Abstract
Islet transplantation could become an ideal treatment for severe diabetes to prevent hypoglycemia shock and irreversible diabetic complications, once some of the major and unresolved obstacles are overcome, including limited donor supplies and side effects caused by permanent immunosuppressant use. Approximately 30 years ago, some groups succeeded in improving the blood glucose of diabetic animals by transplanting encapsulated islets with semi-permeable membranes consisting of polymer. A semi-permeable membrane protects both the inner islets from mechanical stress and the recipient’s immune system (both cellular and humoral immunities), while allowing bidirectional diffusion of nutrients, oxygen, glucose, hormones and wastes, i.e., immune-isolation. This device, which enables immune-isolation, is called encapsulated islets or bio-artificial pancreas. Encapsulation with a semi-permeable membrane can provide some advantages: (1) this device protects transplanted cells from the recipient’s immunity even if the xenogeneic islets (from large animals such as pig) or insulin-producing cells are derived from cells that have the potential for differentiation (some kinds of stem cells). In other words, the encapsulation technique can resolve the problem of limited donor supplies; and (2) encapsulation can reduce or prevent chronic administration of immunosuppressants and, therefore, important side effects otherwise induced by immunosuppressants. And now, many novel encapsulated islet systems have been developed and are being prepared for testing in a clinical setting.
Keywords: Islet transplantation, Encapsulated islets, Bio-artificial pancreas
INTRODUCTION
Islet transplantation is a cell replacement therapy that involves transplantation of isolated islets to recipients with severe diabetes mellitus (DM), especially type 1 diabetes mellitus (T1DM)[1]. Although the therapeutic outcome had been poor for approximately 30 years in the late 20th century, islet transplantation has been done clinically as one of the reliable therapeutic options since the development of the islet transplant protocol at Alberta University and has improved dramatically; it is called the “Edmonton protocol”[1]. Since the success of the Alberta team, islet transplantation has been performed widely for the past 10 years. Approximately 550 islet transplantations have been performed in more than 40 institutions[2,3]. According to a recent report, approximately 70% of patients did not need daily insulin at 1 year after transplantation and the graft function was well maintained with 82% graft survival at 5 years[4].
One of the major obstacles of islet transplantation is the limited human donor supply. Moreover, permanent immunosuppressant use is also problematic because immunosuppressants have some harmful effects on recipients. Firstly, immunosuppressants provide immune-tolerance, but they enable opportunistic infection; that is, an infection caused by a pathogen that usually does not cause disease in a healthy host[5]. A previous clinical report revealed that some of the patients that received islet transplantation had some infectious complications related to opportunistic infection, e.g., pneumonia, herpes infection and abscess formation[2]. Secondly, immunosuppressants also induce some side effects such as those found in patients with malignant disease treated with chemotherapy: mouth ulceration, anemia, leukopenia, diarrhea, headache, neutropenia, nausea, vomiting and fatigue[2]. Thirdly, immunosuppressants have islet toxicity. For example, it is known that tacrolimus and silolimus, which are major immunosuppressants for islet transplantation, impair the islet viability and graft function[6,7]. At present, islet transplantation should be done for patients with uncontrollable blood glucose and severe renal complications, and should not be performed in DM patients with no renal complications. If the problems concerning donors and immunosuppressants can be overcome, islet transplantation could become an ideal therapy for severe DM to prevent hypoglycemic shock and irreversible diabetic complications. We believe immune-isolation technology could be a powerful tool to resolve these problems.
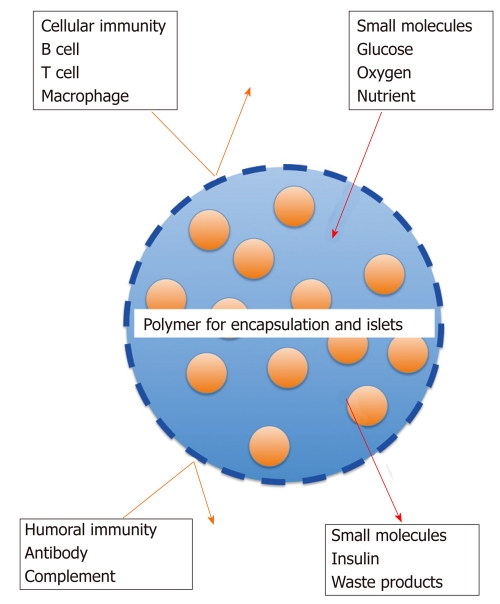
Immune-isolation can be achieved by covering islets with semi-permeable membranes consisting of high polymer[8,9], which is referred to as encapsulated islets or bio-artificial pancreas. Semi-permeable membranes protect the inner islets from both mechanical stress and the recipient’s immune system (both cellular and humoral immunities), while allowing the bidirectional diffusion of glucose, oxygen, nutrients, hormone and wastes[9] (Figure 1). Encapsulated islets could enable successful xenotransplantation with large animals, such as pigs, with a reduction or even absence of chronic administration of immunosuppressants, thus preventing important side-effects induced by immunosuppressants. Since the first encapsulated islet study was reported in 1977[10], encapsulated islets have been developed for the clinical setting.
Figure 1.
Scheme of the mechanism for encapsulated islets. Immune iso-lation can be achieved by covering islets with semi-permeable membranes consisting of high polymer. Semi-permeable membranes protect the inner islets from both mechanical stress and the recipient's immune system (both cellular and humoral immunities), while allowing bidirectional diffusion of glucose, oxygen, nutrients, insulin and waste.
