Abstract
The inositol (1,4,5) trisphosphate 3-kinases comprise a family of enzymes (A, B, and C) that phosphorylate the calcium mobilising molecule inositol (1,4,5) trisphosphate (IP3) to generate inositol (1,3,4,5) tetrakisphosphate. This molecule can function as a second messenger, but its roles are not completely understood. The A isoform of inositol (1,4,5) trisphosphate 3-kinase localises to filamentous actin within dendritic spines in the hippocampus and is implicated in the regulation of spine morphology and long term potentiation, however the mechanisms through which it signals in neuronal cells are not completely understood. We have used NGF driven neurite outgrowth from PC12 cells as a platform to examine the impact of signaling via inositol (1,4,5) trisphosphate 3-kinase activity in a neuronal cell. We have found that the catalytic activity of the enzyme opposes neurite outgrowth, whilst pharmacological inhibition of inositol (1,4,5) trisphosphate 3-kinase leads to a significant increase in neurite outgrowth, and we show that the reduction in neurite outgrowth in response to inositol (1,4,5) trisphosphate 3-kinase activity correlates with reduced ERK activity as determined by western blotting using phosphorylation-specific antibodies. Our findings suggest a novel neuronal signaling pathway linking metabolism of IP3 to signaling via ERK.
Introduction
Numerous ligand-operated signaling pathways involve the second-messenger inositol (1,4,5) trisphosphate (IP3), which is generated by the action of phospholipase C (PLC) on phopsphatidylinositol (4,5) bisphosphate (PIP2). Once generated, IP3 can either be metabolized by IP3 5-phosphatases to generate the inactive inositol bisphosphate (IP2), or can be further phosphorylated by a family of IP3 3-kinases, to generate inositol (1,3,4,5) tetrakisphosphate (IP4). IP3 is a second messenger which functions by binding to the IP3 receptor at the ER, resulting in the release of stored calcium [1]. IP4 is also recognized as a second messenger but its cellular functions are not fully understood [2], [3], [4]. There are three isoforms of IP3 3-kinase. These have been designated A, B and C (IP3 3-KA, IP3 3-KB and IP3 3-KC) [5]. IP3 3-KB and IP3 3-KC are expressed in most tissues, whilst IP3 3-KA expression is enriched in the central nervous system [6], [7], [8]. IP3 3-KB is the most studied of the three isoforms; it has been assigned roles in the regulation and development of various cells in the immune system, functioning principally via the production of IP4. Three studies have implicated IP3 3-KB in the development of T-cells [9], [10], [11], and it has also been implicated in B-cell development, selection and activation [12], [13], [14], as well as the regulation of myelopoesis and neutrophil signaling [15], [16]. However, whilst these studies have been instrumental in defining a physiological role for IP3 3-KB, the cellular roles of IP4 remain unclear and the mechanisms by which it signals require elucidation. Some studies suggest that IP4 production in T-cells is necessary for efficient activation of the ERK pathway [9], [10], whilst it is also necessary for the activation of PLC in order for T-cells to develop correctly [11]. Others suggest that, in B-cells, IP4 signals solely by inhibiting store operated calcium channels [13], [14], whilst it is also reported that IP4 negatively regulates PIP2 mediated activation of the GTPase activating protein Gap1IP4BP resulting in attenuated signaling via ERK [12]. To further complicate the issue, it has been reported that IP4 negatively regulates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) signaling in neutrophils [15], [16]. It is therefore evident that IP4 (as generated by IP3 3-kinase) has the ability to impact on numerous key signaling pathways depending on factors such as the cell type in which IP4 is produced. In the brain, IP3 3-KA is enriched in dendritic spines in the CA1 region of the hippocampus [8], and is upregulated during spatial learning tasks [17]. It is also implicated in the regulation of long term potentiation (LTP) via the generation of IP4 [18], [19], and regulates dendritic spine morphology by functioning as a scaffold for Rac1 [20], and as an F-actin-bundling protein [21]. However the full consequences of its catalytic activity in neurons are not clear.
We chose to investigate the consequences of IP3 3-KA expression in a neuroendocrine cell line during differentiation to a neuronal phenotype. PC12 cells respond to NGF stimulation by halting proliferation, extending neurites and adopting a neuronal phenotype. This differentiation process occurs as a result of the binding of NGF to the TrkA receptor, which activates a number of well characterized signaling pathways [22]. We therefore chose to use NGF driven neurite outgrowth as a platform to investigate the potential consequences of IP3 3-KA activity in neuronal cells. We report that stable high expression of IP3 3-KA dramatically inhibits neurite outgrowth from PC12 cells after NGF stimulation. We present evidence that this inhibition is most likely as a result of IP4 production, and occurs due to the subsequent attenuation of signaling via ERK.
Materials and Methods
Expression constructs
pEGFP-C3-IP3 3-KA, containing the full length rat inositol 1,4,5-trisphosphate 3-kinase A coding sequence cloned into the HindIII and BamH1 restriction sites of pEGFP-C3 (Clontech) [8]. 66-459 pEGFP-C3-IP3 3-KA, containing bp198–1353 of the rat inositol 1,4,5-trisphosphate 3-kinase A coding sequence ligated into the HindIII and BamH1 restriction sites of pEGFP-C3 [8]. D260A/K262A pEGFP-C3-IP3 3-KA, containing the rat inositol 1,4,5-trisphosphate 3-kinase A coding sequence ligated into the HindIII and BamH1 restriction sites (as above), but with D260A and K262A point mutations [23]. All of these constructs were a gift from Dr. Michael Schell, Uniformed Services University of the Health Sciences, USA.
