Abstract
In the present investigation, the epidemiology of malaria among seven tea estates of Nagaon and Udalguri districts of Assam, India has been described. A cross-sectional open study was carried out to understand the malaria epidemiology and associated risk factors among the tea tribes during March to September 2009. Out of 1,182 peripheral blood smears examined, 506 found positive for malaria (slide positivity rate, SPR = 42.8) with Plasmodium falciparum as predominant species. Dimakuchi tea estate was having highest SPR (P = 0.0275) and contributed more number of P. falciparum cases (P < 0.00001). Tea estates studied in both Udalguri and Nagaon districts were equally affected and the SPR recorded were 41.75 and 43.32% respectively. 154 malaria cases detected were having ‘O’ blood group but each blood group was found to have similar susceptibility of acquiring malaria infection (χ2 = 3.603; P = 0.3076) and P. falciparum infection (χ2 = 1.818; P = 0.6110). The SPR was highest among children more than 2 years of age group and variation in SPR among the age groups was statistically significant (χ2 = 17.186; P = 0.0018). No gender biasing was observed in malaria distribution. Anemia was found associated with the infection among both the sexes. The findings suggest that tea estates are endemic for stable malaria transmission primarily due to P. falciparum and the prevalence rate decline with age, suggesting the development of protective immunity. Promising intervention measures could be able to reduce the malaria prevalence effectively in the study areas.
Keywords: Malaria, Plasmodium falciparum, Tea tribes, Tea estates, ABO blood group, Hemoglobin
Introduction
Malaria transmission in northeastern states of India is a daunting epidemiological challenge as its distribution is heterogenous and intensity is governed by many climatic and physiological risk factors (Dutta et al. 2004; Dev et al. 2004). Plasmodium vivax is the major malaria parasite in Indian subcontinent contributing majority of cases (Dua et al. 1996) however in northeast region, the situation is different and >60% malaria cases are contributed by Plasmodium falciparum (Mohapatra et al. 2008; Dhiman et al. 2010). Anopheles dirus, A. minimus and A. fluviatilis are prominent vectors responsible for transmission of the disease in these states (Dev et al. 2003; Dev and Phookan 1996). The malaria transmission is perennial and focal outbreaks are common due to conducive environment to the vector survival and proliferation throughout the year (Dev et al. 2003; Bhattacharya et al. 2006; Dhiman et al. 2010). The antimalarial drug sensitivity studies have suggested the widespread of resistance in the country (Arora et al. 2008; Shah 2008). Similarly, the development of chloroquine and sulfadoxine resistance in P. falciparum has aggravated the situation in the northeastern region (Baruah et al. 2005). The possibility of multi-drug resistance malaria entering in northeastern states through Myanmar border further poses serious health concern (Dua et al. 2003).
The state of Assam (Lat 26°0′N, Long 93°0′E) is largest among all the eight states of northeast region (population 2,66,55,528; area 78,523 km2) and mainly comprised of hilly terrain, deep forests (>20%) and innumerable rivers and tributaries. The economy is agrarian, where tea industry is the backbone and provides employment to approximately 30% people of state’s population. Tea workers are mainly local tribal people and poor people coming from other nearby states, therefore constitute a new community which include the both and mostly referred as ‘tea tribes’. These people live under low socio economic condition and have high level of herd immunity enabling them to serve as reservoir for the malaria transmission (Dev et al. 2003). The movement of non immune tea labours from outside states and engaged in tea work in northeastern states have led to a persistence transmission of malaria in the tea gardens. The knowledge of local malaria epidemiology in the tea gardens seems to be essential in taking up situation specific disease intervention strategies. The tea tribes constitute a special community, which plays a major role in the economic upliftment of the region and represent a section of population which have relatively limited medical facility and are at higher risk of having malaria infection. The present study was carried out among tea tribes in seven tea estates of two districts in Assam to understand the overall burden and epidemiology due to each species of malaria parasite and some risk factors associated with malaria infection.
