantitative PCR to validate the circadian expression of selected gene orthologs in mouse skeletal muscle.
Conclusions
The coordinated regulation of the circadian clock genes Cry1, Per2, and Bmal1 6 hours after RE and diurnal genes 18 hours after RE in the exercised leg suggest that RE may directly modulate circadian rhythms in human skeletal muscle.
Background
Resistance exercise (RE) can improve the overall quality of life and reduce the symptoms of many clinical disorders, including obesity, type II diabetes mellitus [1], coronary heart disease, and stroke [2]. The effects of RE on skeletal muscle are mediated by activation of muscle-specific signaling cascades that increase muscle mass [3], cytoskeletal protein levels, and the force of contraction without increasing the number of myofibers [4]. Exercise-induced transcription of genes involved in growth, vascularization, and metabolism [5-8] indicates that large-scale changes in transcriptional regulation play a key role in muscle remodeling, inducing growth responses and metabolic shifts.
Exercise also appears to help reset circadian rhythms in shift-workers, travelers who have changed time zones, and people with sleep disorders [9-13]. Circadian rhythms are approximately 24-hour fluctuations in gene regulation, physiology, and behavior that have evolved to optimize daily cycles of sleep, activity, feeding, and metabolism [14]. Exercise influences both the phase [15] and the amplitude [16] of circadian rhythms in mice, but this phenomenon is largely unexplored in human tissues.
Circadian rhythms are controlled by a clock mechanism located in central and peripheral tissues. The mechanism comprises an autoregulatory transcriptional feedback loop that includes the circadian-clock genes Clock, Bmal1, Period (Per) and Cryptochrome (Cry). Clock and Bmal1 constitute the positive arm of the feedback loop. These proteins form heterodimers, bind to specific DNA regulatory elements (E-boxes), and initiate transcription of Per 1, 2, and 3 and Cry 1 and 2. In mammals, the Per and Cry gene products, which constitute the negative arm of the transcriptional feedback loop, form homo- and/or heteromeric complexes, translocate to the nucleus, and repress Clock/Bmal1-mediated transcription [17,18] in a temporal manner. Although most studies have focused on the rhythmic expression of these few core clock genes, recent evidence suggests that genes regulated in a circadian fashion (or circadian output genes) constitute 8-10% of all genes expressed in mouse tissues [19,20].
Classically, peripheral clocks are thought to be controlled by a central clock located in the suprachiasmatic nucleus (SCN), which is believed to also synchronize clocks in other brain regions [21]. However, under certain conditions (such as restricted feeding), peripheral tissue clocks can be regulated independently of the SCN [22,23]. The phase-shifting effects of exercise on mammalian circadian rhythms are thought to be mediated by inputs to SCN neurons (these include serotonin [24], neuropeptide Y [25], and melatonin [13,26]) and ultimately lead to acute changes in SCN Per1 and Per2 expression [27]. The extent to which RE may be able to directly affect circadian-regulated genes in peripheral tissues such as human muscle is not known, however.
To understand the global transcriptional effects of RE and time of day on gene expression in human skeletal muscle (hSkM), we used DNA microarrays to analyze biopsies of exercised and non-exercised hSkM collected at different times of day. This study was designed to answer three specific questions. What genes and biological processes are regulated in hSkM by RE and time of day? Which orthologs of genes that undergo temporal regulation in hSkM also undergo circadian regulation in mouse skeletal muscle (mSkM), liver (mLvr), heart (mHrt) or SCN (mSCN)? Are diurnally regulated genes expressed in skeletal muscle directly affected by exercise? To answer these questions, we compared gene expression in the exercised and non-exercised legs of four human subjects after an acute bout of RE. We then filtered and annotated (by biological process) the significantly changed genes and compared these genes to orthologs regulated in microarray studies of rodent models of exercise and circadian gene regulation. Finally, we used quantitative reverse-transcription polymerase chain reaction (RT-PCR) to validate the circadian regulation of selected diurnally regulated hSkM gene orthologs in mSkM.
Results and discussion
Genes regulated in the context of biological processes
Comparison of gene expression profiles of the exercised and non-exercised legs showed that 704 genes were differentially regulated at 6 hours and 1,479 genes at 18 hours after RE (p < 0.05). In the non-exercised leg, comparison of gene expression at 0800 and 2000 hours with the same statistical criteria showed that 608 genes were differentially regulated.
We used MAPPFinder [28] to link gene expression data to the Gene Ontology (GO) hierarchy (Table 1). The program computes a significance score (Z score) that is useful for ranking GO terms by their relative amounts of gene expression changes (see Materials and methods). With 40% of the circadian-rhythm genes classified by the GO hierarchy significantly changed at 6 hours after RE, circadian rhythm displayed the highest Z score (Z = 7.17) in this comparison, indicating that RE may regulate circadian genes directly in the exercised leg (see below). As expected, RE also upregulated a variety of genes involved in intracellular responses (nucleic acid metabolism, G2/M transition of mitotic cell cycle, anti-apoptosis, and transcription) and downregulated genes involved in oxygen and calcium transport, DNA repair, regulation of translational initiation, and glycogen metabolism (Table 1). Many of these processes were similarly regulated in a rat model of RE [8].
Table 1.