PC12 cell culture
Low passage number PC12 cells were acquired from the European Collection of Cell Cultures (ECACC), and routinely grown in RPMI 1640 media (Sigma) supplemented with 10% batch tested French FBS (Biosera), 1% penicillin/streptomycin, and 2 mM L-glutamine on flasks coated with type IV collagen (Sigma), according to ECACC guidelines. PC12 cells were differentiated in the usual media supplemented with 100 ng/ml NGF (Sigma).
Generation of stable PC12 cell lines expressing GFP tagged constructs
PC12 cells were transfected with plasmid DNA using lipofectamine 2000 (Invitrogen) on 90 mm petri dishes and grown in the usual media plus 400 µg/ml neomycin (G418, Invitrogen). In order to generate clonal lines, cultures were left in selective media until isolated colonies were visible. These were screened for GFP expression, and isolated using sterile cloning rings and expanded in selective culture media. Observations of stable cell lines were made using several clonal lines to ensure against artefacts of clonal expression. Individual clonal lines were then selected for FACS sorting.
FACS sorting
Flow cytometric analysis and sorting were performed using a MoFlo Fluorescence Activated Cell Sorter (Cytomation), or the BD FACS Vantage SE system (BD Biosciences). Cells derived from a single clonal line were sorted at 488 nm by virtue of their GFP signal to enrich for the approximate 25% of the cell population expressing the highest levels of fluorescence, and the approximate 25% of the population expressing the lowest levels of fluorescence.
Confocal Microscopy
Cell imaging was performed by confocal microscopy using a Leica TCS-NT confocal laser-scanning microscope attached to a Leica DM RBE upright epifluorescence microscope under a 63× oil immersion objective. Fluorophores were excited at 488 nm and 568 nm using a krypton/argon laser. Images shown are maximum projections of stacks of confocal slices obtained throughout the entire depth of neurites. Images were processed using Adobe Photoshop 6.0 and Adobe Illustrator 10 (2).
Quantitative Analysis of Neurite Outgrowth
Indices of neurite outgrowth were quantified using the Cellomics Arrayscan high content imaging system, using a 10× objective to acquire images of fluorescently labelled cells via automated microscopy. Images were then analysed using a dedicated proprietary algorithm (Cellomics). For experiments to determine the effects of stable expression on neurite outgrowth, cells were plated onto 96 well plates at a density of 2500 cells per well into 100 µl of the usual supplemented media containing 100 ng/ml NGF. Cells were cultured in the presence of NGF and fixed for analysis after four days. For the experiments to determine the effect of C5 inhibition of IP3 3-kinase and XeC inhibition of the IP3 receptor, cells were plated in the presence of NGF and drug or vehicle (DMSO) on day one, and fixed on day four. All experiments were performed a minimum of three times. Cells were fixed with 4% paraformaldehyde containing 10% sucrose and 0.06% Hoescht 33342 dye (to label nuclei). Cells were then washed with a proprietary buffer (Cellomics), and labelled (by indirect immunofluorescence) with a proprietary antibody (Cellomics) to detect neurites, (Cellomics NOG HitKit). The Arrayscan was then used to capture images of Hoescht 33342-labelled nuclei, in order to identify individual cells, and then images were acquired of the fluorescently labelled neurites. Cellomics image analysis software (Neurite Outgrowth Algorithm) was then used to quantify neurite outgrowth. For this study these included the neurite outgrowth index and the average neurite length. The neurite outgrowth index is a measure of neurite bearing cells, and is defined as the percentage of neurons whose summed lengths are greater than a set threshold. This was set at 10 µm longer than the average width of a cell body, which was set at 15 µm. The origin of neurites was determined by the Hoescht label.
The average neurite length was calculated as the sum of neurite lengths from cells with outgrowth divided by the number of cells with outgrowth. Neurites and cell bodies were identified using the Cellomics Neurite Outgrowth HitKit, which uses a proprietary neuronal marker as a primary antibody, visualised with a secondary antibody conjugated to Alexa 488.
Analysis of ERK activity
For experiments to determine ERK activity, serum starved PC12 cells were stimulated with 1 ng/ml NGF and lysed after various time points. ERK activity was then determined by western blot. A rabbit polyclonal antibody recognising p44/42 MAP Kinase (Cell Signalling Technology) was used to detect total ERK, whilst a rabbit polyclonal antibody recognising phospho-p44/42 MAP Kinase (Thr202/Tyr204) was used to detect Phosphorylated ERK. The phospho-specific antibody detects rat p42 and p44 MAP kinase (ERK2 and ERK1) only when phosphorylated at Thr202 and Tyr204 (Cell Signalling Technology). Primary antibody was detected using a horseradish peroxidase conjugated secondary antibody (DAKO) and visualised by enhanced chemiluminescence.
Pharmacological agents
The following small molecule inhibitors were used: Inositol 1,4,5-trisphosphate 3-Kinase inhibitor referred to as “C5”, N2-(m-Trifluorobenzyl), N6-(p-nitrobenzyl) purine (Calbiochem/Merck) [24]. Xestospongin C, an inhibitor of IP3-mediated calcium release (Calbiochem/Merck) [25].
Statistical Analysis
Data presented represent means of three experiments +/− SEM. Statistical analysis was performed using one-way ANOVA with post-hoc analysis, or by two-way ANOVA. Data were analysed using GraphPad Prism.