Materials and methods
Study area
The study was carried out in randomly selected seven tea estates of Udalguri and Nagaon districts of Assam (Fig. 1). Udalguri is situated at north bank of river Brahmaputra from which Hatigargh (26°46′N–91°56′E), Koramore (26°52′N–91°56′E), Dimakuchi (26°45′N–91°51′E) and Majbat (26°47′N–92°16′E) tea estates were selected. All the four selected tea estates of Udalguri are touched by river at least from one side. Nagaon is situated at the south bank of same river from which three tea estates namely Kondoli (26°11′N–92°45′E), Nanoi (26°24′N–92°53′E) and Salonah (26°27′N–92°56′E) were selected. The state of Assam face heavy rainfall, ranging from 2 to 4 m every year, and floods occur regularly. The pre-monsoon rain occurs in March to April, and is proceeded by maximum precipitation (monsoon) during May to September. Temperature ranges from 7°C in winter to 33°C in the pre-monsoon, whereas the relative humidity (RH) varies from 60 to 85%. The vegetation includes evergreen forest covering more than 40% of the total land area. The verbal consents were obtained from the participating subjects and the study was approved by the institutional ethical committee. The present study was carried out during March to September, 2009 and each tea estate was visited twice.
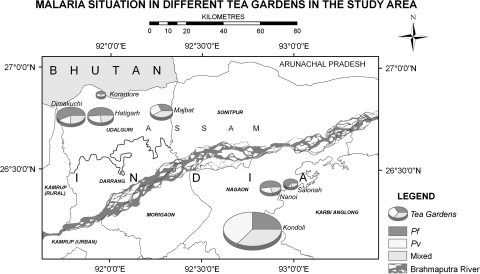
Fig. 1.
Study areas showing malaria situation. The pie charts indicate the location of the study tea estates named along with the each chart. The color in the pie chart represents the actual number of P. falciparum, P. vivax and mixed infection reported in the corresponding tea estate. No additional layers of data have been incorporated in the pie charts
Malaria point prevalence
One thousand one hundred and eighty two blood smears from the subjects either (a) willing to be tested for malaria or (b) having fever history for past few days (clinical cases) were screened for malaria parasite in peripheral blood-smear using microscopic technique. Both thick and thin blood smears stained with Giemsa stain were examined for at least 100 microscopic fields for malaria parasite and species identification. The suspected malaria patients were examined on the spot by immunochromatography based test kits (FirstSign™, M/S Unimed Biosystems) for rapid diagnosis of malaria. Patients found positive for malaria parasite were given treatment as per the guidelines of the National Vector Borne Disease Control Programme (NVBDCP) under medical supervision. Smears were confirmed negative for malaria only after screening 100 fields with each field containing 20–50 white blood cells. The smears having both P. falciparum and P. vivax infection were labeled as mixed infection.
Blood group and hemoglobin (Hb) content
Blood for determining the blood group and Hb content was obtained during the smear collection for malaria prevalence. A single blinded placebo controlled design was used. Standard method was used to determine the ABO blood groups using monoclonal ABO/Rh0D (M/S Tulip Diagnostics). Hb contents were determined using digital Hb 201+ analyser (M/S Hemocue AB, Sweden).
Data analysis and map preparation
The slide positivity rate (SPR) was the percent positive cases among the blood smears examined. The proportion of blood smears found positive for P. falciparum/P. vivax/mixed, was the slide P.falciparum rate (SfR)/slide P. vivax rate (SvR) and slide mixed rate (SmR). χ2 test with Yates correction was used to analyse the difference in malaria distribution and parasite species prevalence. Student’s t test was used to find out the difference in Hb content of patients. The confidence interval was determined using Katz approximation. Statistical analyses were carried out using GraphPad InStat software. The base map of study area was prepared using inputs from global positioning system (GPS) into which the malaria epidemiological data were integrated using ArcGIS (version 7.2).
Results
The malaria cases detected in the present study have been presented in Table 1 and Fig. 1. Out of 1,182 blood smears examined, 506 smears were found positive for malaria infection (SPR = 42.8). P. falciparum cases detected were significantly higher than P. vivax and mixed infections (χ2 = 8.1; df = 2; P = 0.017). Dimakuchi TE was having highest SPR (χ2 = 14.2; df = 6; P = 0.0275) than the other tea estates. However the malaria prevalence in Dimakuchi TE did not differ significantly to that in Kondoli TE (χ2 = 1.208; df = 1; P = 0.2717; 95% CI = 0.8510–1.964) (Table 1). Similarly more Pf cases were detected from Dimakuchi in comparison to the other tea estates (χ2 = 69.707; df = 6; P < 0.0001). The overall SPR observed in tea estates of Udalguri and Nagaon was 41.75 and 43.32 respectively (χ2 = 0.0205; df = 1; P > 0.5).