ISLET ISOLATION AND TRANSPLANTATION
Islets are obtained from the donor pancreas by islet isolation. The procedure of islet isolation consists of pancreas digestion and islet purification steps. Pancreata are obtained from heart or brain dead donors or from living donors (for islet autotransplantation) with minimal warm ischemic time. Donor pancreata are preserved in cold preservation solution until islet isolation is started. University of Wisconsin (UW) solution has been used for this purpose[11,12] and, currently, UW solution is used with oxygenated perfluorochemical called the two-layer method, for better preservation of the pancreas[13]. ET-Kyoto solution, which is also cold preservation solution and used for lung preservation, has been used instead of UW solution for islet isolation in many institutions[14]. ET-Kyoto solution has components similar to extracellular fluid and contains trehalose for a cytoprotective effect and ulinastatin for inhibiting trypsin. Pancreas digestion is performed with enzyme solution. After removing additional organs (duodenum, spleen, lymph nodes and vessels), the blended solution of collagenase and neutral protease is injected into the pancreatic main duct until the pancreas is distended. The pancreas is cut into pieces (approximately 7 to 9 pieces) and put into a Ricordi chamber with some marbles. Pancreas digestion is done by recirculating warmed (at 37 °C) enzyme solution and mechanical shaking of the Ricordi chamber. Pancreas digestion is stopped by cooling until an adequate number of islets are obtained by monitoring samples taken from the recirculating system. Digested pancreas contains many exocrine and connective tissues that may cause portal vein hypertension and thrombosis; thus, removing these cellular components is necessary. Purification is performed using a COBE 2991 cell processor with Ficoll or iodixanol gradient solution[15]. Obtaining many good islets from the donor pancreas is necessary to cure severe diabetes, but it is impossible to obtain all islets contained in the pancreas by current isolation techniques. In the digestion stage, islet loss cannot be prevented if collagenase solution is not fully injected, if the digestion is inadequate, or if the digestion time is longer and isolated islets are injured. In the purification stage, some islets are discarded with other cells such as acinar and ductal cells if the gradient is similar. Over 11 000 islet equivalents per kilogram of body weight are recommended to cure DM[1,12], but it is difficult to acquire this number of islets from one donor pancreas using current techniques. This is the reason why multiple donors are required. Culturing isolated islets before transplantation is done to evaluate them and many institutions perform islet culture[12]. However, while culturing can reduce contamination of acinar cells (improving purity), isolated islets deteriorate rapidly in culture, reducing the number. Fresh islet transplantation is recommended for a good outcome[1] and, if immediate transplantation cannot be done, cold preservation (4 °C in UW solution) before transplantation is recommended over 37 °C culture for preventing islet loss and preserving the size, shape and function of islets[16].
While there are many transplantation sites (kidney[17-19], muscle[20], omentum[21], testis[22], bone marrow[23] and some other organs) for islet transplantation in animal experiments, liver is the only the transplantation site used in a clinical setting. The islets are injected into the recipient liver via the portal vein. There are rare but severe complications due to this method: portal hypertension, portal thrombosis and bleeding[12,24]. Portal thrombosis is an especially life-threatening complication. Recently, the Alberta group reported that portal thrombosis was detected in 3.7% of islet-transplanted patients, and a higher volume of islet graft (over 5.45 mL) and higher increase of the portal pressure at transplantation (over 4.5 mmHg) are risk factors for portal thrombosis[25].
Islet transplantation is classified into three types by the cell sources: autotransplantation, allotransplantation and xenotransplantation. Autotransplantation means transplantation of self-islets when total pancreatectomy cannot be avoided in spite of benign diseases, i.e., chronic pancreatitis[26,27], pancreatic arteriovenous malformation[28] and trauma[29]. The outcome of autotransplantation is excellent because transplanted islets are free from immunity and rejection and there is no need to use immunosuppressants. In allotransplantation, diabetic (especially T1DM) recipients are transplanted islets derived from different individual(s). Rejection cannot be prevented in allotransplantation and thus immunosuppressants are necessary. In xenotransplantation, diabetic recipients are transplanted with islets derived from different animals. As in allotransplantation, rejection cannot be prevented but it is difficult to manage immunity by using any immunosuppressants. To utilize these cell sources, studies about encapsulated islets have been promoted.
MATERIALS FOR ENCAPSULATION
The materials for encapsulation must have two characteristics: Firstly, they must isolate the encapsulated islets from the immune system consisting of immune competent cells (T cells, B cells or macrophages), antibody and complement; and secondly, they must permit the diffusion of small molecules like glucose, oxygen and nutrients, and the diffusion of insulin and waste products (Figure 1 and Table 1). The function of encapsulated islets depends on the materials.
Table 1.