Results
Expression of IP3 3-KA in PC12 Cells Results in an Inhibition of Neurite Outgrowth
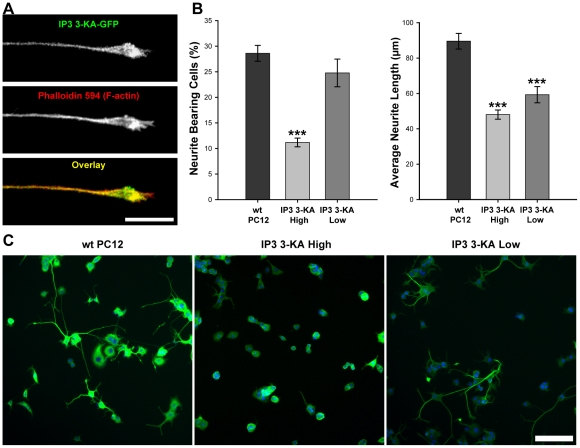
In order to determine how IP3 3-KA activity impacts on NGF driven neurite outgrowth from PC12 cells, we first generated numerous cell lines stably expressing GFP-tagged full length IP3 3-KA. We sorted one of these lines by FACS to produce cell lines with different levels (designated high and low) of recombinant protein expression (as defined by the intensity of the GFP signal). Neuronal differentiation was induced by the addition of NGF, and we investigated the sub-cellular localisation of recombinant IP3 3-KA-GFP by confocal microscopy. We initially observed that very few IP3 3-KA-GFP expressing cells differentiated following the addition of NGF, and that the neurites of those cells that did differentiate were clearly shorter than their wild type counterparts. Where neurites were observed, IP3 3-KA-GFP fluorescence was detected throughout their full length, and was enriched at the growth cone. We then used fluorescently labeled phalloidin to label F-actin, and confirmed that IP3 3-KA-GFP colocalises with F-actin at the tip of growing neurites (Figure 1A).
Figure 1. IP3 3-KA co-localises with F-actin at the PC12 cell growth cone, and inhibits neurite outgrowth.
(A) Growth cone of PC12 cell stably expressing high levels of IP3 3-KA-GFP, fixed ten days after culture in the presence of 100 ng/ml NGF, and labeled with phalloidin-594. IP3 3-KA-GFP co-localises with F-actin. Scale bar is 10 µm. (B) Quantitative analysis of the effects of IP3 3-KA-GFP expression on neurite outgrowth. Neurite outgrowth was quantified using the Cellomics Arrayscan Neurite Outgrowth algorithm to measure the percentage of cells with neurites, and average neurite length (described in full in materials and methods). IP3 3-KA expression results in an inhibition of neurite outgrowth. Data are presented as mean +/− SEM. ***p<0.001, by ANOVA with post-hoc analysis. (C) Arrayscan images of PC12 cells expressing varying levels of IP3 3-KA-GFP, fixed four days after culture in the presence of 100 ng/ml NGF, and labeled using Hoescht 33342 and the Cellomics Neurite Outgrowth HitKit to identify neuritis. Scale bar is 100 µm.
The effects of IP3 3-KA expression on neurite outgrowth were quantified using the Cellomics Arrayscan instrument for high content analysis and the Cellomics Neurite Outgrowth algorithm (detailed in the materials and methods section). Cells were incubated in the presence of NGF to induce neurite outgrowth and fixed after four days in culture. High levels of IP3 3-KA expression resulted in a dramatic decrease (by 61%) in the number of cells with neurites compared with wild type control cells whilst lower levels of IP3 3-KA expression resulted in less reduction (14%) (Figure 1B). We also analysed the average length of neurites and confirmed that expression of IP3 3-KA resulted in cells with shorter neurites than those emanating from control cells (Figure 1B). Cells expressing high levels of IP3-3KA produced neurites that were on average 46% shorter than those produced by control cells, whilst cells expressing lower levels of IP3-3KA had neurites that were shorter on average by 34%. These data show a correlation between level of expression of IP3 3-KA and phenotypic effect. They also demonstrate that IP3 3-KA functions to oppose the actions of NGF on PC12 cells, and that this results in a dramatic reduction in neurite outgrowth, as can clearly be seen in images acquired using the Arrayscan (Figure 1C).