Table 1.
Malaria incidence among the tea estates
| Tea estate (TE) | BSE/n^ (SPR) | Pf/Pv/m# | SfR/SvR/SmR |
|---|---|---|---|
| Hatigargh | 136/58 (42.6) | 30/14/14 | 22.0/10.3/10.3 |
| Koramore | 40/8 (20.0) | 4/2/2 | 10.0/5.0/5.0 |
| Dimakuchi | 102/52 (51.0) | 40/12/0 | 39.2/11.8/0 |
| Majbat | 110/44 (40.0) | 14/12/18 | 12.7/10.9/16.4 |
| Kondoli | 646/288 (44.6) | 72/112/104 | 11.2/17.3/16.1 |
| Nanoi | 104/38 (36.5) | 20/10/8 | 19.2/9.6/7.7 |
| Salonah | 44/18 (40.9) | 12/2/4 | 27.3/4.5/9.1 |
BSE blood smear examined, n^ malaria positive cases, SPR slide positivity rate, Pf P. falciparum, PvP. vivax, m#Pf and Pv mixed infection, SfR/SvR/SmR slide falciparum/vivax/mixed rate
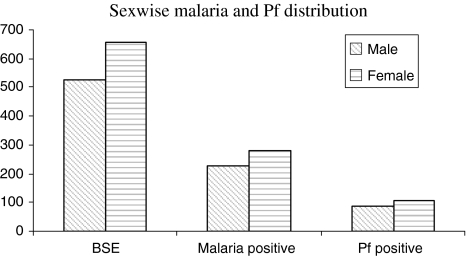
A total 154 malaria cases detected were having O (+ve) blood group, whereas 150, 148 and 54 cases were shared by A (+ve), B (+ve) and AB (+ve) blood group respectively.. There was no significant difference in malaria prevalence among the blood groups (χ2 = 3.603; df = 3; P = 0.3076). Further no significant difference could be found in malaria prevalence due to P. falciparum infection among the blood groups (χ2 = 1.818; df = 3; P = 0.6110). The age wise data on malaria case detected in the present study has been depicted in Table 2. Out of total 506 malaria cases, 79 were children below 5 years of age (SPR = 44.4). The children below 2 years of age were most sufferers with high SPR of 60. The difference in the SPR among the age groups was statistically significant (χ2 = 17.186; df = 4; P = 0.0018). There was no difference of infection due to parasite species in the patients below 5 years and above 15 years of age. However this difference was significant in the patients of 5–10 years (P = 0.0012) and 10–15 years (P = 0.0004) age group as infection due to P. falciparum was high. Sex wise malaria distribution showed that the number of male and female found positive for malaria were 227 (43.15%) and 279 (42.5%) respectively (Fig. 2). There was no significant difference either in malaria prevalence (χ2 = 0.02459; df = 1; P = 0.8754; RR = 1.014; 95% CI = 0.8920–1.153) or SfR (χ2 = 0.06328; df = 1; P = 0.8014; RR = 1.035; 95% CI = 0.8497–1.262) among both the sexes.
Table 2.
Age group wise malaria incidence (all the TE combined)
| Age group (years) | BSE/n^ (SPR) | Pf/Pv/m# |
|---|---|---|
| <2 | 50/30 (60.0) | 11/13/6 |
| >2–5 | 128/49 (38.3) | 15/15/19 |
| >5–10 | 161/70 (43.4) | 35/19/16 |
| >10–15 | 146/79 (54.1) | 39/16/24 |
| >15 | 697/278 (39.9) | 92/101/85 |
Fig. 2.
