Abstract
The present study was envisaged to evaluate the efficacy of ethno-medicinal plant aqueous extracts such as Allium sativum, Lawsonia inermis, and Opuntia ficus indica in vitro in comparison with the chemotherapeutic agent, Oxyclozanide on Fasciola gigantica adults. The efficacy was evaluated by gross visual motility and mortality of F. gigantica with score index, light microscopic examination of carmine stained flukes and histopathology of treated flukes. Based on the in vitro trials conducted using above plant extracts at 1 percent, 2.5 percent and 5 percent concentration, the extracts of O. ficus indica showed flukicidal effect at 2.5 and 5% concentration. However A. sativum and L. inermis were effective at 5% concentration only. The study indicated the potential for developing herbal-based anthelmintics to control F. gigantica in livestock.
Keywords: Fasciola gigantica, Plant extracts, Anthelmintic property, In vitro
Introduction
Fasciolosis is an economically important disease of domestic livestock, in particular cattle and sheep, and occasionally man. The effective control of Fasciola currently includes strategic and tactile use of anthelmintic drugs and careful management of grazing lands, including control of stocking rates and appropriate rotation strategies. Because of the limited availability of drugs, high cost, development of resistance, chemical residue in milk and meat, toxicity problem and failed snail control measures, the majority of world population depends on traditional remedies. It is estimated that some 20,000 species of higher plants are used medicinally throughout the world for controlling various diseases (Githiori et al. 2006). In the search for therapeutically alternatives for Fasciola gigantica, the present study envisaged to evaluate the in vitro activity of extracts from three plant species in comparisons with currently using flukicidal drug oxyclozanide.
Materials and methods
Source of plant extracts
Allium sativum bulbs were purchased from local market. Lawsonia inermis leaves were collected from plants. Opuntia ficus indica stem with spines were collected from plants and identified botanically before use (Prajapati et al. 2007). All the collected materials were grinded in mortar and pestle separately and aqueous extracts were filtered through gauze cloth. The filtrate was labeled and stored at 4°C until further use.
Collection of liver flukes
Fasciola gigantica flukes were collected from the bile duct of infected cattle at the slaughter house in Perambur in Chennai, India. They were kept in saline medium and transferred to the laboratory of department of Veterinary Parasitology. After washing the flukes several times with saline, healthy ones with normal microscopic structure and good motility were selected. They were kept in RPMI 1640 medium until the experiment began.
Experimental design
Anthelmintic activity was studied by in vitro petri dish method as described by Jiraungkoorskul et al. (2005) and Githiori et al. (2006). Five flukes were exposed to each of the following treatment in petridish at room temperature at a concentration of 1, 2.5 and 5% of, A. sativum, L. inermis, O. ficus indica and oxyclozanide drug control and normal RPMI-1640 control. The inhibition of motility and/or mortality of flukes were observed after 3, 12, 15 h and score index was made. The morphological and histopathological variation of flukes was studied after the experiment. This was repeated three times.
Motility criteria
The motility was scored using the following criteria (Jiraungkoorskul et al. 2005).
Score 3—Moving whole body
Score 2—Moving only parts of the body
Score 1—Immobile but alive
Score 0—Died
Specimen preparation for light microscopic analysis (Carmine staining)
After death of flukes, one fluke from each group was used for carmine staining for gross morphological changes. The flukes were washed thoroughly with 0.1 M phosphate buffer saline, pH 7.4 and pressed in between two slides, tied both sides with rubber band and submerged in formalin for at least 12 h for fixing, then they were washed in running tap water overnight. The washed flukes were dehydrated with 70% alcohol three times and stained with acetic alum carmine dye over night. The flukes were destained with 1% acid alcohol, washed in ammonia water, dehydrated with graded series of ethanol, cleared in xylene and mounted with DPX. They were examined for abnormalities under dissection microscope and photographed.
Haematoxylin and eosin staining
A further four flukes from each group were fixed in 10% formaldehyde for 24 h, dehydrated with ascending series of ethanol and cleared with xylene. They were then embedded in paraffin, sectioned at thickness of 5 μm and stained with haematoxylin and eosin. They were examined for abnormalities under light microscope and photographed.
Results
Gross visual motility
Normal control flukes remained active with whole body movements (score 3) from 0 to 12 h in RPMI medium. Only ten percent of the groups showed a decrease in motility by moving only parts of the body (score 2) at 15 h.
Oxyclozanide treated control group flukes become slender, shrunken, paralyzed and then finally died after 2 min at 5 percent concentration. In case of 2.5 percent concentration, the flukes showed paralysis and died after 3 min of exposure. The flukes were shrunken and moved only the parts of the body (score 2) and died after 4 min in 1 percent concentration. The flukes were undergone change in their position and paralyzed before death.
As soon as the flukes exposed to 5 percent concentration of the O. ficus indica extract, they become sluggish and they moved only the parts of their body (score 2) up to 2 min. Then they become immobile (score 1) and died after 5 min. There was no paralysis and change in the position. The flukes did not show any change and remained active by moving only the parts of their body (score 3) at 2.5 percent concentration. There were decrease in their activity and movements of only parts of their body (score 2) after 10 min. Twenty percent of the flukes were died (score 0) and remaining 80% of the flukes were immobile (score 1) at 2 h. All the flukes were died (score 0) by 3 h. The flukes remained active up to 12 h (score 3) as that of negative control and no significant change was observed at 1 percent concentration of O. ficus indica.