The enzymatic activity of IP3 3-KA is principally responsible for inhibiting NGF driven neurite outgrowth
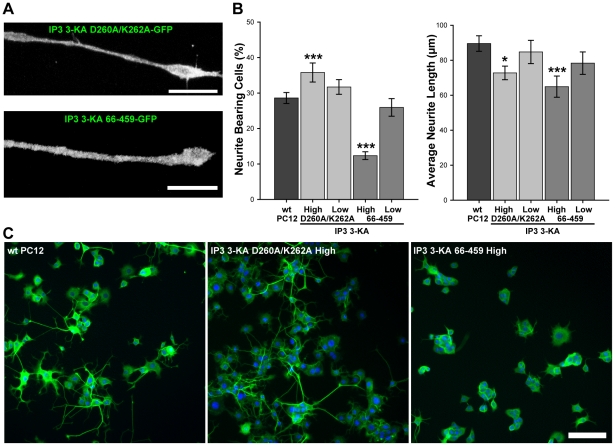
We reasoned that IP3 3-KA could be inhibiting neurite outgrowth either by virtue of its catalytic activity, or by its ability to bind to F-actin. In order to address this issue, we generated cell lines stably expressing truncated or mutated IP3 3-KA-GFP. 66-459 IP3 3-KA-GFP lacks the N-terminal amino acids necessary for binding to F-actin and is therefore rendered cytosolic [8]. D260A/K262A IP3 3-KA-GFP is identical to the full-length construct except for two point mutations in the catalytic domain which render the enzyme catalytically inactive [23], [26]. Cells were again sorted by FACS to produce high and low expressing stable cell lines. In contrast to cells expressing wild type IP3 3-KA-GFP, cells expressing ‘kinase dead’ D260A/K262A IP3 3-KA-GFP differentiated at a similar rate to their wild type counterparts in the presence of NGF. Kinase dead IP3 3-KA-GFP localised in the same way as the wild type construct, and was enriched at the growth cone (Figure 2A, upper panel). Conversely, cytosolic 66-459 IP3 3-KA-GFP was distributed uniformly along neurites, and was not enriched at the growth cone (Figure 2A lower panel). This suggests that the actin binding domain of IP3 3-KA is required to enrich the enzyme at the growth cone of differentiating PC12 cells. We then quantified the effects of the mutant constructs on neurite outgrowth using the same criteria as for the full-length construct. High levels of kinase dead IP3 3-KA-GFP expression resulted in an increase in neurite outgrowth of 25% in the number of cells with neurites compared with control cells (Figure 2B). Lower levels of expression had no effect. This suggests that the kinase dead construct is capable of acting as a partial dominant-negative. In contrast, high levels of expression of the cytosolic IP3 3-KA-GFP construct resulted in a decrease (by 57%) in the number of cells with neurites compared with wild type controls (Figure 2B). These results suggest that the catalytic activity of the IP3 3-KA is essential for the inhibition of differentiation caused by expression of the full-length enzyme. High levels of expression of the kinase dead IP3 3-KA resulted in cells with shorter neurites (by 19%) compared with wild type controls (Figure 2B), whilst low levels of expression had no effect on neurite length. Cells expressing high levels of the cytosolic IP3 3-KA exhibited neurites that were 28% shorter than controls (Figure 2B). This is not as marked an effect as that caused by expression of the full-length enzyme (p<0.001 by two-way ANOVA). Low levels of expression of the cytosolic IP3 3-KA had no effect on neurite length, compared with a 32% reduction exhibited by the cells expressing low levels of the full-length enzyme. It would therefore appear that amino acids 1–65 are necessary to enrich IP3 3-KA at growth cone actin, where it functions to inhibit neurite length, principally by virtue of its catalytic activity but also by regulating the actin cytoskeleton. Representative images of each of these cell lines (Figure 2C) highlight the fact that the catalytic activity of IP3 3-KA is principally responsible for the inhibition of NGF-driven neurite outgrowth from PC12 cells.
Figure 2. The catalytic activity of IP3 3-KA opposes NGF-driven neurite outgrowth from PC12 cells.
(A) Localisation of mutant IP3 3-KA constructs in PC12 cell neurites. IP3 3-KA D260A/K262A-GFP is enriched at the growth cone, whilst IP3 3-KA 66-459-GFP is not. Scale bar is 10 µm. (B) Quantitative analysis of the effects of mutated IP3 3-KA expression on neurite outgrowth. D260A/K262A-GFP overexpression results in an increase in the number of cells with neurites and decreases neurite length. IP3 3-KA 66-459-GFP expression results in a decrease in both indices of neurite outgrowth. Data are presented as mean +/− SEM. *p<0.05, ***p<0.001, by ANOVA with post-hoc analysis. (C) Arrayscan images of PC12 cells expressing mutant IP3 3-KA-GFP, fixed four days after culture in the presence of 100 ng/ml NGF, and labeled using Hoescht 33342 and the Cellomics Neurite Outgrowth HitKit to identify neuritis. Scale bar is 100 µm.
Inhibition of IP3 3-kinase activity enhances neurite outgrowth from PC12 cells
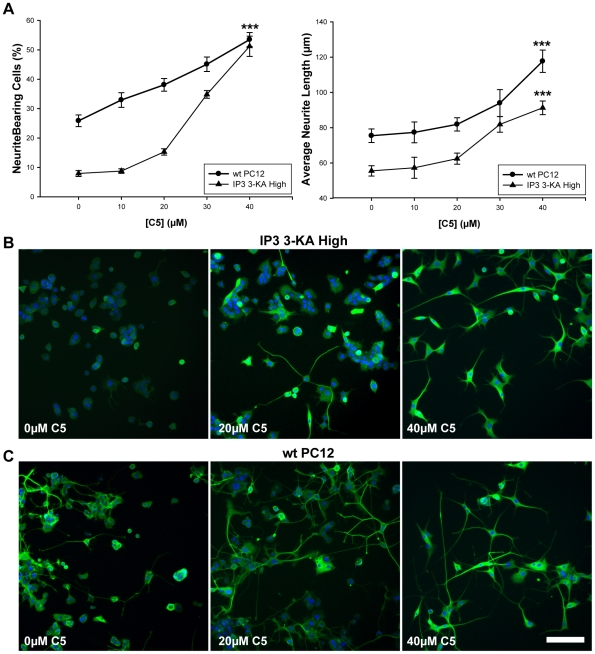
In order to investigate the effects of endogenous IP3 3-kinase activity in PC12 cells, we investigated the effects of a number of reported IP3 3-kinase inhibitors [24], [27] on neurite outgrowth. We found that most of these enhanced neurite outgrowth from IP3 3-KA expressing cells, and chose molecule C5 for further investigation. C5 is a purine-based inhibitor of IP3 3-kinase that is reported to have an IC50 of 10.2 µM in vitro [24]. We therefore investigated the effects of C5 on PC12 neurite outgrowth at concentrations of 10 to 40 µM. Cells expressing high levels of IP3 3-KA were cultured in the presence of NGF and C5. Low doses (10–20 µM) of C5 had little effect. However at higher doses (30–40 µM) C5 overcame the inhibition on neurite outgrowth imposed by expression of IP3 3-KA, giving rise to an increase in the number of cells with neurites, and an increase in average neurite length (Figure 3A). We then investigated the effects of IP3 3-kinase inhibition on neurite outgrowth from wild type PC12 cells and found that increasing doses of C5 resulted in increases in both the number of cells with neurites and the length of neurites (Figure 3A). We found that the number of neurite bearing cells in wild type and IP3 3-KA expressing cell types equalised as C5 dose increased, reflecting a dose dependent affinity of C5 for IP3 3-kinase, and further indicating that the catalytic activity of IP3 3-kinase opposes NGF driven PC12 cell differentiation. However, C5 inhibition did not completely overcome the inhibition of neurite length caused by IP3 3-KA overexpression, again suggesting that it hinders neurite extension partly via its catalytic activity, but also by an additional mechanism (most likely by interfering with the actin cytoskeleton). Representative images of these cells underline the effects of IP3 3-kinase inhibition on neurite outgrowth from PC12 cells, illustrating that as the dose of C5 increases, neurites become increasingly more streamlined, and better defined (Figure 3B and C). These results demonstrate that endogenous IP3 3-kinase activity opposes NGF driven neurite outgrowth from PC12 cells, and suggest that the enzyme functions to oppose the NGF dependant intracellular signals that trigger differentiation.