List of materials for encapsulated islets
| Materials | Shapes of capsule | Donor source and recipient | Results |
| Alginate[36] | Microcapsule | Porcine and bovine to rat | Achieving normoglycemia for 9 mo |
| Polysulphone[41] | Macrocapsule | Porcine to rat | Normoglycemia over 1 mo |
| Polyvinyl alcoho[8] | Macrocapsule | Rat to mouse | Normoglycemia for 30 d |
| Low molecular weight dextran sulfate[47] | Microcapsule | In vitro assay | Inhibition of complement |
In 1980, Lim and Sun[30] first developed microencapsulated islets using alginate and succeeded in achieving normoglycemia in diabetic rats for two-three weeks. Alginate is now the most famous material for encapsulation. Alginates are found in brown algae and in bacterial species[31]. They consist of unbranched binary copolymers of 1-4 linked β-D-mannuronic acid (M) and α-L-guluronic acid (G), of widely varying composition and sequential structure (MMM-blocks, GGGblocks and MGM-blocks). The alkali-, ammonium- and magnesium-alginates are soluble in water. Gelation of alginates occurs when the carboxyl groups of the polymers are cross-linked with multi-valent cations (e.g., Ca2+, Ba2+, La3+, Fe3+) and poly-electrolytes[32]. Alginate is a suitable material for encapsulated islets because alginate does not interfere with the islet function in releasing hormone and has good stability[32]. Moreover, various materials such as poly (ethylene glycol) (PEG) and poly-L-lysine (PLL) have been used to improve the alginate capsule by reducing plasma absorption and making a semi-permeable membrane. The first report about an alginate/PEG capsule was published in 1999. Chandy et al[33] modified alginate encapsulated islets by including PEG and succeeded in improving the stability. Desai and colleagues clarified that islets encapsulated by alginate and PEG had good viability and insulin releasing function in an in vitro assay[34]. The alginate/PLL capsule is the most utilized combination: in 1985, Goosen et al[35] developed three layer capsules consisting of alginate/PLL/alginate layers and proved that the capsules had a good immune isolation effect by blocking the diffusion of serum immunoglobulin, albumin and hemoglobin. Lanza et al[36] transplanted encapsulated xenogeneic islets (porcine and bovine islets) into the peritoneal cavities of diabetic rats and found that there was no destruction of the islets for 9 mo and no fibrous adhesions around the capsule, while achieving normoglycemia. While the size of their capsule was approximately 800 μm, Strand et al[37] succeeded in developing thinner capsules (200 μm) with good immune isolation.
Similar to alginate, polysulphone (PSU) is suitable material for encapsulation. PSU has been used for renal dialysis as its hollow fibers remove tight, molecular-weight waste productions[38]. PSU is focused on as a possible material for encapsulated islets because large amounts of insulin are absorbed by the PSU hollow fibers. Lembert et al[39,40] developed hydroxy-methylated PSU “macroencapsulated” islets which showed good insulin releasing function similar to naked islets while blocking endogenous retrovirus infection. Transplantation of their device also achieved normoglycemia in diabetic rats for over 1 mo[41].
Polyvinyl alcohol (PVA) also has been used as a material for encapsulated islets. PVA is a water-soluble synthetic polymer that has been used as a material for many devices like contact lens solution or artificial tears for the treatment of dry eye. The first report of PVA encapsulated islets was published in 1992 from the Kyoto group. Inoue and colleagues developed a tube type of PVA macrocapsule with a mesh reinforcement. Two thousand rat islets were contained in the capsule and diabetic rats achieved the normoglycemia by allogeneic-transplantation of the device for 12 d[42]. The device also had a good function of insulin release as indicated by the in vitro glucose concentration[43]. After that, they developed various types of PVA capsules and evaluated the function. For example, transplantation of a bag type of PVA capsule encapsulating porcine islets achieved normoglycemia in diabetic rats for 2 wk[44]. Sakurai et al[45] modified the device with an angiogenesis factor (fibroblast growth factor-2) and confirmed neovascularization around the capsule. Qi et al[8] evaluated the function of the sheet type of PVA capsule in vitro and in vivo. Rat islets in the PVA capsule had good function in insulin release and achieved normoglycemia in diabetic mice for 30 d. Sakata et al[46]also attempted to evaluate the therapeutic effect in renal function by transplantation of the device and confirmed an improvement of the hyperglycemia, serum blood urea nitrogen and creatinine and mesangial thickness.
Low molecular weight dextran sulfate (LMW-DS) was first introduced as a material for encapsulated islets in 2003[47]. Ikada et al[47] developed a bio-artificial pancreas (encapsulated islets) using LMW-DS and succeeded in preventing complement attack. The Korsgren group revealed that LMW-DS is useful in preventing instant blood-mediated inflammatory reaction (IBMIR: a rapid thrombotic reaction, in which binding of platelets to the islet surface, activation of the coagulation and complement systems, and leukocyte infiltration of the islets when the islets are exposed to blood occur)[48-50].
Various materials have been studied and shown to have positive results, but encapsulated islets that can be utilized permanently have not been developed yet. Development of a material that can maintain good viability of the encapsulated islets and good immune-isolation for the long term is expected.
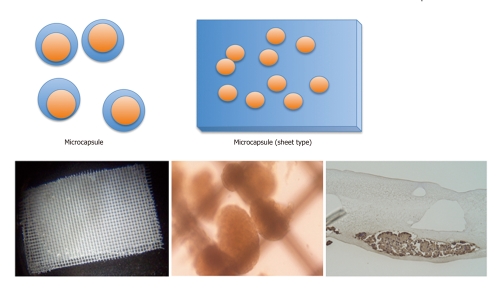
SHAPES OF ENCAPSULATED ISLETS
Encapsulated islets are classified into two types by the size: macrocapsules and microcapsules (Figure 2). Macrocapsules are also divided in two types, intravascular and extravascular types. An intravascular macrocapsule is a perfusion chamber that is directly connected to the host artery and vein[51]. Blood flows into the hollow fibers and islets are placed near the fibers in this system. Islets could receive oxygen and nutrient supply from blood flow and were protected from immunity by the membrane. However, the intravascular macrocapsule had the severe problem of embolization in the hollow fibers caused by the formation of blood clots.
Figure 2.