MAPPFinder analysis
| Upregulated process | C | M | T | Z | Downregulated process | C | M | T | Z | |
| 6 hours after RE | Circadian rhythm | 4 | 10 | 13 | 7.2 | Oxygen transport | 3 | 8 | 17 | 4.5 |
| Nucleic acid metabolism | 59 | 1570 | 2905 | 2.7 | Calcium ion transport | 8 | 44 | 67 | 4.4 | |
| Hearing | 3 | 32 | 54 | 2.3 | DNA repair | 16 | 134 | 195 | 4.2 | |
| G2/M transition of mitotic cell cycle | 3 | 34 | 40 | 2.2 | Regulation of translational initiation | 5 | 26 | 30 | 3.6 | |
| Anti-apoptosis | 4 | 53 | 71 | 2.1 | Di-, trivalent inorganic cation transport | 8 | 66 | 102 | 3.0 | |
| Transcription | 38 | 1025 | 1992 | 2.0 | Glycogen metabolism | 4 | 24 | 25 | 2.9 | |
| 18 hours after RE | Protein amino acid phosphorylation | 60 | 383 | 735 | 4.7 | Peripheral nervous system development | 5 | 11 | 12 | 5.5 |
| Regulation of cell cycle | 42 | 248 | 294 | 4.5 | Glycosphingolipid biosynthesis | 3 | 5 | 5 | 5.0 | |
| Anti-apoptosis | 12 | 53 | 71 | 3.5 | Antigen processing, endogenous antigen via MHC class I | 3 | 6 | 11 | 4.5 | |
| Mitochondrion organization and biogenesis | 5 | 15 | 23 | 3.3 | Iron transport | 4 | 10 | 21 | 4.5 | |
| Protein-mitochondrial targeting | 4 | 11 | 19 | 3.2 | Glutamine family amino acid catabolism | 4 | 13 | 19 | 3.7 | |
| L-Amino-acid transport | 3 | 7 | 9 | 3.1 | Male gonad development | 3 | 9 | 12 | 3.4 | |
| Diurnal | Regulation of transcription, DNA-dependent | 46 | 938 | 1852 | 3.7 | Regulation of protein biosynthesis | 3 | 7 | 12 | 5.7 |
| Cell differentiation | 7 | 68 | 109 | 3.6 | Non-selective vesicle transport | 7 | 57 | 63 | 3.7 | |
| Response to stress | 24 | 452 | 566 | 3.0 | Actin cytoskeleton organization and biogenesis | 3 | 15 | 23 | 3.5 | |
| Hemopoiesis | 3 | 22 | 28 | 2.9 | Cell-cycle arrest | 5 | 37 | 53 | 3.4 | |
| Oncogenesis | 14 | 253 | 282 | 2.4 | Main pathways of carbohydrate metabolism | 7 | 69 | 100 | 3.1 | |
| Nucleic acid metabolism | 61 | 1570 | 2905 | 2.3 | Tricarboxylic acid cycle | 3 | 20 | 23 | 2.8 |
Filtered genes (p < 0.05) were analyzed with MAPPFinder to determine the biological processes that were regulated in each comparison. C, number of genes changed; M, number of genes represented on chip in a process; T, number of genes in the Gene Ontology (GO) process: Z, significance Z-score value. The top six, non-redundant GO terms are shown for each comparison
MAPPFinder analysis identified several biological processes influenced by diurnal gene regulation in hSkM, including transcription, cell differentiation, response to stress, hemopoiesis, oncogenesis, protein biosynthesis, and metabolism (carbohydrate metabolism and tricarboxylic acid cycle) (Table 1). These findings provide human gene targets that correlate with the circadian regulation of metabolism and cancer recently reported in mouse models [19,20,29-31].
Diurnal comparison
Diurnal gene regulation in hSkM: evidence of circadian genes
The significant upregulation of Per1 and Per2 in our diurnal comparison provided the first indication that the 608 genes that changed significantly with time contained known circadian-regulated genes. To filter out potential noise in this comparison and to identify genes with the highest likelihood of being regulated in a circadian fashion, we compared our results to published data on circadian gene regulation in mouse peripheral tissues [19,20]. These comparisons resulted in a list of 44 'putative circadian genes' that were significantly regulated in both our human diurnal dataset and in the mouse circadian studies (Figures 1, 2). We then used quantitative RT-PCR to analyze mRNA expression levels of 12 mouse orthologs in mSkM isolated every 4 hours for 24 hours (Figure 3).
Figure 1.

Venn diagrams of human genes undergoing diurnal regulation compared to their mouse circadian orthologs. (a) Comparison with mouse circadian gene orthologs reported in [19]; (b) comparison with mouse circadian gene orthologs reported in [20]. In the diurnal comparison (0800 vs 2000 h), 608 human genes were changed significantly (p < 0.05) in the non-exercised leg. An additional statistical filter was applied (p < 0.05 and absolute fold change > 20%) that resulted in a list of 239 diurnally regulated (0800 vs 2000 h) genes, that were compared with the mouse circadian orthologs. Gray shading indicates genes represented in Figure 2. Genes listed to the right of each Venn diagram are the intersection of all three tissues (red numbers). mHrts, mouse ortholog is circadian-regulated in heart [19] (n = 462); mLvrs, mouse ortholog is circadian-regulated in liver [19] (n = 575); mLvrp, mouse ortholog is circadian-regulated in liver [20] (n = 335); mSCNp, mouse ortholog is circadian-regulated in the SCN [20] (n = 337).
Figure 2.
