Abstract
The gastrointestinal tract is the largest reservoir of commensal bacteria in the human body, providing nutrients and space for the survival of microbes while concurrently operating mucosal barriers to confine the microbial population. The epithelial cells linked by tight junctions not only physically separate the microbiota from the lamina propria, but also secrete proinflammatory cytokines and reactive oxygen species in response to pathogen invasion and metabolic stress and serve as a sentinel to the underlying immune cells. Accumulating evidence indicates that commensal bacteria are involved in various physiological functions in the gut and microbial imbalances (dysbiosis) may cause pathology. Commensal bacteria are involved in the regulation of intestinal epithelial cell turnover, promotion of epithelial restitution and reorganization of tight junctions, all of which are pivotal for fortifying barrier function. Recent studies indicate that aberrant bacterial lipopolysaccharide-mediated signaling in gut mucosa may be involved in the pathogenesis of chronic inflammation and carcinogenesis. Our perception of enteric commensals has now changed from one of opportunistic pathogens to active participants in maintaining intestinal homeostasis. This review attempts to explain the dynamic interaction between the intestinal epithelium and commensal bacteria in disease and health status.
Keywords: Intestinal barrier, Commensal bacteria, Enterocytes, Tight junctions, Lipopolysaccharide, CD14/TLR4, Inflammatory bowel disease, Colorectal cancer
INTRODUCTION
The gastrointestinal tract is the largest reservoir of commensal bacteria in the human body. Food intake through the oral route serves as a port to the outside environment and allows for entry of exogenous organisms, and nutrients in the gastrointestinal tract support growth and survival of both the host and commensals. With this unique feature, the healthy gut is required to perform digestive and absorptive functions while it concurrently maintains a barrier against luminal microbes. Accumulating evidence indicates that the taxonomically complex intestinal microbes constitute a dynamic community (microbiota) that is now known to have a strong impact on human physiology.
Humans are born germ-free, yet, rapidly after birth, bacteria populates the digestive tract and establishes a microbial ecosystem in the gut[1]. The bacterial density gradually increases along the proximal to distal segments of the gastrointestinal tract and rises to an estimated 1011 to 1012 bacteria per gram of colonic content. The enteric bacterial population consists of up to 100 trillion (1014) cells, which is ten times the number of cells of the human body[2,3]. The gut microbiota is highly diverse and displays an individual-specific composition determined by host genotype and environmental factors. It had been estimated that more than 500 bacterial species inhabit the human gut, based mainly on culturing techniques[4,5]. With the advancement of metagenomic technology, our knowledge of the diversity of bacterial species has expanded rapidly beyond the list obtained from traditional microbiological methods, by which many gut bacteria are not culturable. Around 15 000 to 36 000 species of bacteria have now been identified in the human gastrointestinal tract using culture-independent rRNA sequence analysis[6,7]. A recent paper from the Metagenomics of the Human Intestinal Tract project revealed a total of 3.3 million non-redundant microbial genes in human fecal specimens[8]. Much to our surprise, this number is approximately 150 times larger than the protein-encoding gene set in human cells (approximately 20 000 genes according to data of Human Genome Project)[9,10]. Commonly identified enteric commensal bacteria include the phyla of Firmicutes (species such as Lactobacillus, Clostridium, Enterococcus), Bacteroidetes (species such as Bacteroides), Proteobacteria (species such as Escherichia coli) and Actinobacteria (species such as Bifidobacteria)[6,11].
Commensal bacteria were traditionally considered simply as co-living organisms residing in the gut lumen without much interaction with the host, and their quiet presence in the intestines did not draw interest from the gastroenterological field for several decades. Paradoxically, cardiologists and researchers in critical care medicine have paid much more attention to these bacteria in situations of gut barrier damage. In the event of their invasion to the systemic circulation and/or extraintestinal sterile organs, gut-derived bugs may pose a serious risk to the individual by inadvertently triggering septic shock, systemic inflammatory response syndrome and subsequent multiple organ failure[12,13]. Abnormal enteric bacterial translocation and gut-derived sepsis have been documented clinically and observed in animal models of intestinal ischemia/reperfusion[14-16], bowel obstruction[17,18] and hemorrhagic and traumatic shock[19,20].
The beneficial effects of our co-evolved microorganisms have begun to be seen recently[3,21]. It is now generally believed that commensal bacteria are involved in various physiological functions in the gut, whereas dysbiosis (a term that describes the condition of having microbial imbalances within the body) may cause pathology[6,22]. This review will discuss the classical view and the recent knowledge of host-microbe interaction in the gastrointestinal tract. Early studies investigated the maintenance of a passive intestinal barrier to confine the luminal bacteria and to fend off invasions of opportunistic microbes; current research is focused on the beneficial effects of commensal bacteria on the hosts as well as the influence from an active intestinal barrier on the microfloral population in order to maintain gut homeostasis and, in a broader aspect, to promote the health of the host.
INTESTINAL BARRIERS FOR LUMINAL CONFINEMENT OF COMMENSAL BACTERIA
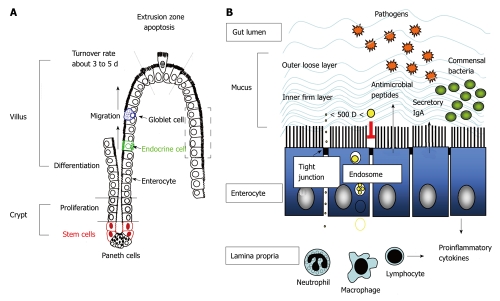
There is no doubt that tight control of the location, number and population of enteric bacteria by the hosts is prerequisite for health-promoting effects. Luminal confinement of commensal microflora is a main task of the gut mucosa. To prevent microbial dissemination or invasion of sterile extraintestinal viscera, physical barriers composed of epithelial cells and mucus layer, chemical barriers with antimicrobial peptides, and immune barriers including secretory IgA, act as front lines of defense. If these foremost barriers fail and bacteria translocation occurs, activation of immune cells in the lamina propria including phagocytes and lymphocytes are next in line to carry out antimicrobial actions (Figure 1).
Figure 1.
Intestinal crypt-villus axis and formation of intestinal barriers for luminal confinement of commensal bacteria. A: Stem cells in the crypt regions undergo proliferation and differentiation into columnar epithelial cells (enterocytes) with high expression of brush border enzymes and transporters, and concurrently migrate upward to the apex of the villi where cell apoptosis and shedding occurs at the so-called “extrusion zone”. The stem cells also differentiate into Paneth cells that migrate downward to the crypt bottom, as well as into mucin-secreting goblet cells and enteroendocrine cells that migrate upwards to the villous tips. During the differentiation and migration process, tight junctional proteins are formed at the cell-cell contact sites to seal off gaps between enterocytes; B: Enteric microbes are restricted in the gut lumen by physical barriers composed of epithelium and mucus, chemical barriers with antimicrobial peptides, and immune barriers such as secretory immunoglobulin A (IgA). The tight junctional complexes between plasma membranes of two cells exclude the influx of bacteria and molecules larger than 500 dalton through paracellular routes, whereas endosomal degradation limits transcellular transport of particles and proteins. If the epithelial barrier is breached and invasion of bacteria occurs, the underlying immune cells in the lamina propria such as phagocytes (macrophages and neutrophils) and lymphocytes are responsible for antimicrobial and inflammatory responses.
Epithelial barrier limits the space for bacterial growth
The luminal surface of the gastrointestinal tract from the stomach to the rectum is covered by a single layer of epithelial cells. These epithelial cells with their well-ordered brush borders constitute a large surface area that is multiplied both by the macroscopic features of valvulae conniventes and the microscopic structures of finger-like villi. The vast interior surface area of the gut lining allows for efficient nutrient uptake for the individual. On the other hand, this large surface area also has to tolerate noxious luminal contents and form a competent barrier and/or defense mechanism in face of a massive load of antigenic substances and microbes. It is worth noting that this amazing balancing act between uptake and exclusion is managed by intestinal epithelial cells with a dynamic turnover pace.
Crypt-villus axis and enterocytic turnover rates: The turnover rates of intestinal epithelial cells (enterocytes) are governed by the pace of crypt cell proliferation and villus/surface cell shedding. The newly proliferated stem cells in the crypt regions differentiate into epithelial cells with high expression of brush border enzymes and transporters, and concurrently migrate upward to the apex of the villi where cell apoptosis and detachment occurs at the so-called “extrusion zone” (Figure 1)[23]. These stem cells also differentiate into Paneth cells that migrate downward to the bottom of the crypt, as well as into goblet cells and enteroendocrine cells in the epithelial layer that migrate upwards to the villous tips. The cell migration process along the crypt-villus axis is dependent on dynamic turnover of focal cell-matrix adhesions. Although the order of apoptosis and sloughing of cells on villous tips is still in debate, accumulating evidence indicates that the apoptotic signaling cascade proceeds along with the purse-string action of cell extrusion[24,25].