Scheme of size of the encapsulated islets. Encapsulated islets are classified into two types by the size: microcapsule (upper) and macrocapsule (lower). Microencapsulated islets are microcapsules containing a single or few islets. The microcapsule has some advantages in the transportation of oxygen and nutrients because of the smaller distance between the capsule surface and islet. The response to glucose change is better than that of a macrocapsule. The macrocapsule is a diffusion chamber containing a large amount of islets. Many shapes including rods, tubes or sheets (shown in the lower figure) are used for the macroencapsulation. One of the merits of a macrocapsule is the ease of implantation and removal with minimum risk when the device is infected. Left: Sheet type of polyvinyl alcohol macroencapsulated islets. Center: Inside of the macrocapsule. Intact islets are encapsulated in it. Right: Immunohistochemical staining of the macrocapsule for insulin. The islets in the macrocapsule are positive for insulin and viable.
The extravascular macrocapsule is a diffusion chamber containing a large number of islets. Many shapes, including rod[52], tube[43,53] or sheet[54] types, are used for the macroencapsulation. One of the merits of macrocapsules is the ease of implantation and removal with minimum risk when the device is infected. On the other hand, the permeability of the macrocapsule is less than that of the microcapsule because of the thicker membrane[38].
Microencapsulated islets are microcapsules containing one or a few islets. Some different protocols for making the devices have been reported. For example, the alginate capsule is made by dropping it into ionic solution (Ca2+, Ba2+, etc.)[55] and the agarose capsule is made by cooling with shaking[56]. Microcapsules have some advantages in the transportation of oxygen and nutrients because of the smaller distance between the capsule surface and the islet[38]. The response to glucose change is better than that by macrocapsules. The disadvantage of microcapsules is that they are difficult to remove completely when necessary.
The sizes of encapsulated islets should be selected according to the transplant sites and other characteristics.
CELL SOURCES
The purpose of encapsulation is to prevent loss of the transplanted islets due to immunity. The improvement of the immunosuppression protocol accounts for the present success of islet transplantation[57]. Encapsulation can protect allogeneic islets from immunity without using immunosuppressants. Human islets are the ideal cell source, but clinical islet transplantation has been limited by the donor supplies. To overcome this hurdle, cell sources in addition to allogeneic islets will be necessary.
One of the candidates is xenogeneic islets, especially those of pig[58]. Historically, porcine insulin was mainly used clinically since insulin was discovered in the early 1920s[59] before the development of recombinant insulin. Porcine insulin differs little from human insulin (only one difference in amino acid)[60]. The pig is a large animal with a large pancreas that contains many islets. Therefore, porcine islets are considered an optimal donor source. There are many publications about encapsulated porcine islet xenotransplantation that could improve the blood glucose control in recipient animals. The first report about improved blood glucose by encapsulated porcine islets was published in 1991. Lanza et al[61] performed encapsulation of porcine islets by using alginate and transplanted the tube type of encapsulated islets into the peritoneal cavity of diabetic rats and confirmed the normalization of the blood glucose. Sun et al[62] succeeded in achieving normoglycemia in a diabetic monkey for over 150 d by transplanting microencapsulated porcine islets. In spite of the positive data for xenotransplantation, there are some obstacles for the clinical setting. The major obstacle is the risk of viral infection, especially porcine endogenous retrovirus (PERV). van der Laan et al[63] first described that PERV infection was detected in NOD/SCID mice that received porcine islet transplantation. Clemenceau et al[64] showed that PERV DNA was transmitted into a mouse and human cell line. On the other hand, many publications described that there was no evidence of and no influence by HERV infection[65-67]. Moreover, a recent study from a Minnesota group revealed that not many virus infections were detected in porcine organs, including islets, except porcine cytomegalovirus (PCMV). They concluded that pigs infected with PCMV should not be used as donors and pigs not older than 21 wk should be used to prevent viral infection[68]. The other obstacle is a reluctance to transplant xenogeneic tissue based on religious beliefs and customs[69]. In summary, while there are still some obstacles to promoting xenotransplantation, encapsulation technology can be a powerful tool for overcoming obstacles to xenotransplantation.
IDEAL TRANSPLANTATION SITE FOR ENCAPSULATED ISLETS
Intrahepatic transplantation is the current standard for islet transplantation and the liver is the only organ that has been successful as a transplantation site for clinical islet transplantation. However, several recent studies have clearly shown that most of the islets (approximately 60% islets) transplanted intraportally are immediately destroyed, mainly due to the IBMIR[49,70]. Moreover, the infusion of islets with some other pancreatic tissues (acinar cells, ductal cells or connective tissues) in the portal vein always has a risk of causing portal hypertension and portal vein embolization[71]. Schneider et al[72] transplanted microencapsulated rat islets (with alginate) into the livers of mice, but only a 1 wk normalization in the blood glucose level was achieved. We revealed[70] that transplanted islets suffer from ischemia due to embolization by the islets themselves. The size of encapsulated islets is enlarged by encapsulation and thus encapsulated islets may tend to suffer from ischemia by the size and loss of permeability of the capsule. A thinner capsule (almost the same size as naked islets) is necessary for successful intraportal transplantation.