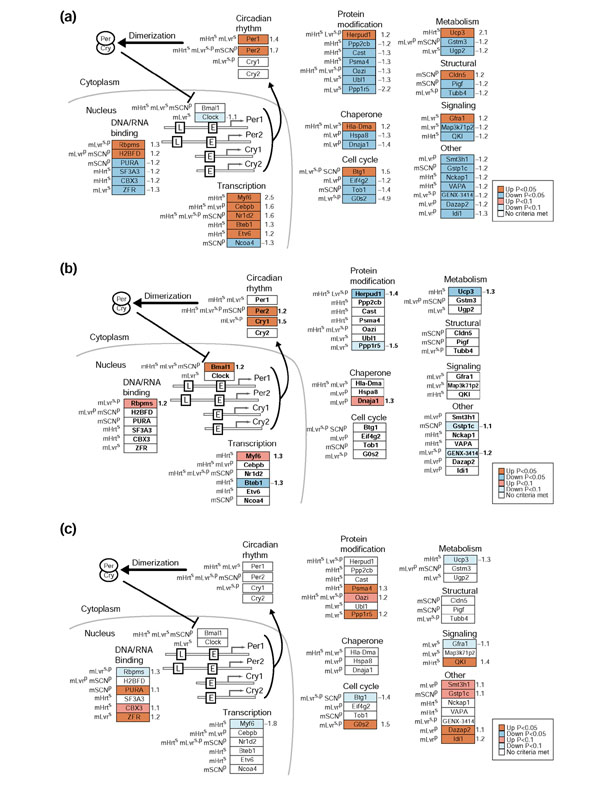
Human genes regulated in the diurnal comparison with orthologs that display circadian regulation in mouse heart and liver [19,20], and SCN [20]. The 608 significantly regulated (p < 0.05) hSkM genes identified in the diurnal comparison (0800 and 2000 hours) were subjected to an additional statistical filter of absolute fold change > 20% (n = 239) and linked to mouse circadian-regulated orthologs. The resultant 44 putative hSkM circadian-regulated genes are represented as boxes and colored in GenMAPP [57] using different filtering criteria. (a) The 44 putative hSkM circadian-regulated genes colored by p values and displaying fold changes from the diurnal comparison (0800 vs 2000 hours non-exercised leg). (b) The 44 putative hSkM circadian-regulated genes colored by p values and displaying fold changes from the comparison 6 hours after RE. (c) The 44 putative hSkM circadian-regulated genes colored by p values and displaying fold changes from the 18 hours after RE comparison. Red, blue, and gray boxes indicate significant upregulation, downregulation, and no significant regulation, respectively, using p-value stringencies defined in the key for each comparison. Numbers to the right of the gene boxes are the fold changes in the diurnal comparison. L, promoter for the light-responsive element; E, E-box (Clock/Bmal1 promoter). Ortholog information is denoted to the left of the gene boxes: mHrts and mLvrs, mouse ortholog was circadian-regulated as described [19] in mouse heart or liver, respectively; mLvrp and mSCNp, mouse ortholog was diurnally regulated as described [20] in mouse liver or SCN, respectively.
Figure 3.

Confirmation of hSkM diurnal gene regulation by analysis of mSkM. Real-time RT-PCR (comparative CT method) was performed on total RNA isolated from wild-type C57BL/6J mouse quadriceps muscle, collected at the indicated zeitgeber times (ZT). (a) Genes with a cycling phase similar to that of Bmal1, a key diurnal clock gene, are shown. (b) Genes with a cycling phase similar to that of Per1 and Per2. One-way ANOVA was applied to all time points and confirmed a statistically significant effect of time on gene expression levels (p < 0.001 for Bmal1 and Hat; p < 0.05 for all others). By normalizing average peak value to the average trough value (12-hour opposite peak), the following fold increases in gene expression were calculated: Bmal1 = 4.1, G0s2 = 7.0, Cry1 = 4.5, Nfil3 = 11.1, Per1 = 3.5, Per2 = 13.0, C/EBPb = 3.5, MyF6 = 3.3, Ier3 = 1.8, Hat = 1.8, and Gadd = 2.1. Values are mean 6 SEM. Gapdh was used as the reference gene and is included as the negative control.
Circadian regulation of selected mouse orthologs of human diurnal genes and core circadian-clock genes
Quantitative RT-PCR indicated that the core circadian-clock genes Bmal1, Per1, and Per2 and selected mouse orthologs of the 44 putative circadian genes exhibited circadian regulation in mSkM (Figure 3). Quantitative RT-PCR also provided a potential explanation for our inability to detect a significant change in the expression of the circadian-regulated core clock genes Cry1 and Bmal1 in hSkM [19,20], as these genes cycle out of phase with Per1 and Per2 in mSkM (Figure 3) and in many other tissues, including mLvr [19] and mHrt [19]. As only two time points were measured in the human samples, genes whose peak and trough times are out of phase with our sampling times (for example, Cry1 and Bmal1) would not be detected.
An interesting finding is that Per1 and Per2 are upregulated in the morning in hSkM (Figure 2), and downregulated in the morning in mSkM (Figure 3b). We cannot determine the exact phase of the potentially circadian-regulated genes in hSkM with only two time points of sampling. However, the observed opposite regulation may reflect opposing phases of core clock gene expression between human and mouse peripheral tissues. Further examination of this phenomenon may provide a molecular explanation for the opposite activity/rest cycles in diurnal versus nocturnal mammals.
Discovery of three conserved putative circadian-regulated genes
To identify conserved circadian-regulated genes, three peripheral tissues were selected for comparison: mHrt [19], mLvr [19,20], and hSkM. Four genes were significantly regulated in all three tissues: Per2, Nr1d2 (nuclear receptor subfamily 1, group D, member 2), Herpud1 (homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1), and Oazi (ornithine decarboxylase antizyme inhibitor) (Figures 1, 2). Per2 is a well-characterized conserved core clock element [18]]. Human Nr1d2 (Rev-Erbβ) is 90% identical to mouse Rev-Erbα, a newly identified component of the circadian clock that represses the transcription of Bmal [32]. Herpud1 appears to be a membrane-bound endoplasmic reticulum protein induced by stress [33]. It contains a ubiquitin-like domain at the amino terminus, indicating its involvement in a protein degradation pathway, a biological process important for maintaining circadian rhythms. The Oazi gene product is an inhibitor of antizyme, which inhibits ornithine decarboxylase (ODC), a key enzyme in polyamine biosynthesis that is essential for normal cell growth [34]. The discovery of common circadian genes in multiple tissues suggests that these genes and their patterns of expression are important in a variety of tissue pathways.