Figure 3. Inhibition of IP3 3-kinase increases neurite outgrowth from PC12 cells, and IP3 3-KA-PC12 cells.
(A) Analysis of the effects of C5 on wild type PC12 cells and cells expressing high levels of IP3 3-KA. Differentiating PC12 cells in the presence of C5 results in an increase in two indices of neurite outgrowth. Data are presented as mean +/− SEM. ***p<0.001, by ANOVA with post-hoc analysis. (B) Arrayscan images of PC12 cells expressing high levels of IP3 3-KA, fixed four days after culture in the presence of 100 ng/ml NGF, and labeled using Hoescht 33342 and the Cellomics Neurite Outgrowth HitKit to identify neurites (using a 10× objective). (C) Arrayscan images of PC12 cells, fixed four days after culture in the presence of 100 ng/ml NGF, and labeled as for (B). Scale bar is 100 µm.
IP3 3-kinase activity attenuates ERK phosphorylation via a mechanism which appears distinct from the removal of IP3
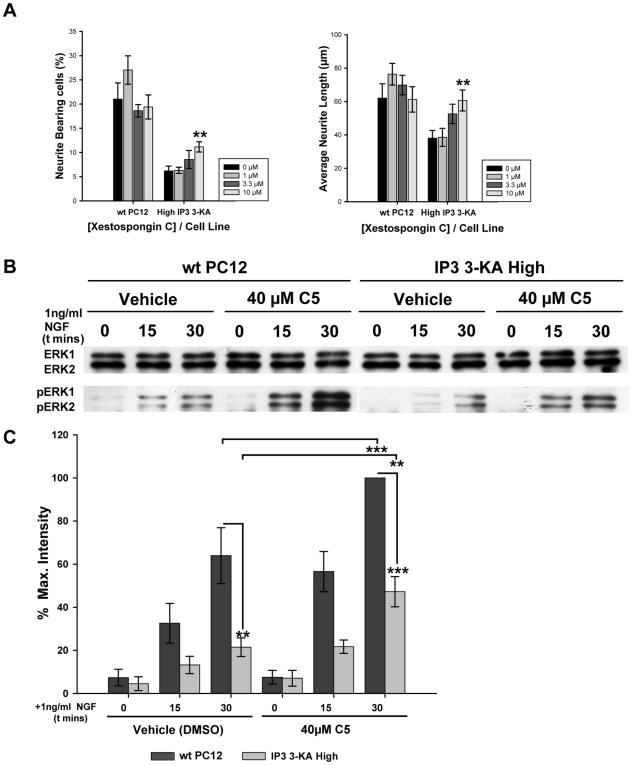
We next questioned the mechanism by which IP3 3-kinase activity opposes NGF driven neurite outgrowth, reasoning that the inhibition of neurite outgrowth caused by IP3KA over-expression was due either to the metabolism of IP3 or to increased production of IP4. We argued that if the increased metabolism of IP3 caused by IP3 3-KA activity was responsible for inhibiting PC12 cell differentiation, then blockade of the IP3 receptor would have a similar effect. We therefore investigated the effects of IP3 receptor blockade during NGF driven neurite outgrowth using the cell permeable IP3 receptor inhibitor Xestospongin C (XeC). We found that IP3 receptor blockade with XeC had no effect on the number of wild type PC12 cells with neurites, or on neurite length (p<0.05 by ANOVA) (Figure 4A). This suggests that increased metabolism of IP3 caused by IP3 3-KA overexpression is insufficient to inhibit PC12 cell differentiation. We also treated cells expressing high levels of IP3 3-KA with NGF in the presence of XeC, and found that rather than compounding the effects of elevated IP3 3-kinase activity, XeC overcame some of the IP3 3-KA dependent inhibition of neurite outgrowth (Figure 4A). This suggests that reducing intracellular calcium by blocking its release from internal stores decreases IP3 3-KA activity, and is in keeping with findings that IP3 3-KA activity is calcium dependent [28], [29]. Our data are therefore consistent with the hypothesis that IP3 3-kinase opposes the actions of NGF on PC12 cells via the generation of IP4, and are in agreement with previous findings that, whilst stimulation of PC12 cells with NGF results in the generation of IP3 and IP4, signaling via IP3 does not appear to contribute substantially to the differentiation process [30], [31]. Our data our also consistent with previous reports that XeC treatment has little effect on NGF-induced neurite outgrowth from PC12 cells [32], [33]. We therefore questioned the mechanisms by which IP4 might prevent PC12 cell differentiation, and focused on the ERK signaling pathway as sustained activation of ERKs is reported to be essential for the NGF mediated differentiation of PC12 cells [34].