Furthermore, other transplant sites should be selected for encapsulated islet transplantation to avoid factors that injure islets by intraportal transplantation and the side effects of intraportal transplantation. In experimental studies, there are many descriptions of positive data on extrahepatic transplantation sites. The Kyoto and other groups[73] succeeded in achieving normoglycemia in diabetic mice by transplantation of encapsulated islets in muscle, subcutaneous tissue[58,74-76], renal subcapsule[77] and omentum[56]. These may be candidate transplantation sites in the near future because the loss of islets due to IBMIR, the risk of portal hypertension and portal vein thrombosis could be prevented. Especially, muscle and subcutaneous tissue are considered the best positions to transplant (easy to approach in comparison with intraperitoneal organs) and to remove when the graft fails in function or becomes an origin of infection. In our opinion, the ideal transplant site for encapsulated islets is a site that is managed easily and has good efficacy in transplantation.
CONCLUSION
Encapsulated islet transplantation was first performed in 1999. Encapsulated human islet transplantation was performed in a 38 year old man who had severe diabetes by a UCLA group[78]. In 2000, Elliott et al[79] transplanted encapsulated porcine islets into diabetic patients and confirmed no PERV infection in patients. Clinical trials have been few, but promising outcomes have been reported. Recently, Calafiore et al[80] performed human islets microencapsulation using alginate and transplanted them into 10 T1DM patients in Italy. Their protocol is based on using clinical grade sodium alginate without pyrogen and endotoxin, using human islets which have high purity and viability (over 80%) and a minimally invasive transplant method by intraportal injection under ultrasound imaging with local anesthesia. This protocol is approved by the Italian Ministry of Health. An Australia group performed transplantation of barium-alginate encapsulated human islets to 4 T1DM patients without immunosuppression and followed them for 2.5 years as a phase 1 study[81]. While no adverse events were detected, there was no improvement of the diabetic condition in the 4 patients. Laparoscopic biopsy revealed no cellular infiltration in the capsule and islet necrosis. As compared with non-capsulated islet transplantation with immunosuppression, the outcome of encapsulated islets is promising. Further improvements of the devices are necessary for the clinical setting, but, when successful, could cure severe DM without using immunosuppressants.
In conclusion, encapsulation technology can utilize allo- or xenogeneic cell sources to overcome limited donor supplies in islet transplantation.
Footnotes
Supported by Research Seeds Quest Program in Japan Science and Technology Agency (NS); the Uehara Memorial Foundation (NS); and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports Science and Technology of Japan, B: 22390253 (SE), C: 22591513 (NS)
Peer reviewers: Cinzia Domeneghini, Professor, University of Milan, Milan I-20133, Italy; Wai-Keung Chow, MD, Division of Gastroenterology, Medical University Hospital, Taichung 400, Taiwan, China
S- Editor Wu X L- Editor Roemmele A E- Editor Li JY
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Paty BW, Senior PA, Bigam DL, Ryan EA. Strategic opportunities in clinical islet transplantation. Transplantation. 2005;79:1304–1307. doi: 10.1097/01.tp.0000157300.53976.2a. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 5.Huurman VA, Kalpoe JS, van de Linde P, Vaessen N, Ringers J, Kroes AC, Roep BO, De Fijter JW. Choice of antibody immunotherapy influences cytomegalovirus viremia in simultaneous pancreas-kidney transplant recipients. Diabetes Care. 2006;29:842–847. doi: 10.2337/diacare.29.04.06.dc05-1647. [DOI] [PubMed] [Google Scholar]
- 6.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 7.Tanemura M, Saga A, Kawamoto K, Machida T, Deguchi T, Nishida T, Sawa Y, Doki Y, Mori M, Ito T. Rapamycin induces autophagy in islets: relevance in islet transplantation. Transplant Proc. 2009;41:334–338. doi: 10.1016/j.transproceed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y, Yamamoto C, Hiura A, Sumi S, Inoue K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25:5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Cell microencapsulation technology: towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Gates RJ, Lazarus NR. Reversal of streptozotocin-induced diabetes in rats by intraperitoneal implantation of encapsulated neonatal rabbit pancreatic tissue. Lancet. 1977;2:1257–1259. doi: 10.1016/s0140-6736(77)92664-2. [DOI] [PubMed] [Google Scholar]