During the differentiation and migration process, epithelial tight junctional proteins are formed at the cell-cell contact sites to seal off gaps between cells. The physical barrier constituted by these closely linked epithelial cells is the rate-limiting factor that determines intestinal permeability. Physiological epithelial apoptosis and extrusion at the villous tips does not compromise barrier function[26,27]. Abundant studies have indicated that tight junctional proteins are present at the base of basolateral membranes between two neighboring enterocytes flanking the extruding cells, and thus barrier functions are sustained at the villous tips[26,27]. Nevertheless, excessive epithelial cell death caused by pathogenic microbes[28-32], metabolic stress[15,16], and nonsteroidal anti-inflammatory drugs, acidic or enzymatic agents[33,34], may lead to villous surface denudation and gut leakiness if crypt proliferation and enterocytic migration were not sufficient to cover the wounded area. Conversely, high rates of cell proliferation and resistance to cell apoptosis are known to be two equally important determining factors during the early stages of colorectal carcinogenesis[35,36]. The balance between these two events, i.e. cell death and proliferation of epithelial cells, is now recognized as a single key determinant for gut homeostasis.
Paracellular epithelial permeability: The intestinal epithelial cells are joined at their apical side by tight junctions (TJs). The tight junctional complexes form the narrowest distance between plasma membranes of two cells, thus excluding the influx of bacteria through paracellular routes. The transmembranous junctional proteins, e.g., claudins, occludin or junction-associated molecule, are linked to intracellular zonula occludens (ZO) which are bridges to cytoskeletal actin and myosin filaments[37,38].
The organization of TJ proteins and perijunctional actinomyosins are regulated by a complex network of signaling pathways. Contraction of the actinomyosin filaments that open up paracellular junctions is mediated by the phosphorylation of myosin light chain (MLC) via activation of myosin light chain kinase (MLCK) or Rho-associated kinase (ROCK)[17,39]. In addition to the physical opening of TJs, ROCK also mediates the endocytosis of TJ proteins into vacuolar apical compartments[39]. Different isoforms of protein kinase C (PKC) are involved in the processes of TJ opening and assembly[40]. The atypical PKC zeta is the sole isoform found located at intercellular contact sites[41,42]. Recent evidence shows that PKC zeta directly interacts with and phosphorylates occludin, causing the redistribution of occludin away from intercellular junctions in cell culture monolayers[43]. A large body of evidence showed that abnormal passage of bacteria across the epithelial layer may occur via the paracellular routes in disease states. Increased epithelial MLC-dependent paracellular permeability was associated with enhanced bacterial translocation to extraintestinal organ routes in experimental models of colitis and bowel obstruction[17,18,44-46]. Increased paracellular permeability and tight junctional disruption were also documented in in vitro cultures of human intestinal epithelial Caco-2 cells challenged with Gram-negative bacterial lipopolysaccharide (LPS)[31].
Transcellular epithelial permeability: Transcellular transport of particles and proteins are limited by endosomal degradation within enterocytes. Dietary proteins are mostly digested by gastric and pancreatic proteases, as well as integral brush border enzymes, and converted to small peptides and amino acids, which are then absorbed by enterocytes via electrogenic or sodium-dependent transporters. Although a small amount of intact protein may be endocytosed into epithelial cells in physiological conditions, most of it is sorted into lysosomal compartments for degradation and therefore, transcytosis of whole proteins is prevented[47-49].
Most commensal bacteria are separated from the epithelial surface by the mucus layer and these bacteria do not internalize into epithelial cells. However, increased translocation of nonpathogenic bacteria via the transcellular routes has been documented in epithelial cells under inflammatory situations and metabolic stresses, such as low dose immunoreactive fibronectin-gamma (IFNγ)[50], tumor necrosis factor-alpha (TNFα) during glutamine deprivation[51], uncoupling of mitochondrial oxidative phosphorylation[52,53], low dose nitric oxide[54,55] and hypoxia[56]. Other studies[57-62] have documented the internalization of bacterial LPS and their binding to intracellular receptors in cell culture models and in mouse enterocytes. Recent reports also showed that commensal bacteria may be engulfed into intestinal epithelial cells in the presence of pathogenic invasive bacterial strains. Using a polarized human intestinal epithelial cell model system, it was demonstrated that Campylobacteri jejuni (a common enteric pathogen identified in humans and chickens) not only penetrates into epithelial cells itself but also promotes the internalization and translocation of non-invasive, nonpathogenic E. coli via a lipid raft-dependent mechanism[63].
Antigen sampling and uptake of bacterial particles by follicle-associated epithelium [mainly by microfold (M) cells] on Peyer’s patches (PP) is another route of transcellular transport for luminal substances. These PPs are specialized lymphoid follicles in the gut with a large number of dendritic cells in the dome region[64]. This particular form of luminal antigen transport across follicle-associated epithelium has been implicated in induction of oral tolerance and was reviewed previously[48,65]. Recent evidence shows that although most enteric bacteria resides in the mucus blanket, there are exceptions, i.e., segmented filamentous bacteria (SFB), a Clostridium-related species that anchors on the gut epithelial cells adjacent to M cells[66,67].
Chemical and immune barriers shape the microbial population
The epithelial cells linked by tight junctions not only physically separate the microbiota from the lamina propria, but also secrete proinflammatory cytokines and reactive oxygen species in response to pathogen invasion and metabolic stress, and serves as a warning system to the underlying immune cells to combat microbes[68-71]. The epithelial layer is now considered as an active participant in innate immunity. Other chemical and immune barriers to restrict and shape the enteric bacterial population include antimicrobial peptides, sIgA, phagocytes and lymphocytes.
Antimicrobial peptides: Antimicrobial peptides (AMPs) or host defense peptides are small cationic peptides that exhibit broad-spectrum antibiotic activity against Gram-positive and Gram-negative bacteria, fungi, yeasts and viruses[72]. Defensins (cryptdins) are stored in the granules of Paneth cells situated besides the proliferative crypt stem cells, and are secreted into the luminal space in response to bacteria and microbial molecules, e.g., oligonucleotides and LPS[73-75]. Triggers for the production of cathelicidin-related AMPs in epithelial cells and neutrophils include bacterial flagellin and LPS[76,77].
Accumulating data indicate a crucial role of AMPs in shaping the commensal bacterial population. A developmental switch of gut AMP expression during the neonatal period is correlated with the establishment of commensal microflora. Previous studies showed that production of mouse cathelin-related antimicrobial peptides (mCRAMP) can be observed in the first two weeks after birth and gradually disappears with the onset of stem cell proliferation and establishment of the crypt-villus axis. The synthesis of mCRAMP was found to play a role in the inhibition of growth of Listeria monocytogenes, which is a commensal bacteria populated in the mother’s vaginal canal but a potential pathogen in the neonatal gut[78]. In addition, Paneth cells and defensin production appear after 2 wk of birth, which accompanies the development of intestinal crypts[79,80].
Human Paneth cell defensins HD-5 and HD-6 are stored in their inactive form and are activated by trypsin after secretion[81], whereas mouse procryptidins (α-de-fensins) are activated by matrix metalloproteinase-7 (MMP-7)[82]. An elegant study using mice overexpressing human α-defensin HD-5 and others lacking functional α-defensins by genetic deficiency of MMP-7 showed that there is no change in the total number of commensal bacteria, but only alterations in the ratio of the two major bacterial phyla Firmicutes and Bacteroides[83]. Interestingly, overexpression of HD-5 inhibited the adherence of SFB to epithelial cells close to M cells on PP in mice[83]. The physiological significance of the attaching SFB has been discussed, including stimulation of sIgA production and regulation of T lymphocyte differentiation[83-85]. Much exploration is needed to understand the interactions between AMP synthesis and the shaping of the commensal bacterial population.
Secretory IgA: The presence of sIgA in the luminal space of the gastrointestinal tract has long been associated with the prevention of infection and dissemination by pathogen neutralization[86]. However, recent evidence shows that sIgA is also involved in homeostatic control of the commensal microbiota. Enteric commensal bacteria were found to be coated with highly specific anti-commensal sIgA[87]. The intestinal IgA production is profoundly affected by the colonization of commensal microflora, as evidenced by the low level of IgA in germ-free animals, which is corrected after inoculation with luminal bacteria[88,89]. Recent studies showed that luminal sIgA selectively adhered to M cells in the mouse and human intestinal PP via a novel IgA receptor and mediated translocation of bacteria and antigenic products to the underlying dendritic cells[90,91]. The luminal bacterial uptake by the sIgA into PPs induces naïve B cells to differentiate into IgA-committed plasma cells[92] and causes a decrease in proinflammatory cytokine expression that accompanies the neutralization of pathogenic bacteria[93]. These IgA-committed B cells in PPs and the mesenteric lymph nodes subsequently drain into the thoracic duct and bloodstream, and finally return home to the intestinal mucosa[94]. The sIgA produced by these lamima propria plasma cells is then transported across the epithelial cells via the polymeric immunoglobulin receptor into gut lumen[95]. The roundtrip, bidirectional transport of sIgA and the bacterial coating mediated by sIgA have been implicated in the mechanism of antigen neutralization that curtails luminal bacterial overgrowth[96].
