Abstract
Out of 44 cases of dermatitis in dogs, 11 cases of parasitic origin were analyzed by cytopathology. Histopathologic examination of punch biopsies was also done for correlation with cytologic findings. Sarcoptic dermatitis was recorded in six cases, wherein, besides sarcoptic mites, neutrophils, macrophages, and plasma cells and keratinizing epithelial cells were also seen. Hematology revealed a relative neutrophilia and mild eosinophilia. Four cases of severe and generalized demodicosis complicated with bacteria and/or Malassezia sp. infection were also recorded. Histopathologically numerous Demodex sp. mites in varying stage of maturation were found damaging the hair follicles along with associated pathological changes and foreign body granulomas in one case. In addition, flea allergy dermatitis was also observed in one dog. In nutshell, cytology was found to be unequivocally effective in diagnosing parasitic dermatitis.
Keywords: Cytopathology, Demodicosis, Flea allergy, Parasitic dermatitis, Sarcoptic mange
Introduction
Parasitic dermatitis is the commonest clinical presentation in small animal practice. Dermatitis caused by sarcoptic and demodectic mange in dogs is widely prevalent in dogs in different parts of India (Rai and Yathiraj 1988; Aujla et al. 2000 and Singh et al. 2011). Cytologic examination has become a very useful tool for practicing veterinarians. Cytologic samples can be collected quickly, easily and inexpensively with little or no risk to the patient. The cytologic interpretation often is valuable in establishing a diagnosis, identifying the disease process, directing therapy, forming a prognosis and determining the procedure to be followed next (Tyler et al. 1999 and Vasudevan et al. 2004). The present study was conducted to find a quick and reliable cytopathologic technique for demonstrating parasitic agent and associated inflammatory response, replacing the conventional skin scrapping procedure.
Materials and methods
The study was conducted on 126 dogs suffering from various cutaneous and subcutaneous affections including those of parasitic dermatitis, mostly presented to the Veterinary Teaching Hospital, GADVASU and some local private veterinary clinics in Ludhiana. Detailed signalment, clinical signs and clinical findings were recorded. The cytologic findings were correlated with radiographic, microbiologic, parasitologic, hematologic and particularly histopathologic findings. Blood was collected from each case and complete blood cell count was performed as per Jain (1986). Skin scrapings, till blood oozed out, were taken from representative sites of cases presented with alopecia; itching and erythematous lesions on a clean glass slide and afterwards a few drops of 10% potassium hydroxide (KOH) were added and covered with a cover slip. The slides were then gently heated for 15–20 sec to clear the debris and examined microscopically for the presence of mites. Identification of Demodex canis and Sarcoptes scabiei was done as per the methods described by Soulsby (1982). Additionally, the smears without KOH were drawn and stained by Ramonwsky’s method for studying inflammatory response and identifying bacteria or yeast (Coles 1986). Histopathologic examination of punch biopsies from selected cases was also done for correlation with cytologic findings. The collected tissue pieces were processed and examined using standard techniques (Luna 1968).
Results and discussion
44 cases of dermatitis of different type were diagnosed and were classified as specific and non-specific dermatitis/dermatoses. After thorough cytologic, microbiologic and/or histopathologic examination, the underlying cause (s) of 36 cases could be ascertained, and hence these cases were grouped under specific dermatitis/dermatoses. Whereas, eight cases (18.2%) were non-specific in their etiology and thus grouped under non-specific dermatitis/dermatoses. In all, 25 cases of dermatitis of infectious nature viz. bacterial (n = 10), demodectic (n = 4), sarcoptic (n = 6), mycotic (n = 4), flea bite dermatitis (n = 1) were diagnosed effectively with the help of cytology, history and clinical signs. Identification of the infectious agent was made at least to the genera level on the basis of morphology and/or staining properties. The details of the cases of parasitic dermatitis is discussed as under.
Sarcoptic mange
Out of the total 44 cases of dermatitis, six cases of sarcoptic dermatitis were diagnosed and confirmed by microscopic examination of the scrapings. The presence of a few to large number of mites at different stages of development was the basis of diagnosis in all of the cases. The cases were observed in five male dogs and one bitch of different breeds ranging between 1 month and 10 years of age. The age related prevalence of scabies indicated that dogs below 6 months of age (five cases) were more susceptible.