Exercise comparisons
RE regulates circadian genes: evidence for RE-induced phase shifting
We hypothesized that RE affects circadian-regulated gene expression directly in skeletal muscle. In support of this, we found that three core circadian clock genes, Cry1, Per2, and Bmal1, were upregulated 6 hours after RE in the exercised leg (1.5-fold, 1.2-fold, and 1.2-fold, respectively; Figure 2b). Although the RE-induced changes of these circadian clock genes were modest in magnitude, they are statistically significant, coordinated, and precede RE-mediated changes in diurnal genes 18 hours after RE in the exercised leg.
RE appeared to shift the expression patterns of diurnal-regulated genes by upregulating genes (n = 12, p < 0.1) that normally were repressed in the morning (n = 29, p < 0.05) or by downregulating genes (n = 5, p < 0.1) normally induced in the morning (n = 15, p < 0.05) (Figure 2c). If we extend this analysis to all the diurnal genes that are significantly changed (p < 0.05) 18 hours after RE, we find all (64 genes) but one reflect this potential phase-shifting effect.
Although we cannot determine whether RE induces a phase advance or phase delay in these genes, our data are consistent with previous studies that show that physical exercise during the day (similar to our study) can induce a circadian phase advance in humans [35] as measured by circulating hormone levels. Our studies now indicate that this phase advance may occur at the level of gene expression in muscle.
Comparison of human and rat genes regulated by RE
To validate our experimental protocol and to identify key genes that may regulate the effects of RE in both humans and rodents, we compared human genes that were regulated by our isotonic RE protocol at 6 hours with rat orthologs that were regulated either transcriptionally or translationally in a published rat eccentric RE study [8] (Figure 4). The rat eccentric exercise protocol induced skeletal muscle hypertrophy [36] and consisted of titanic contractions that were electrically evoked with multistrand electrodes implanted on both sides of the sciatic nerve. Contractions consisted of 10 sets of six repetitions with each repetition lasting 3 seconds. Contractions were stimulated at a frequency of 100 Hz, 6-12 V, 1 msec duration, 9 msec delay. Rat muscles were then harvested 6 hours after the acute bout of exercise [8]. Lists of rat 6-hour RE genes were downloaded from the supplemental data at [37]. Human and rat gene orthologs that were regulated in the same directions are shown in Figure 4.
Figure 4.
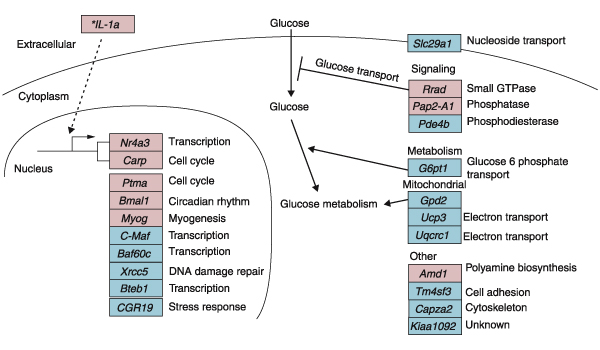
Gene-regulation model 6 hours after RE. The 144 human genes significantly changed (p < 0.05 and fold change > 20%) 6 hours after RE were compared to transcriptionally or translationally regulated rat orthologs (p < 0.05) 6 hours after RE in a rat model of RE [8]. Boxes represent individual human genes; red indicates upregulation and blue downregulation. *IL-1a was not found in the rat exercise data but is included for discussion purposes.
Grouping the orthologs by biological function revealed that two genes, Rrad (Ras associated with diabetes) and G6pt1 (glucose-6-phosphatase, transport protein 1), were regulated in directions suggesting decreased glucose transport, and one gene, Gpd2 (glycerol-3-phosphate dehydrogenase 2 (mitochondrial)) was regulated in a direction that suggested a decrease in glucose metabolism. Other genes involved in the metabolism of glycogen (the cellular storage form of glucose) were also downregulated (Table 1).
Interestingly, all three of these genes have been implicated in human diseases. Rrad was cloned because it was upregulated in patients with type II diabetes [38]. In cultured muscle and fat cells, overexpression of Rrad decreases insulin-stimulated glucose uptake [39]. The upregulation of Rrad provides a potential mechanism for previous reports that acute RE reduces insulin-stimulated glucose uptake [40-42]. Mutations in Gpd2 were found in a family of type II diabetics [43], and mutations in G6pt1 were found in patients with glycogen storage disease [44].
RE regulates potent myogenic genes
Two potent muscle-remodeling genes, myogenin and myostatin, were regulated in opposite directions in response to RE in humans, providing a potential mechanism for exercise-induced muscle hypertrophy. Myogenin was upregulated in the 6-hour comparison, and this observation was validated in the published [8] rat RE study (Figure 4). Myogenin, a muscle-specific transcription factor containing a basic helix-loop-helix domain, is important for muscle development and differentiation [45]. Myostatin, a member of the TGF-β family, is a negative regulator of skeletal muscle size and was downregulated in our study. It is a potent inhibitor of muscle development and proliferation [46]. Downregulation of myostatin may be associated with skeletal muscle growth [47] and is likely to have a major role in muscle remodeling in response to exercise.