Phagocytes and lymphocytes: Once the mucus and epithelial barrier are breached, phagocytes residing in and infiltrated into the lamina propria are next in line for mucosal defense. The phagocytic functions of macrophages and neutrophils are just one part of innate immunity, in which these cells also produce large amounts of reactive oxygen species (ROS, e.g., superoxide and hydrogen peroxide) via catalytic activities of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and myeloperoxidase. Aside from phagocytic sources, intestinal epithelial cells also contain isoforms of NADPH oxidase, e.g., NOX1, and generate superoxide upon stimulation with pro-inflammatory cytokines or with microbial molecules[69-71,97]. These oxidative free radicals are efficient in killing bacteria through lipid peroxidation, protein nitrosylation, and DNA strand breakage, which eventually leads to death of the microbial targets[98,99].
The adaptive arm of the gut immune system, termed gut-associated lymphoid tissues, include lymphocytes scattered in the lamina propria, intraepithelial lymphocytes and those aggregated into lymphoid nodules, such as PP and mesenteric lymph nodes. Depending on the cytokine production profile, the differentiated T helper lymphocytes are mainly subgrouped into Th1, Th2, Th3/Tr1 and Th17. The classical dichotomy of Th1/Th2 paradigm of CD4(+) T-cell subsets are associated with inflammation and allergy, respectively; whereas the Th3/Tr1 subgroups are involved in immunoregulatory and suppressive events. The identification of an additional subset, known as Th17 cells, has further illustrated the complexity and diversity of effector T cells with proinflammatory characteristics.
Studies using germ-free mice have shown that the frequency of Th17 cells in the lamina propria of the large intestine is significantly elevated in the absence of commensal bacteria[100], suggesting that enteric microbes are involved in the reduction of the numbers of this pro-inflammatory T lymphocyte subset. The differentiation of Th17 cells is promoted by interleukin 6 (IL-6) and transforming growth factor-beta, whereas IL-23 is required for the subsequent expansion of committed Th17 cells and production of IL-17[101]. An IL-25-IL-23-IL-17 axis was recently implicated in abnormal reactions towards the individual’s own commensal bacteria that cause autoimmune chronic inflammation in the gut[100]. Commensal-dependent expression of epithelial IL-25 restricted the expansion of Th17 cells by decreasing the expression of macrophage-derived IL-23[100], suggesting that commensal bacteria may promote immune cell hyporesponsiveness through epithelial signaling. Conversely, other reports have demonstrated that specific microbes, i.e., SFB, induce the differentiation of Th17 cells in the intestine of gnotobiotic mice[85,102]. Taken together, these findings indicated that eco-imbalance with particular strains of bacteria or dysbiosis may be a cause for inflammatory responses in the intestine.
COMMENSAL BACTERIA REGULATES INTESTINAL EPITHELIAL BARRIER FUNCTIONS
The traditional concept regarding commensal bacterial as a potential threat to the human body is now changed by evidence of the beneficial effects of gut microbiota in promoting epithelial barrier integrity (Figure 2). At this point, the various health-promoting effects of commensal bacteria have justified the use of the term “symbionts” for these microbes. These enteric bacteria are no longer regarded as an intruder of the human body that requires annihilation and expulsion, but their presence is recognized as part of the human physiology. This consensus has been long-awaited, since the theory that “certain types of bacteria especially those with lactic acid-producing ability in the digestive tract could prolong life” was established by Dr. Eli Metchnikoff, the 1908 Nobel Prize Laureate. Of course, there was no knowledge of the existence of commensal bacteria at the turn of the 1920s, let alone the understanding that Lactobacillus spp. was a constituent of the gut microbiota system. This early theory did lead to the much later recognition by the World Health Organization that particular types of microorganisms which, when administered in adequate amounts, confer a health benefit on the host and the coining of the term “probiotics”.
Figure 2.
Dynamic interactions between host intestine and commensal microbes to achieve balance for maintenance of gut homeostasis. The survival and growth of enteric microbes relies on energy supply from food nutrients, and are dependent on the space and anchor provided by the host intestines. Conversely, luminal microbes are capable of fermenting non-digestible dietary substances, generating short chain fatty acids and essential vitamins, and providing caloric sources for the host. These symbiotic bacteria also play important roles in pathogen competition, regulation of the turnover rate of enterocytes and fortification of epithelial barrier functions, as well as shaping of the mucosal immunity. From the host's point of view, tight physical, chemical and immune barriers of intestines are pivotal in the keeping of the number and location of the microfloral population in check in order to maintain the health-promoting effects, and to prevent bacterial dissemination and the triggering of local and systemic inflammatory responses. The balance of Yin-Yang between the host intestine and commensal microbes is central to maintaining homeostasis.
Enteric microbes are responsible for numerous protective and metabolic functions, and are involved in various structure- and immune-enhancing effects of the gut (Table 1). The presence of commensal bacteria protects against enteric pathogen colonization through competition for nutrients and receptors[103,104], and by synthesis or induction of anti-microbial factors[105,106]. The metabolic role of enteric bacteria involves degradation of non-digestible dietary substances, production of essential vitamins, and generation of short chain fatty acids (SCFAs)[107,108]. In addition, enteric microbes play an active role in the shaping of mucosal immunity, an aspect that has been discussed in detail in other review papers[3,21]. Other important functions of commensal microbes have just begun to emerge, suggesting that luminal bacteria signal the interfacing epithelial layer and control the turnover rate of enterocytes[3,109], and fortify the epithelial regenerative and barrier functions[109-114].
Table 1.
Functions of commensal bacteria in the gut
| Protective functions Pathogen displacement Competition for nutrients Competition for receptors Production of anti-microbial factors |
| Metabolic functions Fermentation of non-digestible dietary substances Generation of short chain fatty acids Salvage of energy source Synthesis of essential vitamins (vitamin K and B12, niacin, biotin and folate) |
| Structural functions Regulation of epithelial cell turnover Promotion of epithelial cell differentiation Fortification of epithelial barrier Stabilization of tight junctions |
| Immune functions Induction of secretory IgA Induction of oral tolerance Shaping of immune microenvironment |
Microbial effects in intestinal epithelial cell turnover rates
When evaluating the effect of commensal microbes on intestinal epithelial cell turnover rates and crypt-villus axis, it is important to consider the balance between cell proliferation and cell death. Increased epithelial cell apoptosis without sufficient proliferation or restitution results in barrier damage, whereas decreased cell death with hyperproliferation runs the risk of tumor formation. A number of reports comparing germ-free, gnotobiotic and conventionally-raised animals have indicated that luminal bacteria signals the epithelial layer to control cell apoptosis, proliferation and differentiation[109-113]. Germ-free piglets display aberrant intestinal morphology with longer villi and shorter crypts than their conventional counterparts. Decreased epithelial apoptosis and crypt cell proliferation were observed in the intestine of germ-free animals compared to those raised conventionally[110,111]. Oral inoculation of commensal bacteria obtained from feces or administration of non-pathogenic E.coli to these gnotobiotic pigs stimulates epithelial apoptosis, increases crypt depth for compensatory proliferation, and induces brush border enzyme activities compared to those raised in a germ-free environment[109-112]. Previous studies also showed that commensals and non-pathogenic E.coli LPS mediate pro-apoptotic effects on epithelial cells in human colon explants depleted of IL-10, as well as human intestinal epithelial cell lines[31,115,116].
Further evidence of a role for commensal bacteria in regulation of epithelial cell turnover and restitution was seen in colitis models with mucosal deformation by oral administration of dextran sodium sulfate (DSS, a sulfated polysaccharide that is directly toxic to colonic epithelial cells[117]). Animals with commensal bacterial depletion are more susceptible to oral DSS-induced mucosal injury, with more extensive denudation of the surface epithelium resulting in ulceration or erosion of mucosa compared to conventionalized counterparts[114]. Impaired epithelial proliferation and regenerative ability were seen in the intestines of germ-free mice upon DSS-induced injury[113]. Moreover, worsened histopathological score, decreased enterocyte proliferation and delayed wound healing were documented in DSS-induced colitis in mice deficient of proinflammatory signal pathways in response to ligands of bacterial LPS or lipoteichoic acid (LTA)[113,114]. Oral ingestion of bacterial products LPS or LTA prior to DSS challenge conferred protection in wild type mice with colons depleted of commensal microflora[114], suggesting that luminally administered bacterial products are important for protection against DSS-induced epithelial injury. Contradictory data were seen in animals with colitis-prone genetic background, showing that IL-10-/- mice fail to develop spontaneous colitis and intestinal histopathology if reared in germ-free conditions, suggesting that the presence of commensal bacteria may trigger chronic intestinal inflammation in the background of IL-10 deficiency[118]. The discrepancy further emphasizes the critical role of commensal bacteria in the shift between immune suppression and inflammation in intestines, and they may stimulate differential responses in enterocytes and immune cells.