- 11.Munn SR, Kaufman DB, Field MJ, Viste AB, Sutherland DE. Cold-storage preservation of the canine and rat pancreas prior to islet isolation. Transplantation. 1989;47:28–31. doi: 10.1097/00007890-198901000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi H. Pancreatic islet transplantation. World J Gastrointest Surg. 2009;1:16–20. doi: 10.4240/wjgs.v1.i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroda Y, Kawamura T, Suzuki Y, Fujiwara H, Yamamoto K, Saitoh Y. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation. 1988;46:457–460. doi: 10.1097/00007890-198809000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi H, Ueda M, Hayashi S, Kobayashi N, Okitsu T, Iwanaga Y, Nagata H, Nakai Y, Matsumoto S. Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function. Cell Transplant. 2008;17:69–81. doi: 10.3727/000000008783907062. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi H, Ikemoto T, Naziruddin B, Jackson A, Shimoda M, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, et al. Iodixanol-controlled density gradient during islet purification improves recovery rate in human islet isolation. Transplantation. 2009;87:1629–1635. doi: 10.1097/TP.0b013e3181a5515c. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, et al. Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation. 2010;89:47–54. doi: 10.1097/TP.0b013e3181be3bf2. [DOI] [PubMed] [Google Scholar]
- 17.Sakata N, Kodama T, Chen R, Yoshimatsu G, Goto M, Egawa S, Unno M. Monitoring transplanted islets by high-frequency ultrasound. Islets. 2011;3:259–266. doi: 10.4161/isl.3.5.17058. [DOI] [PubMed] [Google Scholar]
- 18.Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89:686–693. doi: 10.1097/TP.0b013e3181cb3e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata N, Tan A, Chan N, Obenaus A, Mace J, Peverini R, Sowers L, Chinnock R, Hathout E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc. 2009;41:346–349. doi: 10.1016/j.transproceed.2008.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christoffersson G, Carlsson PO, Phillipson M. Intramuscular islet transplantation promotes restored islet vascularity. Islets. 2011;3:69–71. doi: 10.4161/isl.3.2.14997. [DOI] [PubMed] [Google Scholar]
- 21.McQuilling JP, Arenas-Herrera J, Childers C, Pareta RA, Khanna O, Jiang B, Brey EM, Farney AC, Opara EC. New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch. Transplant Proc. 2011;43:3262–3264. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gores PF, Hayes DH, Copeland MJ, Korbutt GS, Halberstadt C, Kirkpatrick SA, Rajotte RV. Long-term survival of intratesticular porcine islets in nonimmunosuppressed beagles. Transplantation. 2003;75:613–618. doi: 10.1097/01.TP.0000052376.89400.8D. [DOI] [PubMed] [Google Scholar]
- 23.Kover K, Tong PY, Pacicca D, Clements M, Bodker AM, Eidson C, Sheldon M, Southard A, Zaidi A, Moore WV. Bone marrow cavity: a supportive environment for islet engraftment. Islets. 2011;3:93–101. doi: 10.4161/isl.3.3.15567. [DOI] [PubMed] [Google Scholar]
- 24.Brennan DC, Shannon MB, Koch MJ, Polonsky KS, Desai N, Shapiro J. Portal vein thrombosis complicating islet transplantation in a recipient with the Factor V Leiden mutation. Transplantation. 2004;78:172–173. doi: 10.1097/01.tp.0000128332.71657.ea. [DOI] [PubMed] [Google Scholar]
- 25.Kawahara T, Kin T, Kashkoush S, Gala-Lopez B, Bigam DL, Kneteman NM, Koh A, Senior PA, Shapiro AM. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11:2700–2707. doi: 10.1111/j.1600-6143.2011.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980;192:526–542. doi: 10.1097/00000658-198010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farney AC, Najarian JS, Nakhleh RE, Lloveras G, Field MJ, Gores PF, Sutherland DE. Autotransplantation of dispersed pancreatic islet tissue combined with total or near-total pancreatectomy for treatment of chronic pancreatitis. Surgery. 1991;110:427–437; discussion 437-439. [PubMed] [Google Scholar]
- 28.Sakata N, Egawa S, Motoi F, Mikami Y, Ishida M, Aoki T, Ottomo S, Fukuyama S, Rikiyama T, Katayose Y, et al. Institutional indications for islet transplantation after total pancreatectomy. J Hepatobiliary Pancreat Surg. 2008;15:488–492. doi: 10.1007/s00534-007-1309-3. [DOI] [PubMed] [Google Scholar]
- 29.Jindal RM, Ricordi C, Shriver CD. Autologous pancreatic islet transplantation for severe trauma. N Engl J Med. 2010;362:1550. doi: 10.1056/NEJMc0912392. [DOI] [PubMed] [Google Scholar]
- 30.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 31.Leung YF, O'Shea GM, Goosen MF, Sun AM. Microencapsulation of crystalline insulin or islets of Langerhans: an insulin diffusion study. Artif Organs. 1983;7:208–212. doi: 10.1111/j.1525-1594.1983.tb04187.x. [DOI] [PubMed] [Google Scholar]
- 32.de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45:159–173. doi: 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- 33.Chandy T, Mooradian DL, Rao GH. Evaluation of modified alginate-chitosan-polyethylene glycol microcapsules for cell encapsulation. Artif Organs. 1999;23:894–903. doi: 10.1046/j.1525-1594.1999.06244.x. [DOI] [PubMed] [Google Scholar]
- 34.Desai NP, Sojomihardjo A, Yao Z, Ron N, Soon-Shiong P. Interpenetrating polymer networks of alginate and polyethylene glycol for encapsulation of islets of Langerhans. J Microencapsul. 2000;17:677–690. doi: 10.1080/02652040050161675. [DOI] [PubMed] [Google Scholar]
- 35.Goosen MF, O'Shea GM, Gharapetian HM, Chou S, Sun AM. Optimization of microencapsulation parameters: Semipermeable microcapsules as a bioartificial pancreas. Biotechnol Bioeng. 1985;27:146–150. doi: 10.1002/bit.260270207. [DOI] [PubMed] [Google Scholar]
- 36.Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kühtreiber W, Chick WL. Xenotransplantation of cells using biodegradable microcapsules. Transplantation. 1999;67:1105–1111. doi: 10.1097/00007890-199904270-00004. [DOI] [PubMed] [Google Scholar]