Sex-wise malaria and P. falciparum distribution (all the TE combined)
The Hb content of malaria infected individuals was reduced as compared to the control. Among male and female malaria patients, Hb content recorded (mean ± SEM) were 9.82 ± 0.1378 and 8.64 ± 0.09823 g/dl respectively, whereas malaria negative male and female Hb content were 10.79 ± 0.1086 and 9.24 ± 0.09001 g/dl respectively. The decrease in Hb content in malaria infected male as well as female as compared to normal was statistically significant (P < 0.0001; t = 5.593 for male and 4.458 for female). All the malaria positive cases (100%) of either sex were found anemic, however only 1.3% of female and 6% of male who do not have malaria infection, were having Hb content ≥12 and ≥13 g/dl respectively.
Discussion
The north eastern states of India, malaria distribution has been largely limited to the population living in poverty (Dev and Dash 2007; Dash et al. 2010). The people engaged in tea estates contribute most of malaria cases every year and could be given priority in order to strengthen the control programmes in the region. A very high SPR observed in all the tea estates indicates that parasitic load is significantly high and malaria transmission continuous without interruptions. The malaria transmission is persistent in north east region of India and high incidences have been recorded during the months of May to September (Dev et al. 2003, 2004). The rise in malaria prevalence in this period is due to sharp increase in the biting rates of A. minimus, which is regarded as a potential malaria vector in the region (Dev et al. 2004; Dev 1996).
The point prevalence of malaria cases indicated that majority of cases detected correspond to P. falciparum (37.9%). The percentage of P. falciparum cases could be much higher if the mixed infections are also included in the P. falciparum cases. The relative abundance of P. falciparum was less than observed earlier in the same state (Dev 2001). In Indian sub continent the majority of malaria infections are contributed by P. falciparum and P. vivax (Dhiman et al. 2010; Sharma et al. 2004). The SPR in the tea estates of both the districts was similar, indicating the similar kind of malariogenic factors prevailing in both the banks of river Brahmaputra (Dev 1996; Dev and Phookan 1996).
There was uniform distribution of malaria among all the blood groups and both the sexes. The ABO type blood groups have not been significantly contributing to the simple malaria infection, however these have been found associated with the rosette like formation in the blood cells (Facer and Brown 1979; Martin et al. 1979; Montoya et al. 1994). A protective effect of O blood group has been observed through the mechanism of reduced Plasmodium rosetting in Mali (Rowe et al. 2007). Similarly, non-O blood group individuals were found more vulnerable to severe malaria (Fischer and Boon 1998; Ilozumba and Uzozie 2009).
There was no gender reservation for the malaria prevalence and both sexes contributed significantly. Many studies have indicated the similar findings, where males and females were equally affected by malaria infections (Dev et al. 2004; Singh et al. 2004). The males have generally more risk of acquiring the malaria infection due to their poor clothing and maximum outdoor activity (Dhiman et al. 2010). Malaria transmission is stable and perennial in the tea estates and most of the non infected cases have anemia, which indicates that these people harbor repeated attacks of malaria regularly. The malaria infected patients were having significant reduction in the Hb level which is not only because of recent higher density of infection but may be due to malnutrition or persistent asymptomatic infections leading to permanent anemia even if the infection is cleared.
The children below 2 years of age were having high smear parasite rate. Little children are considered to be at more risk of acquiring the malaria infection due to underdeveloped protective immunity, high mobility and outdoor activity in minimum clothing. Similar kinds of results have been obtained earlier, where children of 1–4 year age group were found to be more susceptible to malaria infection (Dhiman et al. 2010; Singh et al. 2006; Owusu-Agyei et al. 2009). Among the children below 5 years of age and adults (>15 year) there were almost equal episodes due to P. vivax and P. falciparum species, but among 5–15 age group, the infection due to P. falciparum was more prominent. The declined chance of acquiring malaria infection with the increase in age of individual is due to progressive development of protective immunity (Sharma et al. 2004; Mayor et al. 2007). Significant variation of malaria prevalence among the different age groups has been reported in northeastern part of India (Dev et al. 2004). The persistence of P. falciparum is attributed to the emergence of drug resistant varieties, inadequate interventions and treatment seeking patterns in the communities (Singh et al. 2004; Mishra et al. 2002).
In north-eastern states, >20% of malaria endemic communities are reported to be asymptomatic cases, which are unlikely to seek malaria treatment (Dev and Phookan 2006; Gogoi et al. 1995). Apparently, inadequate interventions against the parasite reservoir in the communities facilitate the year-round malaria transmission and the associated risk is assessed to be much greater in vector dense areas.