Figure 4. IP3 3-KA activity results in attenuation of ERK phosphorylation.
IP3 does not appear to be required for NGF driven neurite outgrowth. (A) Analysis of the effects of XeC blockade of the IP3 receptor, on PC12 cells expressing high levels of IP3 3-KA and on wild type PC12 cells. XeC has no effect on control PC12 cells, but partially overcomes the inhibition of neurite outgrowth caused by IP3 3-KA expression. Data are presented as mean +/− SEM. **p<0.01, by ANOVA with post-hoc analysis. (B) Determination of ERK activation in control PC12 cells and IP3 3-KA expressing cells using a suboptimal dose of NGF, in the presence of either 40 µM C5 or vehicle. Lower blot represents ERK activation as detected by phospho-specific antibodies against ERK1 and ERK2. Upper blot represents total ERK, as detected using an antibody that detects both ERK1 and ERK2. All antibodies were visualised with an HRP conjugated secondary antibody. The blot shown is representative of three independent experiments. (C) Densitometric quantification of ERK activation, as depicted in (B). **p<0.01, Data are presented as mean +/− SEM. ***p<0.001, by ANOVA with post-hoc analysis. IP3 3-KA expression attenuates ERK activation, whilst C5 inhibition of IP3 3-kinase results in increased ERK activity.
We investigated the effects of C5 inhibition of IP3 3-kinase on ERK phosphorylation in control cells and in cells expressing high levels of IP3 3-KA. Serum starved cells were preincubated with either 40 µM C5 or vehicle for 30 minutes prior to stimulation with a low dose of NGF (1 ng/ml) and subsequently lysed at various time points. The onset of ERK phosphorylation was slow following stimulation with 1 ng/ml of NGF, and reached a peak after 30 minutes. Comparison of cells expressing high levels of IP3 3-KA with wild-type controls revealed that ERK signaling is attenuated in the IP3 3-KA expressing cells after stimulation with 1 ng/ml NGF (Figure 4 B and C). Furthermore, pre-incubation with 40 µM C5 resulted in an increase in the amount of phosphorylated ERKs in both the wild type cells, and cells expressing high levels of IP3 3-KA. Quantification by densitometry revealed that 30 minutes after NGF stimulation, ERK phosphorylation is reduced by 66% in the cells expressing high levels of IP3 3-KA compared with wild type controls (Figure 4C). Furthermore, inhibition of IP3 3-kinase by C5 in wild type cells resulted in a 56% increase in ERK phosphorylation (Figure 4C). A similar effect was seen in cells expressing high levels of IP3 3-KA. These results demonstrate that IP3 3-kinase activity attenuates ERK signaling in NGF stimulated PC12 cells, contributing to an inhibition of neurite outgrowth, most likely as a result of IP4 production.
Discussion
We have demonstrated that elevated expression of IP3 3-KA dramatically inhibits NGF driven PC12 cell differentiation, and that this occurs principally as a result of the kinase activity of the enzyme. We have also shown that inhibiting endogenous IP3 3-kinase leads to enhanced neurite outgrowth from NGF stimulated PC12 cells, suggesting that endogenous IP3 3-kinase functions to oppose the actions of NGF on PC12 cells. This inhibition of PC12 cell differentiation involves the attenuation of signaling through ERK, and most likely occurs via the production of IP4 because inhibition of the IP3 receptor has no effect on neurite outgrowth from wild type PC12 cells. IP3 3-kinase therefore appears to mediate a negative feedback mechanism which functions (via the production of IP4) to oppose the actions of NGF on PC12 cells.
We chose to investigate IP3 3-KA in a model neuronal cell because it is this isoform of IP3 3-kinase that is enriched in the dendritic spines of hippocampal neurons. The majority of previous studies examining the effects of signaling via IP3 3-kinase have been undertaken in various immune cells (B-cells, T-cells and neutrophils [4], [9], [10], [11], [12], [13], [14], [15], [16], and there have been no studies to examine the impact of signaling via IP3 3-kinase in neuronal cells. Whilst PC12 cells do not represent hippocampal neurons, they have provided a platform to investigate the consequences of signaling via IP3 3-kinase in a model system for neuronal differentiation. It is important to note that all of the elements of the pathway we have described are present in neurons in the hippocampus [35] where IP3 is generated post-synaptically by the activation of metabotropic glutamate receptors [36]. We have demonstrated, using a neuronal cell line, that IP3 3-KA can act to attenuate ERK signaling and we suggest that if a similar process occurs within dendritic spines it would have significant consequences in terms of the regulation of post-synaptic excitability, and would be in keeping with the finding that mice lacking IP3 3-KA have enhanced LTP [18]. Further studies are therefore required to determine how the pathway we have investigated in PC12 cells might be applied to the regulation of post-synaptic signaling.