- 37.Strand BL, Gåserød O, Kulseng B, Espevik T, Skjåk-Baek G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul. 2002;19:615–630. doi: 10.1080/02652040210144243. [DOI] [PubMed] [Google Scholar]
- 38.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 2007;13:589–599. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 39.Petersen P, Lembert N, Zschocke P, Stenglein S, Planck H, Ammon HP, Becker HD. Hydroxymethylated polysulphone for islet macroencapsulation allows rapid diffusion of insulin but retains PERV. Transplant Proc. 2002;34:194–195. doi: 10.1016/s0041-1345(01)02724-5. [DOI] [PubMed] [Google Scholar]
- 40.Lembert N, Wesche J, Petersen P, Zschocke P, Enderle A, Planck H, Ammon HP. Macroencapsulation of rat islets without alteration of insulin secretion kinetics. Exp Clin Endocrinol Diabetes. 2001;109:116–119. doi: 10.1055/s-2001-14828. [DOI] [PubMed] [Google Scholar]
- 41.Lembert N, Wesche J, Petersen P, Doser M, Zschocke P, Becker HD, Ammon HP. Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant. 2005;14:97–108. [PubMed] [Google Scholar]
- 42.Inoue K, Fujisato T, Gu YJ, Burczak K, Sumi S, Kogire M, Tobe T, Uchida K, Nakai I, Maetani S. Experimental hybrid islet transplantation: application of polyvinyl alcohol membrane for entrapment of islets. Pancreas. 1992;7:562–568. [PubMed] [Google Scholar]
- 43.Aung T, Kogire M, Inoue K, Fujisato T, Gu Y, Burczak K, Shinohara S, Mitsuo M, Maetani S, Ikada Y. Insulin release from a bioartificial pancreas using a mesh reinforced polyvinyl alcohol hydrogel tube. An in vitro study. ASAIO J. 1993;39:93–96. [PubMed] [Google Scholar]
- 44.Miyamoto M, Inoue K, Gu Y, Tun T, Cui W, Fujiwara I, Ohyanagi H, Hayashi H, Yamazaki T, Setoyama H, et al. Improved large-scale isolation of breeder porcine islets: possibility of harvesting from nonheart-beating donor. Cell Transplant. 1998;7:397–402. doi: 10.1177/096368979800700408. [DOI] [PubMed] [Google Scholar]
- 45.Sakurai T, Satake A, Sumi S, Inoue K, Nagata N, Tabata Y, Miyakoshi J. The efficient prevascularization induced by fibroblast growth factor 2 with a collagen-coated device improves the cell survival of a bioartificial pancreas. Pancreas. 2004;28:e70–e79. doi: 10.1097/00006676-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 46.Sakata N, Gu Y, Qi M, Yamamoto C, Hiura A, Sumi S, Sunamura M, Matsuno S, Inoue K. Effect of rat-to-mouse bioartificial pancreas xenotransplantation on diabetic renal damage and survival. Pancreas. 2006;32:249–257. doi: 10.1097/01.mpa.0000203959.31877.8c. [DOI] [PubMed] [Google Scholar]
- 47.Murakami Y, Iwata H, Kitano E, Kitamura H, Ikada Y. Dextran sulfate as a material for the preparation of a membrane for immunoisolation. J Biomater Sci Polym Ed. 2003;14:875–885. doi: 10.1163/156856203322381384. [DOI] [PubMed] [Google Scholar]
- 48.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77:741–747. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 49.Johansson H, Goto M, Dufrane D, Siegbahn A, Elgue G, Gianello P, Korsgren O, Nilsson B. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6:305–312. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 50.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, Brandhorst H, Wennberg L, Kurokawa Y, Satomi S, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15:225–234. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaco AP, Maki T, Ozato H, Carretta M, Sullivan SJ, Borland KM, Mahoney MD, Chick WL, Muller TE, Wolfrum J. Transplantation of islet allografts and xenografts in totally pancreatectomized diabetic dogs using the hybrid artificial pancreas. Ann Surg. 1991;214:339–360; discussion 361-362. doi: 10.1097/00000658-199109000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones KS, Sefton MV, Gorczynski RM. In vivo recognition by the host adaptive immune system of microencapsulated xenogeneic cells. Transplantation. 2004;78:1454–1462. doi: 10.1097/01.tp.0000142094.63083.fb. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi H, Inoue K, Aung T, Tun T, Yuanjun G, Wenjing W, Shinohara S, Kaji H, Doi R, Setoyama H, et al. Application of a novel B cell line MIN6 to a mesh-reinforced polyvinyl alcohol hydrogel tube and three-layer agarose microcapsules: an in vitro study. Cell Transplant. 1996;5:S65–S69. doi: 10.1016/0963-6897(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 54.Lee JI, Nishimura R, Sakai H, Sasaki N, Kenmochi T. A newly developed immunoisolated bioartificial pancreas with cell sheet engineering. Cell Transplant. 2008;17:51–59. doi: 10.3727/000000008783907035. [DOI] [PubMed] [Google Scholar]
- 55.Johnson AS, O'Sullivan E, D'Aoust LN, Omer A, Bonner-Weir S, Fisher RJ, Weir GC, Colton CK. Quantitative assessment of islets of Langerhans encapsulated in alginate. Tissue Eng Part C Methods. 2011;17:435–449. doi: 10.1089/ten.tec.2009.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, Ko S, Nagao M, Harb G, Nakajima Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006;15:359–365. doi: 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- 57.Laugharne M, Cross S, Richards S, Dawson C, Ilchyshyn L, Saleem M, Mathieson P, Smith R. Sirolimus toxicity and vascular endothelial growth factor release from islet and renal cell lines. Transplantation. 2007;83:1635–1638. doi: 10.1097/01.tp.0000266555.06635.bf. [DOI] [PubMed] [Google Scholar]