A recent study has indicated that commensal bacteria promote epithelial restitution and wound closure through mechanisms that involve ROS[119]. Epithelial restitution is dependent on cell migration, a process that requires phosphorylation of focal adhesion kinase (FAK) for the dynamic turnover of focal cell-matrix adhesions[120]. It was demonstrated that commensal bacteria stimulate the production of epithelial-derived oxidative free radicals that induce oxidation and inactivation of FAK phosphatases, which in turn results in increased phosphorylation of FAK[119]. Another report has shown that hydrogen peroxide promotes intestinal epithelial cell migration via induction of FAK phosphorylation by a phosphatidylinositol 3 kinase-dependent mechanism[121]. In addition, NOX1 (a superoxide-generating oxidase which is highly expressed on colonic epithelial cells) plays a crucial role in regulation of epithelial proliferation and differentiation by modulating Wnt/Notch signaling[122]. It seems plausible that bacterial contact on epithelial surface or microbial influx to the mucosa due to barrier dysfunction may serve as triggers for ROS production from enterocytes and phagocytes to promote cell renewal and wound healing.
The enteric microbiota thrives in a largely anaerobic luminal environment and generates a spectrum of SCFAs, including butyrate, succinate and propionate, as well as other terminal products such as lactate[107]. SCFAs are important energy sources for the colonic epithelium and for the host, and also regulate colonic epithelial cell growth and differentiation[108,123]. Butyrate was shown to increase alkaline phosphatase activity, a marker of colonocyte differentiation, in highly proliferative epithelial cells, correlated with cell cycle arrest[124,125]. Besides its role in promoting cell differentiation, butyrate plays a role in prevention of colonic cancer by terminating cell cycle progression and promoting apoptosis of transformed colonocytes through mechanisms associated with inhibition of histone deacetylase activity and induction of p21WAF1/Cip1 proteins[124,126,127].
Microbial effects in fortification of epithelial tight junctional structures
Strong evidence that commensal bacteria regulate epithelial permeability came from studies with probiotics in various disease models. Probiotics are defined as non-pathogenic microorganisms that confer health benefits for the host[128] and several strains of commensal bacteria have been included in the category so far. Pretreatment with multispecies or single strain of probiotics (e.g., VSL3, nonpathogenic Escherichia coli Nissle 1917, or Lactobacillus rhamnosus) inhibited gut leakiness and prevented the colonic cell apoptosis in colitis mice models induced by DSS challenge[129-131] and IL-10 gene deficiency[132]. The maintenance of epithelial barrier was associated with restoration of tight junctional structures and increased expression of ZO-1 and MLCK[130,131]. Oral administration of probiotics containing Lactobacillus sp., Enterococcus faecalis (previously Streptococcus faecalis) and Bifidobacterium brevis prevented the increase of transepithelial macromolecular flux in rat intestines caused by acute or chronic psychological stress[133,134]. Studies in vitro have shown that probiotics, such as E.coli strain Nissle 1917 and Lactobacillus plantarum, reduced the epithelial hyperpermeability caused by enteropathogenic Escherichia Coli in human intestinal epithelial cells by silencing PKCzeta and reorganizing ZO-2[135,136]. Beneficial effects of probiotics in maintaining colonic barrier function and reducing bacterial influx and plasma endotoxin levels were also seen in clinical studies and endotoxemic rat models[137,138]. Several strains of lactobacillus stabilize tight junctional structures after free radical-induced or cyclooxygenase-dependent epithelial barrier dysfunction[139-141]. It is noteworthy that administration of these probiotics does not lead to changes in intestinal epithelial permeability in healthy control animals[133], emphasizing that the presence of probiotics is critical for the prevention of intestinal barrier dysfunction only upon injury. In addition, bacterial fermentation products of SCFAs also directly increase the transepithelial resistance of intestinal epithelial monolayers in vitro by accelerating the assembly of tight junctions that is regulated by AMP-activated protein kinase and PI3K signaling pathways[142,143]. A lactobacillus-derived molecule, polyphosphate, was recently identified to suppress oxidant-induced intestinal permeability in mouse small intestine[144]. The findings of specific molecules secreted by probiotics and/or commensal bacteria may benefit the development of natural product supplementations to enhance the intestinal barrier functions.
ABERRANT RECOGNITION OF MICROBIAL PRODUCTS RESULTS IN INTESTINAL PATHOLOGY
Chronic inflammation
Intestinal epithelial cells are constantly bombarded with pathogenic, cytotoxic, metabolic stresses which trigger apoptotic and necrotic cell death, leading to gut barrier damage, microbial influx and inflammatory responses[15,16,31,32,145,146]. Evidence supporting the notion that gut permeability defects precedes the onset of mucosal inflammation was found in spontaneous enterocolitis models of IL-10-/- and SAMP1/YitC mice[147-149]. Moreover, mucosal inflammation was seen in areas adjacent to epithelium with TJ disruption (loss of endogenous E-cadherin) due to the expression of a dominant negative N-cadherin mutant lacking an extracellular domain in mice[150]. Recent studies using epithelial-specific knockout models provide direct evidence of the cause-and-effect relationship between cell death-dependent epithelial barrier defects and intestinal inflammation. Mice with conditional deletion of caspase-8 or Fas-Associated protein with Death Domain on intestinal epithelial cells spontaneously developed epithelial cell necrosis and inflammatory lesions in the ileum and colon[145,146]. On the other hand, a number of studies have demonstrated that pro-inflammatory cytokines (e.g., IFNγ and TNFα) and phagocytic mediators (e.g., free radicals and proteases) cause tight junctional breakdown and intestinal permeability rise[139,151,152], and thus argue in favor of inflammation as the cause for epithelial barrier disruption. Regardless of the starting point, a feed-forward vicious cycle between barrier dysfunction and inflammatory reaction is crucial for the perpetuation and aggravation of chronic inflammation in intestines.
Several lines of evidence suggest a critical role of dysbiosis in the pathogenesis of inflammatory bowel disease (IBD). In IBD patients, not only the quantity of commensal bacteria in the intestine is reduced (about ten-fold lower than control subjects), but also the diversity of the microbiota is altered[6,153,154]. Reduction of major classes of commensals, Firmicutes and Bacteroidetes, and increase of mucosal adherent bacteria are documented in patients[6,153-155]. Experimental models such as IL-2- or IL-10-deficient mice that spontaneously develop colitis do not develop disease when raised in a germ-free environment[156,157]. In addition, monoassociation with Bacteroides vulgatus or E. coli is sufficient to induce colitis in human leukocyte antigen-B27 transgenic rats[158]. Recent findings that transmission of colitogenic commensal bacteria is able to trigger colitis in the genetically intact recipient mice further strengthen this view. Mice with genetic deficiency in RAG-1 and T-bet displayed dysbiosis and developed spontaneous colonic inflammation that resembles human ulcerative colitis[159]. Interestingly, T-bet-competent wild type pups develop colitis after being crossfostered to female mutant mice, suggesting a communicable nature of this form of colitis by the gut microbiota[160].
Aberrant bacterial signaling by microbe-associated molecular pattern receptors, e.g., nucleotide-binding oligomerisation domain 2 (NOD2) and toll-like receptors (TLRs), on mucosal cells is incriminated in the development of chronic intestinal inflammation. Mutations in the gene encoding NOD2 were identified in patients with Crohn’s disease[161,162]. NOD2 has been known as a cytosolic innate receptor able to sense peptidoglycan from Gram-positive and -negative bacteria inside enterocytes to trigger RIP2- and nuclear factor kappa B (NF-κB)-mediated pro-inflammatory responses and to induce antimicrobial defensin synthesis[163,164]. Recent studies demonstrated that NOD2-deficient mice display altered microbiota composition, and elevated bacterial load in the feces and terminal ileum compared to their wild-type counterparts[165,166], supporting that NOD2 dysfunctions and its subsequent dysbiosis may result in the breakdown of gut homeostasis and predispose to chronic inflammation.