Interleukin-1 signaling and exercise
The interleukin-1 gene (IL-1) was upregulated 6 hours after RE (Figure 4). The potential importance of IL-1 regulation in response to RE is indicated by the fact that two genes regulated by IL-1, Nr4a3 [48] and Carp [49], had the highest fold changes (3.5-fold and 2.3-fold, respectively, p < 0.05) 6 hours after RE. These two genes were also regulated in the rat exercise study (Figure 4). Furthermore, the vascular endothelial growth factor gene (Vegf) [50], which is also regulated by IL-1, had the highest fold change at 18 hours after RE (1.6-fold). Vegf is believed to be an important mediator of endurance exercise-induced angiogenesis in skeletal muscle [51]. However, resistance exercise protocols do not result in increases in capillaries per muscle area but do result in a redistribution of blood flow to hypertrophied muscle fiber types [4].
Hierarchical cluster analysis indicates potential co-regulated gene clusters
To identify potential co-regulated genes, we performed hierarchical clustering analysis [52] with expression values in the non-exercised leg at 2000 hours as the baseline. Two clusters included genes upregulated at 6 hours after RE (Figure 5), one centered on Nr4a3 (Figure 5a) and the other on Rrad (Figure 5b). These two genes displayed the highest fold changes 6 hours after RE. Cry1, a member of the core clock mechanism (Figure 2), was found in the Nr4a3 cluster.
Figure 5.
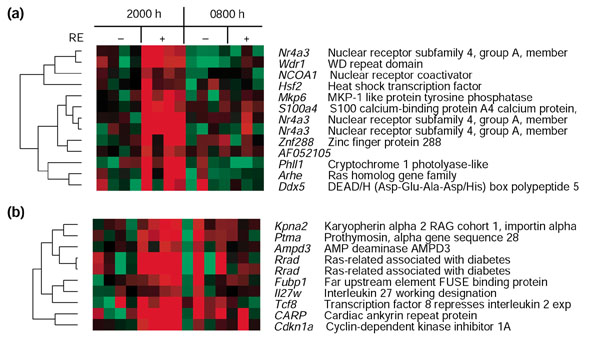
Cluster of genes upregulated 6 hours after RE. Columns indicate each subject and rows indicate individual genes. Each gene is represented by the difference of the genes expression value and the average expression value of the four non-exercised control legs 6 hours after RE. Red indicates upregulated genes, and green indicates downregulated genes. Gene names and descriptions (or GenBank IDs) appear to the right.
Two clusters contained core circadian genes Per1 (Figure 6a) and Per2 (Figure 6b) which were upregulated at 0800 hours. A third cluster (Figure 6c) contained genes downregulated at 0800 but not at 2000 hours. The Per2 cluster contained genes that were upregulated both at 0800 hours and in response to exercise (2000 hours + RE).
Figure 6.
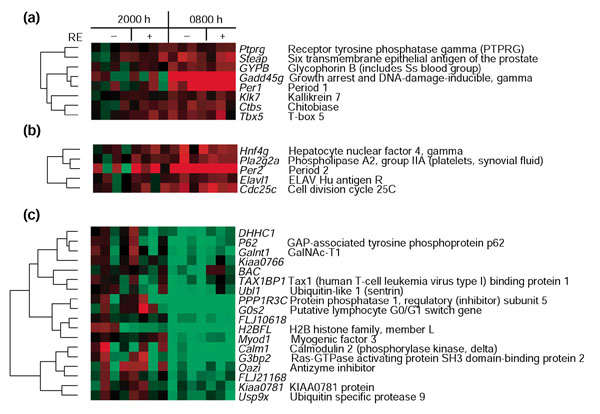
Cluster of genes regulated in the 0800 hours biopsies. Columns indicate each subject and rows indicate individual genes. Each gene is represented by the difference of the gene-expression value and the average expression value of the four non-exercised control legs 6 hours after RE. Red indicates upregulated genes; green indicates downregulated genes. Gene names and descriptions (or GenBank IDs) appear to the right. (a) Per1 0800 hours upregulated cluster. (b) Per2 0800 hours upregulated cluster. (c) 0800 hours downregulated cluster.
Conclusions
Analysis of our data in the context of biological processes and pathways allowed us to define physiologically the transcriptional basis of muscle remodeling induced by RE and its potential circadian gene regulation. This large-scale expression analysis of acute RE and circadian gene regulation in hSkM suggests that RE can directly regulate circadian clock genes (Per2, Cry1, and Bmal1) and circadian output genes. Our findings support the emerging idea that peripheral clocks can regulate themselves independently of the SCN [22,23]. If the SCN were responsible for the phase shifting, the same changes in gene expression would have occurred in both the control and exercised legs, which was not observed. However, acute phase advances in SCN Per1 and Per2 expression in response to exercise have been observed in hamsters [27]. Whereas the SCN is still probably involved in the long-term effects of clock phase shifting in peripheral tissue, our evidence suggests that the skeletal muscle clock responds quickly to RE by transcriptional regulation of specific clock genes.
Although circadian studies in human tissue are critical for understanding the effects of circadian gene regulation on human physiology, access to ample tissue samples is difficult, costly, and painful. We therefore found it valuable to validate and compare our results with those of similar experiments in rodents, in which tissue can be harvested at multiple time points to establish the circadian cycle. Such comparisons were also valuable in identifying gene orthologs regulated 6 hours after RE in both humans and rats. Cross-species, cross-tissue comparisons are essential for defining transcriptional regulatory pathways of key genes and will allow us to define new genes and pathways responsible for tissue regulation and remodeling.