- 58.Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90:1054–1062. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 59.Rosenfeld L. Insulin: discovery and controversy. Clin Chem. 2002;48:2270–2288. [PubMed] [Google Scholar]
- 60.Brogden RN, Heel RC. Human insulin. A review of its biological activity, pharmacokinetics and therapeutic use. Drugs. 1987;34:350–371. doi: 10.2165/00003495-198734030-00003. [DOI] [PubMed] [Google Scholar]
- 61.Lanza RP, Butler DH, Borland KM, Staruk JE, Faustman DL, Solomon BA, Muller TE, Rupp RG, Maki T, Monaco AP. Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppression. Proc Natl Acad Sci USA. 1991;88:11100–11104. doi: 10.1073/pnas.88.24.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun AM, Vacek I, Sun YL, Ma X, Zhou D. In vitro and in vivo evaluation of microencapsulated porcine islets. ASAIO J. 1992;38:125–127. [PubMed] [Google Scholar]
- 63.van der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, et al. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature. 2000;407:90–94. doi: 10.1038/35024089. [DOI] [PubMed] [Google Scholar]
- 64.Clémenceau B, Jégou D, Martignat L, Saï P. Microchimerism and transmission of porcine endogenous retrovirus from a pig cell line or specific pathogen-free pig islets to mouse tissues and human cells during xenografts in nude mice. Diabetologia. 2002;45:914–923. doi: 10.1007/s00125-002-0832-7. [DOI] [PubMed] [Google Scholar]
- 65.Denner J, Specke V, Karlas A, Chodnevskaja I, Meyer T, Moskalenko V, Kurth R, Ulrichs K. No transmission of porcine endogenous retroviruses (PERVs) in a long-term pig to rat xenotransplantation model and no infection of immunosuppressed rats. Ann Transplant. 2008;13:20–31. [PubMed] [Google Scholar]
- 66.Garkavenko O, Dieckhoff B, Wynyard S, Denner J, Elliott RB, Tan PL, Croxson MC. Absence of transmission of potentially xenotic viruses in a prospective pig to primate islet xenotransplantation study. J Med Virol. 2008;80:2046–2052. doi: 10.1002/jmv.21272. [DOI] [PubMed] [Google Scholar]
- 67.Irgang M, Laue C, Velten F, Kurth R, Schrezenmeier J, Denner J. No evidence for PERV release by islet cells from German landrace pigs. Ann Transplant. 2008;13:59–66. [PubMed] [Google Scholar]
- 68.Abrahante JE, Martins K, Papas KK, Hering BJ, Schuurman HJ, Murtaugh MP. Microbiological safety of porcine islets: comparison with source pig. Xenotransplantation. 2011;18:88–93. doi: 10.1111/j.1399-3089.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 69.Sattar SP, Ahmed MS, Madison J, Olsen DR, Bhatia SC, Ellahi S, Majeed F, Ramaswamy S, Petty F, Wilson DR. Patient and physician attitudes to using medications with religiously forbidden ingredients. Ann Pharmacother. 2004;38:1830–1835. doi: 10.1345/aph.1E001. [DOI] [PubMed] [Google Scholar]
- 70.Sakata N, Obenaus A, Chan N, Mace J, Chinnock R, Hathout E. Factors affecting islet graft embolization in the liver of diabetic mice. Islets. 2009;1:26–33. doi: 10.4161/isl.1.1.8563. [DOI] [PubMed] [Google Scholar]
- 71.Walsh TJ, Eggleston JC, Cameron JL. Portal hypertension, hepatic infarction, and liver failure complicating pancreatic islet autotransplantation. Surgery. 1982;91:485–487. [PubMed] [Google Scholar]
- 72.Schneider S, von Mach MA, Kraus O, Kann P, Feilen PJ. Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats. Artif Organs. 2003;27:1053–1056. doi: 10.1046/j.1525-1594.2003.07159.x. [DOI] [PubMed] [Google Scholar]
- 73.Balamurugan AN, Gu Y, Tabata Y, Miyamoto M, Cui W, Hori H, Satake A, Nagata N, Wang W, Inoue K. Bioartificial pancreas transplantation at prevascularized intermuscular space: effect of angiogenesis induction on islet survival. Pancreas. 2003;26:279–285. doi: 10.1097/00006676-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 74.Kawakami Y, Iwata H, Gu YJ, Miyamoto M, Murakami Y, Balamurugan AN, Imamura M, Inoue K. Successful subcutaneous pancreatic islet transplantation using an angiogenic growth factor-releasing device. Pancreas. 2001;23:375–381. doi: 10.1097/00006676-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Gu Y, Tabata Y, Miyamoto M, Hori H, Nagata N, Touma M, Balamurugan AN, Kawakami Y, Nozawa M, et al. Reversal of diabetes in mice by xenotransplantation of a bioartificial pancreas in a prevascularized subcutaneous site. Transplantation. 2002;73:122–129. doi: 10.1097/00007890-200201150-00023. [DOI] [PubMed] [Google Scholar]
- 76.Wang W, Gu Y, Hori H, Sakurai T, Hiura A, Sumi S, Tabata Y, Inoue K. Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation. 2003;76:290–296. doi: 10.1097/01.TP.0000073613.25658.4D. [DOI] [PubMed] [Google Scholar]
- 77.Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81:1345–1353. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 78.Soon-Shiong P. Treatment of type I diabetes using encapsulated islets. Adv Drug Deliv Rev. 1999;35:259–270. doi: 10.1016/s0169-409x(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 79.Elliott RB, Escobar L, Garkavenko O, Croxson MC, Schroeder BA, McGregor M, Ferguson G, Beckman N, Ferguson S. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 2000;9:895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- 80.Calafiore R, Basta G, Luca G, Lemmi A, Racanicchi L, Mancuso F, Montanucci MP, Brunetti P. Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transplant Proc. 2006;38:1156–1157. doi: 10.1016/j.transproceed.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]