Grossly, the lesions varied from papules to thick encrustation distributed on the different body parts. Elbow, hock, pinna, abdomen and limbs were the common sites involved (Fig. 1). The lesions localized on the elbow, pinna, and limbs had a tendency to form scabs, whereas the lesions on the abdomen were papular to pustular eruptions. This may be attributed to inaccessibility of the sites for scratching by the dogs. In all the cases, there was intense itching, erythema, keratinization, excoriation and poor hair condition. Similar sites of predilection and gross lesions in sarcoptic mange have been reported by most workers (Muller et al. 1983; Aiello and Mays 1998; Aujla et al. 2000; Medleau and Hnilica 2001 and Pin et al. 2006). The skin lesions were more pronounced in the 10 year-old male dog of non-descript breed.
Fig. 1.
Sarcoptic dermatitis: papules to thick encrustations on the abdomen, elbow, pinna and face
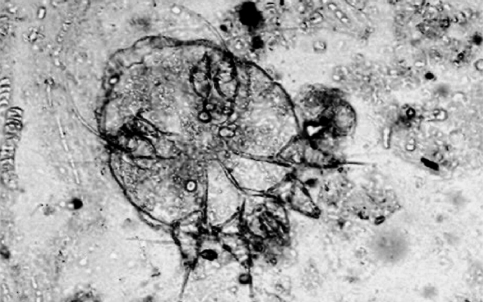
Cytological findings revealed that skin scrapings of all these positive cases were having a number of roughly circular shaped mites (Sarcoptes scabiei) with the last two pairs of legs not extending beyond the body margins (Fig. 2). In addition to the adult mites, nymphs, and larvae were also noticed. In addition to typical sarcoptic mites, the presence of inflammatory cells comprising of neutrophils, macrophages and plasma cells besides, keratinizing epithelial cells were the other cytologic findings. Hematologically relative neutrophilia with a mild eosinophilia were observed in three of the cases. Mites secrete allergenic substances that elicit an intensely pruritic hypersensitivity reaction in sensitized dogs (Medleau and Hnilica 2001) and the increased number of eosinophils in this study may be ascribed to such a reaction.
Fig. 2.
Female Sarcoptes scabiei mite: circular shaped adult mite with the posterior two pairs of legs having bristles that do not extend beyond the body margins. ×33
Demodectic mange
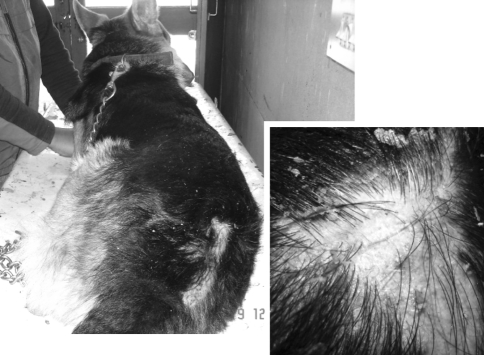
Four cases of severe and generalized demodicosis were observed in one Pug (Fig. 3), two German shepherds and one Gaddi (Fig. 4) ranging between 1.5 and 9 years of age. The male dogs (three out of four) were more susceptible than the bitches. The dogs were treated by field veterinarians but their condition didn’t improve. All of the cases were complicated with secondary etiological agents. The face, fore limb, ventral abdomen, and thorax and the hind limb were the most common sites involved. In all of the cases there was mild to moderate itching.
Fig. 3.
Demodicosis: severe and generalized form with thickening, hyperpigmentation and wrinkling of the skin
Fig. 4.
Generalized demodicosis: another case, highly lichenified, hyperpigmented and elephant-skin-like appearance
Both localized and generalized forms of demodicosis are common in young dogs (Aujla et al. 2000) whereas adult-onset generalized demodicosis occurs in dogs greater than 18 month-old with the highest incidence in immunocompromised dogs due to an underlying condition like endocrine disturbances (Medleau and Hnilica 2001). In the present study, the affected dogs were above 18 month-old and the disease had a generalized form. This may be due to the fact that animal owners bring their animals to the veterinary hospital usually after several therapeutic trials by local veterinary practitioners or even by themselves or due to some kind of immunosuppression.