Materials and methods
Subjects
Four healthy men, 31-51 years old, were recruited for study. The subjects had not carried out RE training for at least 3 months before enrolment, had no history of chronic illnesses, and showed no abnormalities on the screening physical examination or routine hematology and chemistry tests. Subjects were admitted to the General Clinical Research Center at San Francisco General Hospital, where they were fed a constant metabolic diet that provided 1.2 g of protein/kg body weight and 35 kcal/kg body weight per day for nine days before the bout of exercise. Equilibration on the diet was evidenced by constancy of urine urea nitrogen excretion. The study protocol was approved by the Committee on Human Research of the University of California, San Francisco, and informed consent was obtained from each subject.
Exercise protocol
Maximum strength (one-repetition maximum) during isotonic knee extension from 90° to 170° was tested in the right leg 8 days before the study exercise session. Subjects did not perform any RE between the testing and the study session. On the ninth hospital day, beginning at 1330 h, subjects performed a vigorous bout of RE with the right leg. Exercise consisted of 10 sets of eight repetitions of isotonic knee extension (Cybex 4850 leg extension, Ronkonkoma, NY) at 80% of the predetermined one-repetition maximum, with 3-min rest periods between sets, over 30-45 min. The isotonic knee extensions include both concentric and eccentric phases.
Muscle biopsies
Muscle biopsies were performed 6 hours (between 1930 and 2000 hours) and 18 hours (between 0730 and 0800 hours the next day) after RE in both the exercised and non-exercised leg. Tissue (200-300 mg) was obtained from the lateral portion of each vastus lateralis muscle approximately 20 cm above the knee with a 4-mm Bergstrom needle (Stille, Stockholm, Sweden) under local anesthesia with 1% lidocaine. Blood and visible fat were removed, and the tissue was immediately frozen in liquid nitrogen and stored at -80°C for later analysis. Although relatively homogeneous when compared to other tissues, skeletal muscle is a complex tissue consisting of many cell types, and thus our results must be interpreted in that context.
Sample preparation and analysis
Total RNA was extracted from frozen tissue with a polytron homogenizer and Trizol (Invitrogen, Carlsbad, CA). Fragmented, biotin-labeled cRNA samples were generated from 5-14 μg total RNA and hybridized to Affymetrix human U95Av2 arrays. For each array, the .cel files were generated with Affymetrix Microarray Suite 5.0 and analyzed with Robust Microarray Analysis (RMA) [53].
Preparation of expression array samples
Total RNA was extracted from 5-100 mg frozen tissue with a polytron homogenizer and Trizol (Invitrogen) and purified with an RNEasy kit (Qiagen, Santa Clara, CA). Depending on the amount of starting material, 5-14 μg total RNA was reverse transcribed with an oligo-dT primer containing a T7 RNA polymerase promoter (Affymetrix) and then converted into double-stranded cDNA (ds cDNA) with a ds cDNA synthesis kit (Invitrogen). After the second-strand synthesis, ds cDNA was extracted with phenol-chloroform-isoamyl alcohol and recovered by ethanol precipitation. Biotinylated cRNA was generated from ds cDNA by in vitro transcription (IVT) with an Enzo BioArray high-yield RNA transcript labeling kit (Affymetrix). After a further round of purification with the Qiagen RNEasy kit, IVT reactions yielded 30-70 μg biotinylated cRNA, which was fragmented into lengths of around 100 base-pairs (bp) before hybridization.
Analysis of biotinylated cRNA with HG-U95Av2 microarray
IVT reaction products (5 μg) were hybridized to Affymetrix Test2 arrays before hybridization to the HG-U95Av2 chip (Affymetrix) to ensure full-length transcript representation of GAPDH. Each chip was hybridized to 15 μg fragmented cRNA. Arrays were hybridized and scanned with a GeneArray Scanner (Hewlett-Packard/Affymetrix) at the Genomics Core Facility of the General Clinical Research Center at San Francisco General Hospital.
Statistical analysis and comparisons
Three comparisons were made (Figure 7). Gene expression in the exercised leg was compared with that in the non-exercised leg at 6 and 18 hours after RE. The diurnal effects on gene expression were assessed by comparing gene transcript levels in the non-exercised leg at 2000 and 0800 hours. Two-tailed paired t tests were used to compare each sample with its respective baseline value. These t tests were validated using permutation t tests. Human U95Av2 chip probe set to gene annotations were obtained from NetAffx [54].
Figure 7.

Diagram of the experimental protocol. Each volunteer performed a bout of resistance exercise (RE) consisting of 10 sets of eight repetitions of isometric knee extension at 80% of the predetermined one-repetition maximum with a single leg. Biopsies were obtained 6 and 18 hours after RE in both the exercised and non-exercised (contralateral) leg. Arrows denote the three comparisons of gene expression in the biopsy samples.
Gene Ontology (GO) analysis with MAPPFinder
Genes that were significantly upregulated or downregulated (p < 0.05) were annotated with GO [55] information with the MAPPFinder 1.0 program [28] (Table 1). MAPPFinder is an accessory program to GenMAPP [56], a freely available software tool that colors biological pathways with gene expression data [57]. MAPPFinder Z-scores, a statistical measure of significance for gene expression in a given group, were calculated by subtracting the number of genes expected to be randomly changed in a GO term from the observed number of changed genes in that GO term. This value was then divided by the standard deviation of the observed number of genes under a hypergeometric distribution. Output from the MAPPFinder analysis was manually filtered to remove processes that represented the same genes (typically parent-child processes). The top six biological processes for each comparison are listed in Table 1. For a biological process to be included in the table, it was required that at least three genes changed significantly (nested results) and the Z-score was > 2.