Accumulating evidence points out that changes in the expression levels of receptors to Gram-negative bacterial LPS in the intestinal mucosa may be involved in the pathogenesis of IBD and colorectal cancer[167-170]. The multi-unit receptor for LPS (CD14/TLR4/MD-2 complex) was originally detected on blood monocytes in the context of the pathogenesis of septic shock[171,172]. It becomes clear now that intestinal epithelial cells and resident macrophages bear a distinct expression pattern of receptors unlike circulating monocytes and peritoneal macrophages. Recent data show that in purified enterocytes isolated from normal human biopsy samples, CD14 protein is constitutively expressed, whereas TLR4 is barely detectable[167-170,173]. Moreover, human intestinal macrophages isolated from normal jejunal specimens do not express innate immune receptors, such as receptors for LPS (CD14), Fcα (CD89), Fcγ (CD64, CD32, CD16), CR3 (CD11b/Cd18) and CR4 (CD11a/CD18)[174]. Low TLR4 levels have also been reported in the lamina propria macrophages in comparison to blood monocytes in normal human subjects[175]. It is noteworthy that these intestinal resident macrophages show downregulated LPS-induced production of proinflammatory cytokines, but retain potent phagocytic and bactericidal activities in physiological conditions[174,176]. The distinct characteristics of LPS receptors on enterocytes and mucosal macrophages may reflect its tolerance to the presence of commensal bacteria, which is crucial for limiting unwanted inflammation and for maintaining gut homeostasis.
Polymorphism of CD14 and TLR4 genes was identified in subsets of IBD patients[177-183], suggesting that abnormal bacterial LPS signaling may play a role in the pathogenesis. Since both intestinal epithelial cells and lamina propria macrophages express CD14 and TLR4 proteins at variable levels, their changes related to chronic colitis will be discussed in a cell type-specific fashion. Upregulated epithelial TLR4 expression was observed in IBD patients compared to normal subjects[167,168]. A similar increase in TLR4 was found in crypt epithelial cells in DSS-induced mouse colitis models[184,185]. Moreover, CD14 mRNA and protein levels in the intestinal epithelial cells of DSS-induced and spontaneous colitic mice were also higher than those in healthy animals[184,186]. These finding suggest that at the interface with commensal microbes, altered expression of LPS receptor components (CD14 and TLR4) on enterocytes may trigger epithelial-derived proinflammatory signals.
A wide array of differential expression patterns and subcellular location of LPS receptors was seen in different intestinal epithelial cell lines that correlated with their responsiveness to LPS for proinflammatory cytokine synthesis. For example, Caco-2 cells that express cell surface CD14 but have low levels of TLR4 mRNA and proteins, similar to normal human enterocytes, neither activate their NF-κB pathway nor produce IL-8 after LPS challenge[57,68,116,187], showing one of the possible mechanisms for endotoxin tolerance by enterocytes. Transfection of TLR4/MD2 to Caco-2 cells restores the responsiveness to LPS and synergistic activation of NF-κB and IL-8 reporter genes[187]. Moreover, HT29 cells that express membrane-bound CD14 and cytoplasmic TLR4 are responsive to IFNγ for upregulation of intracellular TLR4 levels and the cells are sensitized for LPS-induced IL-8 production[57,116]. Among human intestinal epithelial cell lines that express constitutively high cell surface levels of TLR4, such as SW480 and T84 cells, exposure to LPS stimulates the activation of NF-κB and AP-1 signaling and the production of TNFα and IL-8[57,8,187]. It is clear from in vitro data that induction or heightened expression of individual LPS receptor components on intestinal epithelial cells may overrule their hyporesponsiveness to luminal bacterial LPS as a trigger for proinflammatory signals. Augmented expression of LPS receptors was also noted in lamina propria macrophages in inflamed tissues of IBD patients[168,175,188,189]. Heightened TLR4 expression was localized to intestinal macrophages in biopsy or surgical specimens obtained from both ulcerative colitis and Crohn’s disease patients[175]. In Crohn’s disease patients, recent studies found increased subsets of CD14+ macrophages in comparison to the typical resident macrophages (CD14-CD33+) in the intestinal lamina propria[168,188,189]. The CD14+ population of macrophages exhibit potent antigen-presenting ability to evoke differentiation of Th17 cells[188] and produce large amounts of proinflammatory cytokines (e.g., TNFα and IL-23) that stimulate lamina propria mononuclear cells to synthesize IFNγ in a positive feedback loop[189]. These abnormal CD14+ macrophages may decrease the threshold to mount an inflammatory response upon exposure to low concentrations of LPS and to commensal bacteria, and may amplify the production of proinflammatory cytokines from different cell types through the positive feedback loop of IL-23/IFNγ[189,190].
Other reports indicated that a decrease in IL-10-producing intestinal macrophage subsets (CD11b+F4/80+CD11c-) also plays a role in the development of chronic intestinal inflammation[191,192]. Studies in IL-10-deficient colitis mouse models have demonstrated that bone marrow-derived macrophages from IL-10-/-mice produce large amounts of IL-12 and IL-23 upon stimulation with heat-killed bacterial antigens, whereas those from wild type mice produce high levels of IL-10 but neither IL-12 nor IL-23[190], which is correlated to the phenomenon where IL-10-/- mice fail to develop spontaneous colitis and intestinal histopathology if reared in germ-free conditions[118]. These findings suggest that commensal microbes or bacterial LPS may stimulate different subsets of macrophages, leading to varied patterns of macrophage-derived cytokine production (IL-10 vs IL-12/IL-23) that determine the progress to immune hyporesponsiveness or development of colitis[118,190]. It remains unknown whether the low baseline levels of Fcα and Fcγ on normal intestinal resident macrophages are also upregulated in IBD patients, which may increase opsonization and phagocytosis for more efficient antigen presenting capability to stimulate long-term immune memory and chronic reactions.
Dysregulation of enterocytic apoptosis, proliferation and tumorigenesis
The abnormal TLR4 overexpression on enterocytes and intestinal macrophages in IBD patients suggests that bacterial LPS stimulation may initiate mucosal-derived proinflammatory signals in the pathogenesis of chronic colitis. Based on this theory, a number of laboratories investigated the possibility that targeted deficiency of TLR4 signaling might decrease gut inflammation. Unexpectedly, mice with spontaneous mutation or targeted knock-out of TLR4 and MyD88 displayed poorer colitis scores and lower survival rates in DSS models[114,193,194]. Besides the heightened mucosal inflammatory responses, the lack of TLR4 signaling also resulted in other abnormalities, such as elevated epithelial cell apoptosis, decreased crypt cell proliferation, and impaired epithelial restitution accompanied with more severe mucosal ulceration in the DSS-induced colitis model[114,193,194]. The findings in these TLR4-/- and MyD88-/- mice were similar to those with commensal bacteria depletion in DSS-induced colonic injury, whereby more extensive denudation of the surface epithelium results in ulceration or erosion of mucosa accompanied by pronounced compensatory crypt proliferation[114]. These novel observations point out that presence of commensal bacteria and LPS-mediated TLR4 signaling may also be involved in epithelial cell survival that is critical in maintaining epithelial barrier integrity in physiological conditions and recovery to gut homeostasis in diseased states.
Many studies have shown that a lack of NF-κB sig-naling leads to increased epithelial apoptosis and impaired epithelial restitution after DSS challenge in colitis development[114,193-196]. Mice with epithelial-specific deficiency of IKKγ/NEMO develop spontaneous chronic intestinal inflammation associated with increased epithelial apoptosis and bacterial translocation[195]. Targeted ablation of IKKβ in intestinal epithelial cells also resulted in severe cell apoptosis upon radiation[197] or ischemic challenge[198], further supporting a universal role of IKKβ for cell survival against various types of stresses. Another study also showed that enterocyte-specific knockout of Raf-1 leads to NF-κB inactivation that is responsible for increased epithelial apoptosis and impaired epithelial proliferation and regeneration after oral DSS challenge[194]. Taken together, the aforementioned studies indicated that epithelial-derived TLR4/NF-κB pathways are involved in anti-apoptotic events.
From a physiological point of view, LPS signaling in the normally tolerant gut epithelial cells may serve as a warning system to the underlying immune cells while trying to promote epithelial restitution and maintain epithelial barrier functions via multiple pathways for proinflammatory, anti-apoptotic and proliferative effects. Short-term epithelial TLR4/NF-κB signaling is crucial for preventing pathogenic epithelial cell death and epithelial barrier disruption, which may help limit the exposure of the immune cells to bacterial antigens and toxins that could cause full-blown reactions. On the other hand, a chronic epithelial-derived LPS signaling may shift the normal cell cycle into tumorigenic phenotypes in the long run.
A strong link between inflammation and cancer formation was suggested by the higher incidence of gastric and colorectal cancer in patients with early onset of IBD[199,200]. Accumulating evidence indicates that TLR4 expression in intestinal epithelial cells is upregulated in patients with colorectal cancer[169,170], suggesting that altered expression pattern and malformed signals of epithelial LPS receptor components may also play crucial roles in tumorigenesis. Aberrant reactions to bacterial LPS by CD14/TLR4 may induce an imbalance of apoptosis and proliferation, resulting in cancer formation. Recent data showed that TLR4-/- and MyD88-/- mice failed to develop colitis-associated and carcinogen-induced colorectal tumors[201-204]. TLR4 may be responsible for upregulated production of cyclooxygenases and activation of epidermal growth factor receptors which may contribute to cancer formation[193,201]. A recent study pointed out that MyD88-dependent signaling controls the expression of several key modifier genes of intestinal tumorigenesis and has a critical role in both spontaneous and carcinogen-induced tumor development[202].