Gross lesions were characterized by encrustation, complete alopecia, thickening, and wrinkling of the skin around the axilla, head and limbs (Figs. 3, 4). In two of the cases the affected sites were oily, hyperpigmented and elephant-skin-like. Crusting and blood tinged discharge was also observed in one of the cases. A typical symmetrical hair loss with hyperpigmentation and moderate erosion was seen on the back and lateral side of one German shepherd. The skin on the neck and ventral aspect of this dog was hairless, highly lichenified, wrinkled and loose, which aroused a suspicion of secondary yeast infection. Although lichenification can be seen with many chronic infectious, allergic, or seborrheic skin disorders, yet lichenification of the ventral neck, chest, and axillary areas is particularly characteristic of yeast dermatitis (Bruner and Blakemore 1999).
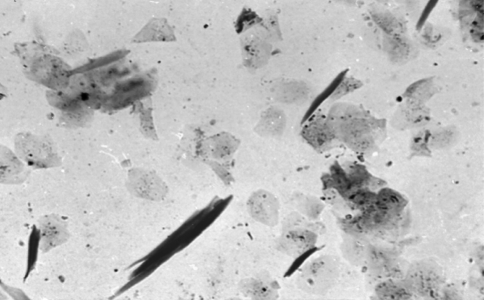
Cytologic findings of the deep scrapings and oozing material from intact papules collected from three cases were full of Demodex canis (Fig. 5). The mites were observed at different stages of development. Adult mites (Fig. 5 Inset) were characteristically cigar shaped with four pair of stubby legs in the thorax part of their body (Soulsby 1982). The skin scrapings collected from a German shepherd having symmetrical alopecia on the back and ramp were negative for Demodex, but Malassezia were observed adhering on the superficial epithelium (Fig. 8). However, this case was found positive for Demodex canis and negative for Malassezia species on histopathological examination of punch biopsy. Inference drawn from these findings is that some skin diseases can have multiple etiologies that can be ruled out by using several tests. The commonly used scraping technique may fail to spot the mites localized deep in the follicles; however, it is usually found effective to detect mites and yeast localized on the surface of the skin or a little deeper. Similarly, those superficial pathogens (Malassezia and Sarcoptic mange) found on the stratum corneum of the skin can be washed off during the routine paraffin technique. Consequently, the pathologist may not find any yeast in the processed biopsy sample (Patterson and Frank 2002). In such conditions scraping method should be complemented with punch biopsies and clinical signs to augment precise diagnosis.
Fig. 5.
Several mites at different stages of development. ×66. Inset: Demodex canis with a characteristic cigar shape and four pairs of stubby legs on thorax part. ×100
Fig. 8.
Skin imprint showing superficial epithelial cells, adhered Malassezia organisms as well as keratin fragments. Wright–Leishman, ×50
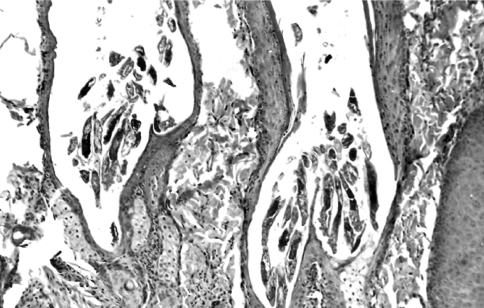
Histopathology of the skin biopsies collected from representative sites showed characteristic findings of demodicosis. In all of the cases, there were several mites in the follicles, folliculitis, perifolliculitis and furunculosis (Fig. 6). Hyperplasia of the sebaceous glands, parakeratosis, hyperkeratosis, acanthosis, and telogen hairs were also apparent.
Fig. 6.
Many mites in different stages of development in the hair follicles, folliculitis, perifolliculitis, furunculosis, hyperplasia of sebaceous glands and thinning of the external root sheath of the follicles. H&E, ×25
In one case, granulomas with foreign body giant cells, mononuclear cells, fibroblasts, and neutrophils were observed in the dermis where the mites breached the follicles and localized in the vicinity of the follicles (Fig. 7). Pigmentary incontinence, panniculitis and hemorrhage were also found associated with the granulomas.
Fig. 7.