The tea tribes constitute a community having poor socio economic status and continue to have high prevalence of malaria infection with considerable anemia. The present study strongly evidences that despite of comprehensive malaria control programme there are regions in India, where the control programmes are inadequate. These are hyper-endemic for malaria and transmission rates are comparable to those found in many regions of Africa. Personal and community based malaria protection measures at individual level, curtailing malaria transmission by reducing gametocyte carriage using effective drug regime and controlling adult vectors and their breeding sites at authority level can be important in these highly endemic areas.
Acknowledgments
The authors are grateful to the medical officers of all the tea estates for their support during the study.
Conflict of interests None.
Contributor Information
Bipul Rabha, Phone: +91-3712-258538-34, FAX: +91-3712-258538-34, Email: rabha_bipul05@rediffmail.com.
D. Goswami, Email: digantagos@yahoo.co.in
Sunil Dhiman, Email: sunildhiman81@gmail.com.
N. G. Das, Email: ngdas_drdo@rediffmail.com
P. K. Talukdar, Email: pranabktal@yahoo.co.in
M. J. Nath, Email: manashjnath@gmail.com
Indra Baruah, Email: indrabaruah@gmail.com.
R. K. Bhola, Email: bhola_zoology@yahoo.co.in
Lokendra Singh, Email: director_drl@yahoo.com.
References
- Arora U, Sonal GS, Dhillon GPS, Thakor HG. Emergence of drug resistance in India. J Indian Med Assoc. 2008;106:678–683. [PubMed] [Google Scholar]
- Baruah I, Talukdar PK, Das SC. The drug sensitivities of Plasmodium falciparum in the Sonitpur District, Assam, India. Southeast Asian J Trop Med Public Health. 2005;36:587–590. [PubMed] [Google Scholar]
- Bhattacharya S, Sharma C, Dhiman RC, Mitra AP. Climate change and malaria in India. Curr Sci. 2006;90:369–375. [Google Scholar]
- Dev V. Malaria survey in Tarajulie tea estate and adjoining hamlets in Sonitpur district, Assam. Indian J Malariol. 1996;33:21–29. [PubMed] [Google Scholar]
- Dev V. Malaria-attributable morbidity in Assam, North-eastern India. Ann Trop Med Parasitol. 2001;95:789–796. doi: 10.1080/00034980120111136. [DOI] [PubMed] [Google Scholar]
- Dev V, Dash AP. Rainfall and malaria transmission in North-eastern India. Ann Trop Med Parasitol. 2007;101:457–459. doi: 10.1179/136485907X176526. [DOI] [PubMed] [Google Scholar]
- Dev V, Phookan S. Malaria prevalence in tea estates of Brahmaputra Valley of Assam, India. J Parasit Dis. 1996;20:189–192. [Google Scholar]
- Dev V, Bhattacharyya PC, Talukdar R. Transmission of malaria and its control in the Northeastern region of India. J Assoc Physician India. 2003;51:1073–1076. [PubMed] [Google Scholar]
- Dev V, Phookan S, Sharma VP, Anand SP. Physiographic and entomologic risk factors of malaria in Assam, India. Am J Trop Med Hyg. 2004;71:451–456. [PubMed] [Google Scholar]
- Dev V, Phookan S, Sharma VP, Dash AP, Anand SP. Malaria parasite burden and treatment seeking behavior in ethnic communities of Assam, Northeastern India. J Infect. 2006;52:131–139. doi: 10.1016/j.jinf.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Dev V, Sangma BM, Dash AP. Persistent transmission of malaria in Garo hills of Meghalaya bordering Bangladesh, North-east India. Malar J. 2010;9:263. doi: 10.1186/1475-2875-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman S, Goswami D, Rabha B, Gopalakrishnan R, Baruah I, Singh L. Malaria epidemiology along Indo-Bangladesh border in Tripura state, India. Southeast Asian J Trop Med Public Health. 2010;41(6):1279–1289. [PubMed] [Google Scholar]
- Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–819. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Dua VK, Dev V, Phookan S, Gupta NC, Sharma VP, Subbarao SK. Multi-drug resistant Plasmodium falciparum malaria in Assam, India: timing of recurrence and antimalarial drug concentrations in whole blood. Am J Trop Med Hyg. 2003;69:555–557. [PubMed] [Google Scholar]
- Dutta J, Singh Z, Verma AK, Bishnoi MS. Malaria—resurgence and problems. Indian J Community Med. 2004;29:171–172. [Google Scholar]
- Facer CA, Brown J. ABO blood groups and falciparum malaria. Trans R Soc Trop Med Hyg. 1979;73:599–600. doi: 10.1016/0035-9203(79)90066-X. [DOI] [PubMed] [Google Scholar]
- Fischer PR, Boon P. Short report: severe malaria associated with blood group. Am J Trop Med Hyg. 1998;58(1):122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- Gogoi SC, Dev V, Choudhury B, Phookan S. Susceptibility of Plasmodium falciparum to chloroquine in tea garden tribes of Assam, India. Southeast Asian J Trop Med Public Health. 1995;26(2):228–230. [PubMed] [Google Scholar]
- Ilozumba PCO, Uzozie CR. Prevalence of malaria parasitemia and its association with ABO blood groups in Odoakpu area of Onitsha south local government area, Anambra state, Nigeria. Niger Ann Nat Sci. 2009;8(2):1–8. [Google Scholar]
- Martin SK, Miller LH, Hicks CU, David-West A, Ugbode C, Deane M. Frequency of blood group antigens in Nigerian children with falciparum malaria. Trans R Soc Trop Med Hyg. 1979;73:216–218. doi: 10.1016/0035-9203(79)90217-7. [DOI] [PubMed] [Google Scholar]
- Mayor A, Aponte JJ, Fogg C, Saute F, Greenwood B, Dgedge M, Menendez C, Alonso PL. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar J. 2007;6:3. doi: 10.1186/1475-2875-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Mohanty S, Das BS. The influence of health care facilities on malaria mortality in and around Rourkela, India. Ann Trop Med Parasitol. 2002;96:835–837. doi: 10.1179/000349802125002248. [DOI] [PubMed] [Google Scholar]
- Mohapatra PK, Prakash A, Bhattacharyya DR, Goswami BK, Ahmed A, Sharma B, Mahanta J. Detection & molecular confirmation of a focus of Plasmodium malariae in Arunachal Pradesh, India. Indian J Med Res. 2008;128:52–56. [PubMed] [Google Scholar]
- Montoya F, Restrepo M, Montoya AE, Rojas W. Blood groups and malaria. Rev Inst Med Trop Sao Paulo. 1994;36:33–38. doi: 10.1590/S0036-46651994000100006. [DOI] [PubMed] [Google Scholar]
- Owusu-Agyei S, Asante KP, Adjuik M, Adjei G, Awini E, Adams M, Newton S, Dosoo D, Dery D, Agyeman-Budu A, Gyapong J, Greenwood B, Chandramohan D. Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar J. 2009;8:220. doi: 10.1186/1475-2875-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JA, Handel IG, Thera MA, Deans AM, Lyke KE, Kone A, Diallo DA, Raza A, Kai O, Marsh K, Plowe CV, Doumbo OK. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci USA. 2007;104(44):17471–17476. doi: 10.1073/pnas.0705390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I. Chloroquine resistant vivax malaria in an infant: a report from India. J Vector Borne Dis. 2008;45:176–177. [PubMed] [Google Scholar]
- Sharma SK, Chattopadhyay R, Chakrabarti K, Pati SS, Srivastava VK, Tyagi PK, Mahanty S, Mishra SK, Adak T, Das BS, Chitnis C. Epidemiology of malaria transmission and development of natural immunity in a malaria- endemic village, San Dulakudar, in Orissa state, India. Am J Trop Med Hyg. 2004;71(4):457–465. [PubMed] [Google Scholar]
- Singh N, Nagpal AC, Saxena A, Singh MP. Changing scenario of malaria in Central India: the replacement of Plasmodium vivax by Plasmodium falciparum (1986–2000) Trop Med Int Health. 2004;9:364–371. doi: 10.1046/j.1365-3156.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Mishra AK, Chand SK, Singh MP, Bharti PK, Ahluwalia TP, Dash AP (2006) Epidemiology of malaria in an area of low transmission in Central India. Am J Trop Med Hyg 75:812–816 [PubMed]