Mice with epithelial-specific IKKβ deficiency had a lower incidence of tumor formation, partly due to increased levels of epithelial apoptosis, compared to wild type animals after injection with azoxymethane (AOM) followed by treatment with DSS[205]. It is noteworthy that deletion of IKKβ in myeloid cells led to smaller tumor size, but no change of tumor incidence compared to wild type mice after AOM-DSS challenge[205]. These findings suggest that IKKβ in different cell types contributes to tumorigenesis via variable cellular functions, of which epithelial-specific IKKβ promotes tumor formation by conferring resistance to cell apoptotic pathways, whereas IKKβ signals in myeloid cells are involved in boosting epithelial cell cycle progression and cell division[205]. Therefore, it is important to identify the different types of mucosal cells (enterocytes or macrophages) responding to LPS when explaining the pathogenesis of intestinal inflammation and colorectal cancer formation.
In summary, LPS/TLR4-mediated signals which are normally downregulated in the gut epithelium are now linked with various pathological phenomena and disease states such as chronic inflammation, anti-apoptosis, hyperproliferation and tumorigenesis in the gastrointestinal tract.
CONCLUSION
With such a variety of species and the large numbers of commensal bacteria which undergoes wax and wane processes throughout the host’s life, homeostasis of the gut is maintained by dynamic cross-talks between luminal microbes and intestinal epithelium. This delicate balance is complicated by the need to maintain both oral tolerance and mucosal defense. It remains to be resolved whether the expression of pattern recognition receptors, such as NOD2, CD14 and TLR4, on enterocytes along the crypt-villus axis is differentially regulated in order to respond to microbes for proliferative, differentiative and apoptotic signals at different stages. Aberrant recognition and abnormal signaling caused by luminal bacteria may result in epithelial barrier dysfunction and/or carcinogenesis. The understanding of the interaction between host epithelium and commensal bacteria will provide us with novel information for the development of prophylactic and therapeutic interventions for patients with chronic inflammation and colorectal cancer.
Footnotes
Supported by National Science Council, No. NSC99-2628-B-002-008-MY3 and NSC100-2325-B-002-035
Peer reviewers: Dr. Stelios F Assimakopoulos, Department of Internal Medicine, University Hospital of Patras, Patras 26504, Greece; Dr. Sya N Ukena, Hannover Medical School, Hannover 30625, Germany; Maria Teresa Martin, PhD, Professor, Universitat Autonoma de Barcelona, Barcelona 08193, Spain
S- Editor L- Editor E- Editor
References
- 1.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andoh A, Benno Y, Kanauchi O, Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- 5.Manson JM, Rauch M, Gilmore MS. The commensal microbiology of the gastrointestinal tract. Adv Exp Med Biol. 2008;635:15–28. doi: 10.1007/978-0-387-09550-9_2. [DOI] [PubMed] [Google Scholar]
- 6.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mount DW, Pandey R. Using bioinformatics and genome analysis for new therapeutic interventions. Mol Cancer Ther. 2005;4:1636–1643. doi: 10.1158/1535-7163.MCT-05-0150. [DOI] [PubMed] [Google Scholar]
- 10.Southan C. Has the yo-yo stopped? An assessment of human protein-coding gene number. Proteomics. 2004;4:1712–1726. doi: 10.1002/pmic.200300700. [DOI] [PubMed] [Google Scholar]
- 11.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 12.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- 13.Leaphart CL, Tepas JJ. The gut is a motor of organ system dysfunction. Surgery. 2007;141:563–569. doi: 10.1016/j.surg.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Yu LCH. Protective Mechanism against Gut Barrier Dysfunction in Mesenteric Ischemia/Reperfusion. Adapt Med. 2010;2:11–22. [Google Scholar]
- 15.Hsiao JK, Huang CY, Lu YZ, Yang CY, Yu LC. Magnetic resonance imaging detects intestinal barrier dysfunction in a rat model of acute mesenteric ischemia/reperfusion injury. Invest Radiol. 2009;44:329–335. doi: 10.1097/RLI.0b013e3181a16762. [DOI] [PubMed] [Google Scholar]
- 16.Huang CY, Hsiao JK, Lu YZ, Lee TC, Yu LC. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab Invest. 2011;91:294–309. doi: 10.1038/labinvest.2010.177. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Lu YZ, Wu LL, Yu LC. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol. 2010;10:39. doi: 10.1186/1471-230X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu LL, Chiu HD, Peng WH, Lin BR, Lu KS, Lu YZ, Yu LC. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med. 2011;39:2087–2098. doi: 10.1097/CCM.0b013e31821cb40e. [DOI] [PubMed] [Google Scholar]
- 19.Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–169. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 20.Luyer MD, Buurman WA, Hadfoune M, Speelmans G, Knol J, Jacobs JA, Dejong CH, Vriesema AJ, Greve JW. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686–3692. doi: 10.1128/IAI.73.6.3686-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly D, Conway S. Bacterial modulation of mucosal innate immunity. Mol Immunol. 2005;42:895–901. doi: 10.1016/j.molimm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 24.Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 26.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 27.Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell F, McKenna M, Rose T, Montrose MH. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Moss SF, Sordillo EM, Abdalla AM, Makarov V, Hanzely Z, Perez-Perez GI, Blaser MJ, Holt PR. Increased gastric epithelial cell apoptosis associated with colonization with cagA + Helicobacter pylori strains. Cancer Res. 2001;61:1406–1411. [PubMed] [Google Scholar]
- 29.Paesold G, Guiney DG, Eckmann L, Kagnoff MF. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell Microbiol. 2002;4:771–781. doi: 10.1046/j.1462-5822.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 30.Flynn AN, Buret AG. Tight junctional disruption and apoptosis in an in vitro model of Citrobacter rodentium infection. Microb Pathog. 2008;45:98–104. doi: 10.1016/j.micpath.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Yu LC, Flynn AN, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 2005;19:1822–1835. doi: 10.1096/fj.05-4226com. [DOI] [PubMed] [Google Scholar]
- 32.Yu LC, Huang CY, Kuo WT, Sayer H, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects human intestinal epithelial cells against Giardia duodenalis-induced apoptosis. Int J Parasitol. 2008;38:923–934. doi: 10.1016/j.ijpara.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omatsu T, Naito Y, Handa O, Hayashi N, Mizushima K, Qin Y, Hirata I, Adachi S, Okayama T, Kishimoto E, et al. Involvement of reactive oxygen species in indomethacin-induced apoptosis of small intestinal epithelial cells. J Gastroenterol. 2009;44 Suppl 19:30–34. doi: 10.1007/s00535-008-2293-3. [DOI] [PubMed] [Google Scholar]
- 34.Redlak MJ, Power JJ, Miller TA. Prevention of deoxycholate-induced gastric apoptosis by aspirin: roles of NF-kappaB and PKC signaling. J Surg Res. 2008;145:66–73. doi: 10.1016/j.jss.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Renehan AG, O'Dwyer ST, Haboubi NJ, Potten CS. Early cellular events in colorectal carcinogenesis. Colorectal Dis. 2002;4:76–89. doi: 10.1046/j.1463-1318.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 36.Oumouna-Benachour K, Oumouna M, Zerfaoui M, Hans C, Fallon K, Boulares AH. Intrinsic resistance to apoptosis of colon epithelial cells is a potential determining factor in the susceptibility of the A/J mouse strain to dimethylhydrazine-induced colon tumorigenesis. Mol Carcinog. 2007;46:993–1002. doi: 10.1002/mc.20351. [DOI] [PubMed] [Google Scholar]
- 37.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Dodane V, Kachar B. Identification of isoforms of G proteins and PKC that colocalize with tight junctions. J Membr Biol. 1996;149:199–209. doi: 10.1007/s002329900020. [DOI] [PubMed] [Google Scholar]
- 42.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriez R, Salvador-Cartier C, Theodorou V, Fioramonti J, Eutamene H, Bueno L. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071–1079. doi: 10.1016/S0002-9440(10)61196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- 46.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita M, Reinhart F, Neutra M. Convergence of apical and basolateral endocytic pathways at apical late endosomes in absorptive cells of suckling rat ileum in vivo. J Cell Sci. 1990;97(Pt 2):385–394. doi: 10.1242/jcs.97.2.385. [DOI] [PubMed] [Google Scholar]
- 48.Yu LCH. The epithelial gatekeeper against food allergy. Pediatr Neonatol. 2009;50:247–254. doi: 10.1016/S1875-9572(09)60072-3. [DOI] [PubMed] [Google Scholar]
- 49.Yu LC. Intestinal epithelial barrier dysfunction in food hypersensitivity. J Allergy (Cairo) 2012;2012:596081. doi: 10.1155/2012/596081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 51.Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL. Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut. 2003;52:224–230. doi: 10.1136/gut.52.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nazli A, Yang PC, Jury J, Howe K, Watson JL, Söderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947–957. doi: 10.1016/S0002-9440(10)63182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16:1138–1148. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