Several demodectic mites (m) in extra follicular space invoking granulomatous response comprising of giant cells (g), mononuclear cells, fibroblasts and neutrophils. H&E
Flea bite dermatitis
Flea allergy dermatitis was observed on the caudodorsal lumbosacral area of a 3 year-old male German shepherd. The area was inflamed, excoriated and alopecic (Fig. 9). On closer examination, several fleas were seen actively moving in between the hair. Flea allergy dermatitis is a hypersensitivity type reaction against the proteolytic enzymes and histamine-like substances found in the saliva of fleas. It is the most common dermatologic disease in USA (Sischo et al. 1989; Aiello and Mays 1998 and Medleau and Hnilica 2001). The low prevalence of flea allergy dermatitis observed in the present study could be due to the continuous exposure of the dogs to fleas in the study area with the concomitant immunologic tolerance (Aiello and Mays 1998).
Fig. 9.
Flea bite allergy dermatitis: erythema and hair loss on the caudodorsal lumbosacral area
Cytologically, smears made from scrapings of the affected sites were negative for mange mites and fungi. Characteristically, several eosinophils and epithelial cells and a few neutrophils were seen. Increased eosinophils in cytologic specimens collected from hypersensitivity dermatitis and parasitic dermatitis has been described previously (Tyler et al. 1999 and Thrall 2000).
From the present study it was concluded that diagnostic cytology was an efficient technique for diagnosing parasitic dermatitis along with the associated inflammatory response and thus could be used as an alternative to the conventional skin scrapping and histopathology methods.
References
- Aiello SE, Mays A. Integumentary system. Merck veterinary manual. 8. NJ: Merck & Co.,; 1998. pp. 589–722. [Google Scholar]
- Aujla RS, Singla LD, Juyal PD, Gupta PP. Prevalence and pathology of mange-mite infections in dogs. J Vet Parasitol. 2000;14:45–49. [Google Scholar]
- Bruner RS, Blakemore JC. Malassezia dermatitis in dogs. Vet Med. 1999;94:613–622. [Google Scholar]
- Coles EH. Veterinary clinical pathology. 4. Philadelphia: W.B. Saunders Company; 1986. [Google Scholar]
- Jain NC. Schalm’s veterinary haematology. 4. Philadelphia: Lea and Febiger; 1986. [Google Scholar]
- Luna LG. Manual of histologic methods of the armed forces institute of pathology. 3. New York: McGraw-Hill; 1968. [Google Scholar]
- Medleau L, Hnilica KA. Small animal dermatology: a color atlas and therapeutics guide. 1. Philadelphia: W.B. Saunders Company; 2001. [Google Scholar]
- Muller GH, Kirk RW, Scott DW. Small animal dermatology. 3. Philadelphia: W.B. Saunders; 1983. [Google Scholar]
- Patterson AP and Frank LA (2002) How to diagnose and treat malassezia dermatitis in dogs. Vet Med: 612–622
- Pin D, Bensignor E, Carlotti DN, Cadiergues MC. Localized sarcoptic mange in dogs: a retrospective study of 10 cases. J Small Anim Pract. 2006;47:611–614. doi: 10.1111/j.1748-5827.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- Rai MJ, Yathiraj S. Clinical evaluation of ivermectin for treatment scabies in canines. Indian Vet J. 1988;65:626–628. [Google Scholar]
- Singh SK, Dimri U, Sharma MC, Swarup D, Sharma B. Determination of oxidative status and apoptosis in peripheral blood of dogs with sarcoptic mange. Vet Parasitol. 2011;178:330–338. doi: 10.1016/j.vetpar.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Sischo WM, Ihrke PJ, Franti CE. Regional distribution of ten common skin diseases in dogs. J Am Vet Med Assoc. 1989;195:752–756. [PubMed] [Google Scholar]
- Soulsby EJL. Helminthes, arthropods and protozoa of domestic animals. 7. London: Bailliere Tindall; 1982. pp. 478–495. [Google Scholar]
- Thrall MA. Cytologic examination of cutaneous and subcutaneous lumps and lesions. Vet Med. 2000;95:224–241. [Google Scholar]
- Tyler RD, Cowell RL, Meinkoth JH. Cutaneous and subcutaneous lesions: masses, cysts, ulcers and fistulous tracts. In: Cowell RL, Tyler RD, Meinboth JH, editors. Diagnostic cytology and haematology of the dog and cat. 2. Mosby: St. Louis; 1999. pp. 20–51. [Google Scholar]
- Vasudevan B, Ragul J, Madheswaran R, Muralimanohar B, Balachandran C. Cytological and histopathological diagnosis of canine skin tumors. Indian J Vet Pathol. 2004;28:130–133. [Google Scholar]