We have not investigated the other signaling pathways activated by the binding of NGF to the TrkA receptor. TrkA also signals via phosphoinositide 3-kinase (PI 3-kinase) and PIP3, and IP4 is reported to inhibit PIP3 signaling since depletion of IP4 enhances PIP3 signaling in neutrophils [16]. Furthermore, IP3 3-KB is reported to regulate myelopoiesis via the production of IP4 and the subsequent down-regulation of signaling via PIP3 and AKT [15]. It is possible then that increased IP4 production in PC12 cells expressing elevated levels of IP3 3-KA could also inhibit signaling via PIP3, and contribute to the inhibition of neurite outgrowth. However, sustained ERK activation is considered to be the principal signal for NGF driven neurite outgrowth from PC12 cells, and it is possible that there is a direct link between IP4 production and ERK attenuation. The most likely candidates for this role are the IP4 binding proteins Gap1IP4BP and GAP1M. These are closely related proteins that are both reported to exhibit GTPase activating (GAP) activity towards Ras, whilst Gap1IP4BP is also reported to exhibit GAP activity towards the Ras related protein Rap1 [37]. As the sustained activation of ERKs responsible for NGF driven differentiation of PC12 cells is mediated by Rap1 [34], it may be that the dual GAP activity of Gap1IP4BP on both Ras and Rap is limiting the ERK dependent outgrowth process. In support of this hypothesis, it is reported that Gap1IP4BP is down-regulated during the differentiation of PC12 cells to a neuronal phenotype, suggesting that Gap1IP4BP exists as an obstacle to NGF driven neurite outgrowth [38]. It would therefore be interesting to determine whether changes in IP4 concentration (as a result of IP3 3-kinase activity) affect GAP1IP4BP activity towards Rap1 and Ras in NGF stimulated PC12 cells. In summary, our findings suggest that IP3 3-kinase functions to negatively regulate the NGF dependent signals that lead to the differentiation of PC12 cells. This most likely occurs via the production of IP4 leading to attenuation of signaling through ERK. We suggest that this signaling pathway may have relevance in other neuronal cells where it may be involved in the regulation of post synaptic signaling.
Acknowledgments
We would like to thank Rob Jepras for FACS sorting of cell lines, and Mike Schell for the DNA constructs. We also kindly thank Aviva Tolkovsky for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: Funded in part by a commercial funder (GlaxoSmithKline), who provided part funding for a Ph.D. studentship, which has now been completed. At present, no authors are employed by GlaxoSmithKline, and are not involved with consultancy, patents or products in development or marketed products related to GlaxoSmithKline. The authors are free to adhere to the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by funding from the BBSRC and CASE Studentship with GlaxoSmithKline (www.bbsrc.ac.uk, www.gsk.co.uk). The BBSRC had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. GSK contributed to a small percentage of the funding. Kalpana Patel was employed by GSK at the time and contributed to the design of the study. The design of the study on the part of GSK was to investigate the use of fluorescent reporter constructs in PC12 cells for high throughput screening. The decision to pursue investigations of the role of inositol (1,4,5) trisphosphate 3-kinase A in PC12 cell differentiation was not part of the original study design regarding GSK. GSK did not design the study as presented here, however some data was collected and analysed on GSK equipment by Richard Eva. Specifically, neurite outgrowth quantification and analysis was performed on GSK equipment by Richard Eva, who was at the time a PhD student at the University of Bristol. All the data presented in figures 4, 5 and 6 was acquired at the University of Bristol. GSK had no role in the decision to publish or preparation of the manuscript. Kalpana Patel was employed by GSK at the time the data was acquired, but not at the time of the drafting of the manuscript. Cells expressing GFP tagged Inositol (1,4,5) trisphosphate 3-kinase A were sorted for expression levels by Rob Jepras on a FACS machine at GSK. Rob Jepras had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- 2.Irvine RF, Lloyd-Burton SM, Yu JC, Letcher AJ, Schell MJ. The regulation and function of inositol 1,4,5-trisphosphate 3-kinases. Adv Enzyme Regul. 2006;46:314–323. doi: 10.1016/j.advenzreg.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schell MJ. Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling. Cell Mol Life Sci. 2010;67:1755–1778. doi: 10.1007/s00018-009-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AT, Chamberlain PP, Cooke MP. Beyond IP3: roles for higher order inositol phosphates in immune cell signaling. Cell Cycle. 2008;7:463–467. doi: 10.4161/cc.7.4.5518. [DOI] [PubMed] [Google Scholar]
- 5.Pattni K, Banting G. Ins(1,4,5)P3 metabolism and the family of IP3-3Kinases. Cell Signal. 2004;16:643–654. doi: 10.1016/j.cellsig.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Yamada M, Kakita A, Mizuguchi M, Rhee SG, Kim SU, et al. Specific expression of inositol 1,4,5-trisphosphate 3-kinase in dendritic spines. Brain Res. 1993;606:335–340. doi: 10.1016/0006-8993(93)91004-c. [DOI] [PubMed] [Google Scholar]
- 7.Go M, Uchida T, Takazawa K, Endo T, Erneux C, et al. Inositol 1,4,5-trisphosphate 3-kinase highest levels in the dendritic spines of cerebellar Purkinje cells and hippocampal CA1 pyramidal cells. A pre- and post-embedding immunoelectron microscopic study. Neurosci Lett. 1993;158:135–138. doi: 10.1016/0304-3940(93)90247-i. [DOI] [PubMed] [Google Scholar]