- 54.Inaba T, Alexander JW, Ogle JD, Ogle CK. Nitric oxide promotes the internalization and passage of viable bacteria through cultured Caco-2 intestinal epithelial cells. Shock. 1999;11:276–282. doi: 10.1097/00024382-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci USA. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells CL, VandeWesterlo EM, Jechorek RP, Erlandsen SL. Effect of hypoxia on enterocyte endocytosis of enteric bacteria. Crit Care Med. 1996;24:985–991. doi: 10.1097/00003246-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol. 2002;160:165–173. doi: 10.1016/S0002-9440(10)64360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drewe J, Beglinger C, Fricker G. Effect of ischemia on intestinal permeability of lipopolysaccharides. Eur J Clin Invest. 2001;31:138–144. doi: 10.1046/j.1365-2362.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira RC, Forsyth LE, Richman PI, Wells C, Spencer J, MacDonald TT. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology. 1990;98:1255–1263. doi: 10.1016/0016-5085(90)90342-x. [DOI] [PubMed] [Google Scholar]
- 61.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomita M, Ohkubo R, Hayashi M. Lipopolysaccharide transport system across colonic epithelial cells in normal and infective rat. Drug Metab Pharmacokinet. 2004;19:33–40. doi: 10.2133/dmpk.19.33. [DOI] [PubMed] [Google Scholar]
- 63.Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1:2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keita AV, Gullberg E, Ericson AC, Salim SY, Wallon C, Kald A, Artursson P, Söderholm JD. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab Invest. 2006;86:504–516. doi: 10.1038/labinvest.3700397. [DOI] [PubMed] [Google Scholar]
- 65.van Wijk F, Knippels L. Initiating mechanisms of food allergy: Oral tolerance versus allergic sensitization. Biomed Pharmacother. 2007;61:8–20. doi: 10.1016/j.biopha.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Rozee KR, Cooper D, Lam K, Costerton JW. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982;43:1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanford SE. Light and electron microscopic observations of a segmented filamentous bacterium attached to the mucosa of the terminal ileum of pigs. J Vet Diagn Invest. 1991;3:328–333. doi: 10.1177/104063879100300410. [DOI] [PubMed] [Google Scholar]
- 68.Eckmann L, Jung HC, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 69.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C433–C443. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- 70.Kuwano Y, Tominaga K, Kawahara T, Sasaki H, Takeo K, Nishida K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K. Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med. 2008;45:1642–1652. doi: 10.1016/j.freeradbiomed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 71.Kawahara T, Kuwano Y, Teshima-Kondo S, Takeya R, Sumimoto H, Kishi K, Tsunawaki S, Hirayama T, Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J Immunol. 2004;172:3051–3058. doi: 10.4049/jimmunol.172.5.3051. [DOI] [PubMed] [Google Scholar]
- 72.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 73.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanabe H, Ayabe T, Bainbridge B, Guina T, Ernst RK, Darveau RP, Miller SI, Ouellette AJ. Mouse paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun. 2005;73:2312–2320. doi: 10.1128/IAI.73.4.2312-2320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Ménard S, Balsari A. Degranulation of paneth cells via toll-like receptor 9. Am J Pathol. 2004;165:373–381. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol. 2010;185:1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ouhara K, Komatsuzawa H, Shiba H, Uchida Y, Kawai T, Sayama K, Hashimoto K, Taubman MA, Kurihara H, Sugai M. Actinobacillus actinomycetemcomitans outer membrane protein 100 triggers innate immunity and production of beta-defensin and the 18-kilodalton cationic antimicrobial protein through the fibronectin-integrin pathway in human gingival epithelial cells. Infect Immun. 2006;74:5211–5220. doi: 10.1128/IAI.00056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ménard S, Förster V, Lotz M, Gütle D, Duerr CU, Gallo RL, Henriques-Normark B, Pütsep K, Andersson M, Glocker EO, et al. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008;205:183–193. doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA. 1994;91:10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darmoul D, Brown D, Selsted ME, Ouellette AJ. Cryptdin gene expression in developing mouse small intestine. Am J Physiol. 1997;272:G197–G206. doi: 10.1152/ajpgi.1997.272.1.G197. [DOI] [PubMed] [Google Scholar]
- 81.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, Yadav SP, Crabb JW, Ganz T, Bevins CL. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 82.Shirafuji Y, Tanabe H, Satchell DP, Henschen-Edman A, Wilson CL, Ouellette AJ. Structural determinants of procryptdin recognition and cleavage by matrix metalloproteinase-7. J Biol Chem. 2003;278:7910–7919. doi: 10.1074/jbc.M210600200. [DOI] [PubMed] [Google Scholar]
- 83.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snel J, Bakker MH, Heidt PJ. Quantification of antigen-specific immunoglobulin A after oral booster immunization with ovalbumin in mice mono-associated with segmented filamentous bacteria or Clostridium innocuum. Immunol Lett. 1997;58:25–28. doi: 10.1016/s0165-2478(97)02715-6. [DOI] [PubMed] [Google Scholar]
- 85.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corthésy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 87.van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rey J, Garin N, Spertini F, Corthésy B. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172:3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 91.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 92.Massacand JC, Kaiser P, Ernst B, Tardivel A, Bürki K, Schneider P, Harris NL. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS One. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthésy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 94.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 95.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- 97.Kim KA, Kim JY, Lee YA, Song KJ, Min D, Shin MH. NOX1 participates in ROS-dependent cell death of colon epithelial Caco2 cells induced by Entamoeba histolytica. Microbes Infect. 2011;13:1052–1061. doi: 10.1016/j.micinf.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 98.Stark G. Functional consequences of oxidative membrane damage. J Membr Biol. 2005;205:1–16. doi: 10.1007/s00232-005-0753-8. [DOI] [PubMed] [Google Scholar]
- 99.Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Des. 2006;12:3551–3570. doi: 10.2174/138161206778343082. [DOI] [PubMed] [Google Scholar]
- 100.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leatham MP, Banerjee S, Autieri SM, Mercado-Lubo R, Conway T, Cohen PS. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157: H7 growth in the streptomycin-treated mouse intestine. Infect Immun. 2009;77:2876–2886. doi: 10.1128/IAI.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jankowska A, Laubitz D, Antushevich H, Zabielski R, Grzesiuk E. Competition of Lactobacillus paracasei with Salmonella enterica for adhesion to Caco-2 cells. J Biomed Biotechnol. 2008;2008:357964. doi: 10.1155/2008/357964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A. Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr. 2009;49:23–30. doi: 10.1097/MPG.0b013e3181924d1e. [DOI] [PubMed] [Google Scholar]
- 107.Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol. 2009;587:4169–4174. doi: 10.1113/jphysiol.2009.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–58. doi: 10.1097/MOG.0b013e3282f323f3. [DOI] [PubMed] [Google Scholar]
- 109.Shirkey TW, Siggers RH, Goldade BG, Marshall JK, Drew MD, Laarveld B, Van Kessel AG. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood) 2006;231:1333–1345. doi: 10.1177/153537020623100807. [DOI] [PubMed] [Google Scholar]
- 110.Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci. 2007;85:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- 111.Danielsen M, Hornshøj H, Siggers RH, Jensen BB, van Kessel AG, Bendixen E. Effects of bacterial colonization on the porcine intestinal proteome. J Proteome Res. 2007;6:2596–2604. doi: 10.1021/pr070038b. [DOI] [PubMed] [Google Scholar]
- 112.Kozakova H, Kolinska J, Lojda Z, Rehakova Z, Sinkora J, Zakostelecka M, Splichal I, Tlaskalova-Hogenova H. Effect of bacterial monoassociation on brush-border enzyme activities in ex-germ-free piglets: comparison of commensal and pathogenic Escherichia coli strains. Microbes Infect. 2006;8:2629–2639. doi: 10.1016/j.micinf.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, Laboisse CL. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu LC, Turner JR, Buret AG. LPS/CD14 activation triggers SGLT-1-mediated glucose uptake and cell rescue in intestinal epithelial cells via early apoptotic signals upstream of caspase-3. Exp Cell Res. 2006;312:3276–3286. doi: 10.1016/j.yexcr.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 117.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 118.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 119.Swanson PA, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci USA. 2011;108:8803–8808. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanders MA, Basson MD. Collagen IV regulates Caco-2 cell spreading and p130Cas phosphorylation by FAK-dependent and FAK-independent pathways. Biol Chem. 2008;389:47–55. doi: 10.1515/BC.2008.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Basuroy S, Dunagan M, Sheth P, Seth A, Rao RK. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2010;299:G186–G195. doi: 10.1152/ajpgi.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Tréton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 124.Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006;132:487–497. doi: 10.1007/s00432-006-0092-x. [DOI] [PubMed] [Google Scholar]