Gene expression analysis in mSkM
Adult male C57BL/6J mice were subjected to a 12-hour light/12-hour dark cycle for 2 weeks before tissue collection (n = 3 per time point). Mice were sacrificed every 4 hours for 24 hours, and quadriceps muscle was dissected and rapidly frozen on dry ice. Total RNA was extracted from frozen samples with Trizol (Sigma) and diluted to 0.1 mg/ml. TaqMan real-time RT-PCR assays were performed using the comparative amplification detection threshold of target gene expression (CT) method, an ABI 7700 Sequence Detector, and TaqMan EZ RT-PCR kit reagents (Applied Biosystems, Foster City, CA). Probe and primer sets were designed with Primer Express software (Applied Biosystems). mRNA levels were measured by determining the cycle number at which CT was reached. In each sample, CT was normalized to GAPDH expression, performed in parallel (ΔCT). Normalized ΔCT values from each time point were then subtracted from the average ΔCT value at all time points (ΔΔCT) to determine the relative abundance values (2-ΔΔCT).
Linking databases for determining rat and mouse orthologs
To relate diurnally regulated hSkM genes to known circadian-regulated genes (potential hSkM circadian genes), we linked human U95Av2 probe sets to mouse U74Av2 ortholog probe sets using three public databases: NCBI Homologene [58], TIGR Resourcerer [59], and NetAffx. A similar approach was used to link the human and rat orthologs regulated 6 hours after RE (U95Av2 to U34A probe sets). 'Overlap genes' were defined as those exhibiting a fold change greater than 20% at a significance level of p < 0.05 that corresponded to significantly changed rodent orthologs based on the statistical filters used in the published studies [8,19,20].
Hierarchical clustering analysis
For hierarchical clustering, we used data from the 3,260 genes (of 12,626 examined) that resulted from a p < 0.05 in any of three comparisons. The analysis was performed with Cluster and TreeView [52]. As input for Cluster, the average (N = 4) log2 truncated value of the unexercised 2000-hour biopsies (control leg) was used as the baseline. All 3,260 genes were clustered by correlation uncentered similarity metric, using complete linking clustering. Figures were generated with TreeView.
Additional data files
The following additional data files are available with the online version of this article: two Excel sheets containing the Affymetrix data (MAS 4.0, target intensity 800) from rat 6 hours after exercise [8] total and polysomal (Additional data file 1); two Excel sheets containing log2 RMA signal values, fold changes, P values and probe level annotation, and descriptions of column titles, respectively (Additional data file 2); two sheets with information for linking orthologous probe sets between Affymetrix Hs. U95A, Mm. U74A and Rn. U34A arrays (Additional data file 3); two sheets (Hs RE/rat total RE and Hs RE/rat polysomal RE) of 6 h after exercise-regulated gene orthologs regulated in this study and those published by Chen et al. [8] (Additional data file 4); four Excel sheets containing the putative circadian overlap genes (Additional data file 5); and, finally, five sheets each with the unfiltered output results from the MAPPFinder analysis (Additional data file 6). All of the data, including RMA expression values, annotated chip information, GenMAPP expression dataset [57], MAPPFinder results [28], lists of links between the human and rodent probe sets, and full lists of ortholog matches, are also available for download from the GenMAPP site [60].
Supplementary Material
The Affymetrix data (MAS 4.0, target intensity 800) from rat 6 hours after exercise
Log2 RMA signal values, fold changes, P values and probe level annotation, and descriptions of column titles
Information for linking orthologous probe sets between Affymetrix Hs. U95A, Mm. U74A and Rn. U34A arrays
Hs RE/rat total RE and Hs RE/rat polysomal RE of 6 h after exercise-regulated gene orthologs regulated in this study and those published by Chen et al.
The putative circadian overlap genes
The unfiltered output results from the MAPPFinder analysis
Acknowledgments
Acknowledgements
We thank Karyn A. Esser for insightful discussions and help in revising the manuscript, Bethany Taylor for assistance with manuscript preparation and submission, Stephen Ordway and Gary Howard for editorial assistance, and Chandi Griffith for hybridizing and scanning arrays. J.S.T. is an Investigator and E.L.M. is a Research Associate in the Howard Hughes Medical Institute. This work is supported by the J. David Gladstone Institutes, the San Francisco General Hospital General Clinical Research Center (RR-00083), the National Heart, Lung, and Blood Institute (HL61689, HL60664), the NHLBI Programs for Genomic Applications (BayGenomics HL66621), and Diabetes, Endocrinology & Metabolism Training Grant (DK07418).