- 8.Schell MJ, Erneux C, Irvine RF. Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem. 2001;276:37537–37546. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- 9.Wen BG, Pletcher MT, Warashina M, Choe SH, Ziaee N, et al. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proc Natl Acad Sci U S A. 2004;101:5604–5609. doi: 10.1073/pnas.0306907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pouillon V, Hascakova-Bartova R, Pajak B, Adam E, Bex F, et al. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol. 2003;4:1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- 11.Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, et al. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316:886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 12.Marechal Y, Pesesse X, Jia Y, Pouillon V, Perez-Morga D, et al. Inositol 1,3,4,5-tetrakisphosphate controls proapoptotic Bim gene expression and survival in B cells. Proc Natl Acad Sci U S A. 2007;104:13978–13983. doi: 10.1073/pnas.0704312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AT, Beisner DR, Liu D, Cooke MP. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J Immunol. 2009;182:4696–4704. doi: 10.4049/jimmunol.0802850. [DOI] [PubMed] [Google Scholar]
- 14.Miller AT, Sandberg M, Huang YH, Young M, Sutton S, et al. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol. 2007;8:514–521. doi: 10.1038/ni1458. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y, Loison F, Hattori H, Li Y, Erneux C, et al. Inositol trisphosphate 3-kinase B (InsP3KB) as a physiological modulator of myelopoiesis. Proc Natl Acad Sci U S A. 2008;105:4739–4744. doi: 10.1073/pnas.0800218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim IH, Park SK, Sun W, Kang Y, Kim HT, et al. Spatial learning enhances the expression of inositol 1,4,5-trisphosphate 3-kinase A in the hippocampal formation of rat. Brain Res Mol Brain Res. 2004;124:12–19. doi: 10.1016/j.molbrainres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Jun K, Choi G, Yang SG, Choi KY, Kim H, et al. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem. 1998;5:317–330. [PMC free article] [PubMed] [Google Scholar]
- 19.Szinyei C, Behnisch T, Reiser G, Reymann KG. Inositol 1,3,4,5-tetrakisphosphate enhances long-term potentiation by regulating Ca2+ entry in rat hippocampus. J Physiol. 1999;516(Pt 3):855–868. doi: 10.1111/j.1469-7793.1999.0855u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim IH, Park SK, Hong ST, Jo YS, Kim EJ, et al. Inositol 1,4,5-trisphosphate 3-kinase a functions as a scaffold for synaptic Rac signaling. J Neurosci. 2009;29:14039–14049. doi: 10.1523/JNEUROSCI.2483-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson HW, Schell MJ. Neuronal IP3 3-kinase is an F-actin-bundling protein: role in dendritic targeting and regulation of spine morphology. Mol Biol Cell. 2009;20:5166–5180. doi: 10.1091/mbc.E09-01-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 23.Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, et al. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem. 2002;277:35947–35960. doi: 10.1074/jbc.M205622200. [DOI] [PubMed] [Google Scholar]
- 24.Chang YT, Choi G, Bae YS, Burdett M, Moon HS, et al. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. Chembiochem. 2002;3:897–901. doi: 10.1002/1439-7633(20020902)3:9<897::AID-CBIC897>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 26.Togashi S, Takazawa K, Endo T, Erneux C, Onaya T. Structural identification of the myo-inositol 1,4,5-trisphosphate-binding domain in rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1997;326(Pt 1):221–225. doi: 10.1042/bj3260221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayr GW, Windhorst S, Hillemeier K. Antiproliferative plant and synthetic polyphenolics are specific inhibitors of vertebrate inositol-1,4,5-trisphosphate 3-kinases and inositol polyphosphate multikinase. J Biol Chem. 2005;280:13229–13240. doi: 10.1074/jbc.M500545200. [DOI] [PubMed] [Google Scholar]
- 28.Woodring PJ, Garrison JC. Expression, purification, and regulation of two isoforms of the inositol 1,4,5-trisphosphate 3-kinase. J Biol Chem. 1997;272:30447–30454. doi: 10.1074/jbc.272.48.30447. [DOI] [PubMed] [Google Scholar]
- 29.Vanweyenberg V, Communi D, D'Santos CS, Erneux C. Tissue- and cell-specific expression of Ins(1,4,5)P3 3-kinase isoenzymes. Biochem J. 1995;306(Pt 2):429–435. doi: 10.1042/bj3060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rong R, Ahn JY, Chen P, Suh PG, Ye K. Phospholipase activity of phospholipase C-gamma1 is required for nerve growth factor-regulated MAP kinase signaling cascade in PC12 cells. J Biol Chem. 2003;278:52497–52503. doi: 10.1074/jbc.M306744200. [DOI] [PubMed] [Google Scholar]
- 31.Contreras ML. Nerve growth factor stimulates the production of inositol 1,3,4- and 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in PC12 cells. J Neurochem. 1993;61:1035–1042. doi: 10.1111/j.1471-4159.1993.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishima T, Nishimura T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: role of sigma-1 receptors and IP3 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1656–1659. doi: 10.1016/j.pnpbp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS One. 2008;3:e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 35.Irvine RF, McNulty TJ, Schell MJ. Inositol 1,3,4,5-tetrakisphosphate as a second messenger–a special role in neurones? Chem Phys Lipids. 1999;98:49–57. doi: 10.1016/s0009-3084(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 37.Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, et al. GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem. 2006;281:9891–9900. doi: 10.1074/jbc.M512802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashita S, Kobayashi M, Kubo Y, Hinohara Y, Sezaki M, et al. Versatile roles of R-Ras GAP in neurite formation of PC12 cells and embryonic vascular development. J Biol Chem. 2007;282:3413–3417. doi: 10.1074/jbc.C600293200. [DOI] [PubMed] [Google Scholar]