- 125.Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005;50:490–498. doi: 10.1007/s10620-005-2463-6. [DOI] [PubMed] [Google Scholar]
- 126.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, Lupton JR. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–1420. doi: 10.1093/carcin/bgn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 129.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 130.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92:2400–2408. doi: 10.3168/jds.2008-1698. [DOI] [PubMed] [Google Scholar]
- 132.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 133.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laudanno OM, Cesolari JA, Godoy A, Sutich E, Sarangone S, Catalano J, San Miguel P. Bioflora probiotic in immunomodulation and prophylaxis of intestinal bacterial translocation in rats. Dig Dis Sci. 2008;53:2667–2670. doi: 10.1007/s10620-007-0179-5. [DOI] [PubMed] [Google Scholar]
- 135.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 136.Anderson RC, Cookson AL, McNabb WC, Kelly WJ, Roy NC. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett. 2010;309:184–192. doi: 10.1111/j.1574-6968.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 137.Schiffrin EJ, Parlesak A, Bode C, Bode JC, van't Hof MA, Grathwohl D, Guigoz Y. Probiotic yogurt in the elderly with intestinal bacterial overgrowth: endotoxaemia and innate immune functions. Br J Nutr. 2009;101:961–966. doi: 10.1017/s0007114508055591. [DOI] [PubMed] [Google Scholar]
- 138.Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 139.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Montalto M, Maggiano N, Ricci R, Curigliano V, Santoro L, Di Nicuolo F, Vecchio FM, Gasbarrini A, Gasbarrini G. Lactobacillus acidophilus protects tight junctions from aspirin damage in HT-29 cells. Digestion. 2004;69:225–228. doi: 10.1159/000079152. [DOI] [PubMed] [Google Scholar]
- 141.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 143.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, Kohgo Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011;6:e23278. doi: 10.1371/journal.pone.0023278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 146.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 148.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reuter BK, Pizarro TT. Mechanisms of tight junction dysregulation in the SAMP1/YitFc model of Crohn's disease-like ileitis. Ann N Y Acad Sci. 2009;1165:301–307. doi: 10.1111/j.1749-6632.2009.04035.x. [DOI] [PubMed] [Google Scholar]
- 150.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 151.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 152.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 156.Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, Kwon J, Grenther WB, Balish E, Horak I, Sartor RB. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461–G1472. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 157.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gutiérrez A, Holler E, Zapater P, Sempere L, Jover R, Pérez-Mateo M, Schoelmerich J, Such J, Wiest R, Francés R. Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn's disease is affected by NOD2/CARD15 genotype. Inflamm Bowel Dis. 2011;17:1641–1650. doi: 10.1002/ibd.21537. [DOI] [PubMed] [Google Scholar]
- 162.Lacher M, Helmbrecht J, Schroepf S, Koletzko S, Ballauff A, Classen M, Uhlig H, Hubertus J, Hartl D, Lohse P, et al. NOD2 mutations predict the risk for surgery in pediatric-onset Crohn's disease. J Pediatr Surg. 2010;45:1591–1597. doi: 10.1016/j.jpedsurg.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 163.Lecat A, Piette J, Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem Pharmacol. 2010;80:2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 164.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 165.Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, Schreiber S, Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 166.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–274. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Doan HQ, Bowen KA, Jackson LA, Evers BM. Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Res. 2009;29:2473–2478. [PMC free article] [PubMed] [Google Scholar]
- 170.Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, Kudo E, Shimada M, Sano T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908–915. doi: 10.1038/sj.bjc.6605558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 172.Cuschieri J, Billgren J, Maier RV. Phosphatidylcholine-specific phospholipase C (PC-PLC) is required for LPS-mediated macrophage activation through CD14. J Leukoc Biol. 2006;80:407–414. doi: 10.1189/jlb.1105622. [DOI] [PubMed] [Google Scholar]
- 173.Martín-Villa JM, Ferre-López S, López-Suárez JC, Corell A, Pérez-Blas M, Arnaiz-Villena A. Cell surface phenotype and ultramicroscopic analysis of purified human enterocytes: a possible antigen-presenting cell in the intestine. Tissue Antigens. 1997;50:586–592. doi: 10.1111/j.1399-0039.1997.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 174.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 176.Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, Sellers MT, Orenstein JM, Shimada T, Graham MF, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 177.Brand S, Staudinger T, Schnitzler F, Pfennig S, Hofbauer K, Dambacher J, Seiderer J, Tillack C, Konrad A, Crispin A, et al. The role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn's disease. Inflamm Bowel Dis. 2005;11:645–652. doi: 10.1097/01.mib.0000168372.94907.d2. [DOI] [PubMed] [Google Scholar]
- 178.Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Devière J, et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut. 2004;53:987–992. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.De Jager PL, Franchimont D, Waliszewska A, Bitton A, Cohen A, Langelier D, Belaiche J, Vermeire S, Farwell L, Goris A, et al. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007;8:387–397. doi: 10.1038/sj.gene.6364398. [DOI] [PubMed] [Google Scholar]
- 180.Baumgart DC, Buning C, Geerdts L, Schmidt HH, Genschel J, Fiedler T, Gentz E, Molnar T, Nagy F, Lonovics J, et al. The c.1-260C& gt; T promoter variant of CD14 but not the c.896A& gt; G (p.D299G) variant of toll-like receptor 4 (TLR4) genes is associated with inflammatory bowel disease. Digestion. 2007;76:196–202. doi: 10.1159/000112646. [DOI] [PubMed] [Google Scholar]
- 181.Browning BL, Huebner C, Petermann I, Gearry RB, Barclay ML, Shelling AN, Ferguson LR. Has toll-like receptor 4 been prematurely dismissed as an inflammatory bowel disease gene? Association study combined with meta-analysis shows strong evidence for association. Am J Gastroenterol. 2007;102:2504–2512. doi: 10.1111/j.1572-0241.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 182.Hsiao CH, Wei SC, Wong JM, Lai HS, Chang MH, Ni YH. Pediatric Crohn disease: clinical and genetic characteristics in Taiwan. J Pediatr Gastroenterol Nutr. 2007;44:342–346. doi: 10.1097/MPG.0b013e31802c6997. [DOI] [PubMed] [Google Scholar]
- 183.Guo QS, Xia B, Jiang Y, Morré SA, Cheng L, Li J, Crusius JB, Peña AS. Polymorphisms of CD14 gene and TLR4 gene are not associated with ulcerative colitis in Chinese patients. Postgrad Med J. 2005;81:526–529. doi: 10.1136/pgmj.2004.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 185.Ortega-Cava CF, Ishihara S, Rumi MA, Aziz MM, Kazumori H, Yuki T, Mishima Y, Moriyama I, Kadota C, Oshima N, et al. Epithelial toll-like receptor 5 is constitutively localized in the mouse cecum and exhibits distinctive down-regulation during experimental colitis. Clin Vaccine Immunol. 2006;13:132–138. doi: 10.1128/CVI.13.1.132-138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meijssen MA, Brandwein SL, Reinecker HC, Bhan AK, Podolsky DK. Alteration of gene expression by intestinal epithelial cells precedes colitis in interleukin-2-deficient mice. Am J Physiol. 1998;274:G472–G479. doi: 10.1152/ajpgi.1998.274.3.G472. [DOI] [PubMed] [Google Scholar]
- 187.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 188.Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Kitazume MT, Takayama T, Okamoto S, Koganei K, Sugita A, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 189.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 191.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 192.Takada Y, Hisamatsu T, Kamada N, Kitazume MT, Honda H, Oshima Y, Saito R, Takayama T, Kobayashi T, Chinen H, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 193.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Edelblum KL, Washington MK, Koyama T, Robine S, Baccarini M, Polk DB. Raf protects against colitis by promoting mouse colon epithelial cell survival through NF-kappaB. Gastroenterology. 2008;135:539–551. doi: 10.1053/j.gastro.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 196.Matharu KS, Mizoguchi E, Cotoner CA, Nguyen DD, Mingle B, Iweala OI, McBee ME, Stefka AT, Prioult G, Haigis KM, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009;137:1380–1390.e1-3. doi: 10.1053/j.gastro.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 199.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 200.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 201.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 203.Li Q, Yu YY, Zhu ZG, Ji YB, Zhang Y, Liu BY, Chen XH, Lin YZ. Effect of NF-kappaB constitutive activation on proliferation and apoptosis of gastric cancer cell lines. Eur Surg Res. 2005;37:105–110. doi: 10.1159/000084541. [DOI] [PubMed] [Google Scholar]
- 204.Morais C, Pat B, Gobe G, Johnson DW, Healy H. Pyrrolidine dithiocarbamate exerts anti-proliferative and pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol Dial Transplant. 2006;21:3377–3388. doi: 10.1093/ndt/gfl543. [DOI] [PubMed] [Google Scholar]
- 205.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]