References
- Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Piña IL, Stein RA, Williams M, Bazzarre T. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription. An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101:828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- Dusterhoft S, Putman CT, Pette D. Changes in FGF and FGF receptor expression in low-frequency-stimulated rat muscles and rat satellite cell cultures. Differentiation. 1999;65:203–208. doi: 10.1046/j.1432-0436.1999.6540203.x. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol. 2002;545:27–41. doi: 10.1113/jphysiol.2002.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Hoese EK, Youngstedt SD, Liu L. Phase-shifting human circadian rhythms with exercise during the night shift. Physiol Behav. 1995;58:1287–1291. doi: 10.1016/0031-9384(95)02031-4. [DOI] [PubMed] [Google Scholar]
- Copinschi G, Spiegel K, Leproult R, Van Cauter E. Pathophysiology of human circadian rhythms. Novartis Found Symp. 2000;227:143–157. doi: 10.1002/0470870796.ch9. discussion 157-162. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol. 1999;277:R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs. 2001;15:311–328. doi: 10.2165/00023210-200115040-00005. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Sturis J, Byrne MM, Blackman JD, L'Hermite-Baleriaux M, Leproult R, Oliner C, Refetoff S, Turek FW, Van Cauter E. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol. 1994;266:E964–E974. doi: 10.1152/ajpendo.1994.266.6.E964. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–378. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–82. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Reid MS, Dement WC. Serotonergic afferents mediate activity-dependent entrainment of the mouse circadian clock. Am J Physiol. 1997;273:R265–R269. doi: 10.1152/ajpregu.1997.273.1.R265. [DOI] [PubMed] [Google Scholar]
- Biello SM, Mrosovsky N. Phase response curves to neuropeptide Y in wildtype and tau mutant hamsters. J Biol Rhythms. 1996;11:27–34. doi: 10.1177/074873049601100103. [DOI] [PubMed] [Google Scholar]
- Carr DB, Reppert SM, Bullen B, Skrinar G, Beitins I, Arnold M, Rosenblatt M, Martin JB, McArthur JW. Plasma melatonin increases during exercise in women. J Clin Endocrinol Metab. 1981;53:224–225. doi: 10.1210/jcem-53-1-223. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawler SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M, Takahashi JS. Circadian rhythms: the cancer connection. Nature. 2002;420:373–374. doi: 10.1038/420373a. [DOI] [PubMed] [Google Scholar]
- Keith LG, Oleszczuk JJ, Laguens M. Circadian rhythm chaos: a new breast cancer marker. Int J Fertil Womens Med. 2001;46:238–247. [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response textit in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Matsufuji S, Hayashi S, Tanahashi N, Tanaka K. Degradation of ornithine decarboxylase by the 26S proteasome. Biochem Biophys Res Commun. 2000;267:1–6. doi: 10.1006/bbrc.1999.1706. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto S, Masubuchi S, Honma S, Honma KI. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am J Physiol Regul Integr Comp Physiol. 2001;281:R197–R205. doi: 10.1152/ajpregu.2001.281.1.R197. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Childrens National Medical Center - microarray resources - KEsser Rat Exercise link http://microarray.cnmcresearch.org/resources.htm
- Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Moyers JS, Bilan PJ, Reynet C, Kahn CR. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol. 1996;494:891–898. doi: 10.1113/jphysiol.1996.sp021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp S, Daugaard JR, Richter EA. Eccentric exercise decreases glucose transporter GLUT4 protein in human skeletal muscle. J Physiol. 1995;482:705–712. doi: 10.1113/jphysiol.1995.sp020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckey JD, Ploug T, Galbo H. Attenuated insulin action on glucose uptake and transport in muscle following resistance exercise in rats. Acta Physiol Scand. 1999;167:77–82. doi: 10.1046/j.1365-201x.1999.00592.x. [DOI] [PubMed] [Google Scholar]
- Novials A, Vidal J, Franco C, Ribera F, Sener A, Malaisse WJ, Gomis R. Mutation in the calcium-binding domain of the mitochondrial glycerophosphate dehydrogenase gene in a family of diabetic subjects. Biochem Biophys Res Commun. 1997;231:570–572. doi: 10.1006/bbrc.1997.6147. [DOI] [PubMed] [Google Scholar]
- Kure S, Suzuki Y, Matsubara Y, Sakamoto O, Shintaku H, Isshiki G, Hoshida C, Izumi I, Sakura N, Narisawa K. Molecular analysis of glycogen storage disease type Ib: identification of a prevalent mutation among Japanese patients and assignment of a putative glucose-6-phosphate translocase gene to chromosome 11. Biochem Biophys Res Commun. 1998;248:426–431. doi: 10.1006/bbrc.1998.8985. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- Kawada S, Tachi C, Ishii N. Content and localization of myostatin in mouse skeletal muscles during aging, mechanical unloading and reloading. J Muscle Res Cell Motil. 2001;22:627–633. doi: 10.1023/a:1016366409691. [DOI] [PubMed] [Google Scholar]
- Borghaei RC, Sinai RS, Mochan E, Pease EA. Induction of mitogen-inducible nuclear orphan receptor by interleukin 1 in human synovial and gingival fibroblasts. Biochem Biophys Res Commun. 1998;251:334–338. doi: 10.1006/bbrc.1998.9477. [DOI] [PubMed] [Google Scholar]
- Chu W, Burns DK, Swerlick RA, Presky DH. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem. 1995;270:10236–10245. doi: 10.1074/jbc.270.17.10236. [DOI] [PubMed] [Google Scholar]
- Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–D89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of sigh density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- NetAffx http://www.affymetrix.com/analysis/index.affx
- Gene Ontology Consortium http://www.GeneOntology.org
- GenMAPP http://www.GenMAPP.org
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comp Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Tsai J, Sultana R, Lee Y, Pertea G, Karamycheva K, Antonescu V, Cho J, Parvizi P, Cheung F, Quackenbush J. RESOURCERER: a database for annotating and linking microarray resources within and across species. Genome Biol. 2001;2:software0002.1–0002.4. doi: 10.1186/gb-2001-2-11-software0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GenMAPP data http://www.genmapp.org/Zambon_2003/data.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Affymetrix data (MAS 4.0, target intensity 800) from rat 6 hours after exercise
Log2 RMA signal values, fold changes, P values and probe level annotation, and descriptions of column titles
Information for linking orthologous probe sets between Affymetrix Hs. U95A, Mm. U74A and Rn. U34A arrays
Hs RE/rat total RE and Hs RE/rat polysomal RE of 6 h after exercise-regulated gene orthologs regulated in this study and those published by Chen et al.
The putative circadian overlap genes
The unfiltered output results from the MAPPFinder analysis