All the flukes were remained active after exposure to 5 percent concentration of A. sativum extract (score 3). Eighty percent of the flukes moved only their parts of the body (score 2) while 20 percent of them are immobile (score 1) at the end of 1 h. After 2 h 80 percent flukes were immobile (score 1) and finally all the flukes died (score 0). In case of 2.5 percent concentration, the flukes were motile in the beginning (score 3) and 40 percent flukes were motile and folded their body (score 3) after 1 h. There was decrease in mortality at third hour (score 2).But they maintained motility up to 12 h as that of normal control flukes. All the flukes were active and no change in their motility noticed (score 3) and remained as that of normal control flukes in 1 percent concentration. The flukes become shrunken immediately after exposure to 5 percent concentration of L. inermis extract and they were motile (score 3). Forty percent of the flukes barely moved and remain alive (score 1) and 60% of the flukes were active (score 3) at the end of first h. After 2 h, 40 percent of the flukes were moved only the parts of their body (score 2), 40 percent of flukes immobile (score 1), and remaining 20 percent died (score 0). Flukes treated with 2.5 percent and 1 percent did not show any changes and they are remained active up to 12 h as that of normal control flukes.
Gross microscopical changes
The morphology of normal adult control F. gigantica was compared with treated groups. The oxyclozanide treated flukes showed spiny eruptions on the surface of the body, shrinkage of the fluke and breaking of testicular branches. Rupture of uterus and caeca but the spines were present at 5 percent concentration of O. ficus indica. No morphological changes were observed, and they looked like as that of normal control fluke at 2.5 and 1 percent concentration. Flukes treated with 5 percent concentration of A. sativum extract showed rupture of intestinal caeca. No changes were noticed in 2.5 and 1 percent concentration. No abnormal gross changes were detected in Lawsonia extract treated flukes, in all the concentrations.
Histopathological changes
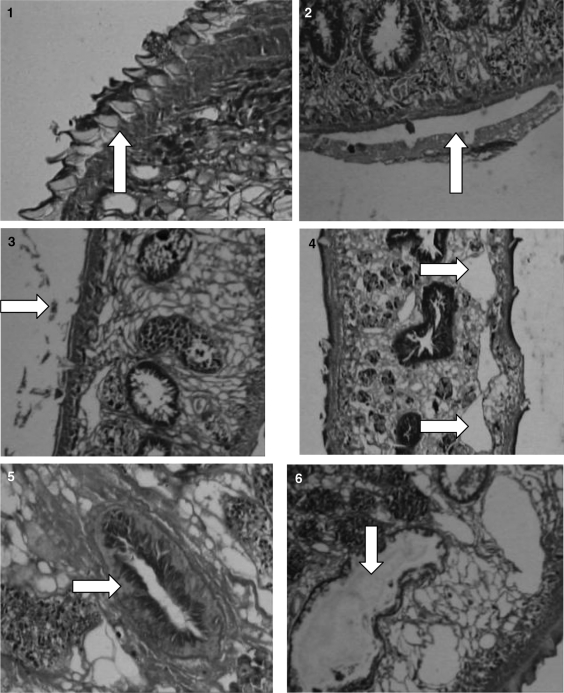
The flukes showed the normal microscopic structure of various organs and tegument in control group (Figs. 1, 5). Vacuole formation was noticed in the longitudinal section of fluke in oxyclozanide treated group (Fig. 4). Testes were massively degenerated and also there was disruption of villi in 5% oxyclozanide treated fluke. At 2.5 and 1% concentration, there was vacuolation in parenchyma, degeneration of testes, disruption of villi, but in a less intensive manner. There was complete desquamation of gut epithelial cells in flukes at 5 percent concentration of O. ficus indica (Fig. 6), whereas no changes were noticed in 2.5 and 1 percent concentration. Blebbing of the tegument was noticed in flukes treated with 5 percent concentration of A. sativum (Fig. 2). Treated fluke with 2.5 and 1 percent were normal. Detachment of spines in flukes at 5% concentration of Lawsonia was seen (Fig. 3). But no abnormality could be detected at 2.5 and 1 percent treated flukes.
Figs. 1–6.
1. Control fluke showing tegument with spines. 2. 5% Allium sativum treated fluke showing blebbing of tegument. 3. 5% Lawsonia inermis showing detachment of spines. 4. Vacuole formation in 5% oxyclozanide treated flukes. 5. Intestinal caeca with villi of control fluke. 6. 5% Opuntia treated fluke showing extensive degeneration of gut epithelium
Discussion
In recent years, there has been a resurgence of interest in traditional health care practices in the western as well as in the developing world. In animal health, this has led to further interest in ethno veterinary research and development that covers traditional practices, ethnobotany and application of animal care practices in local tradition (Schillhorn van Veen 1997). In the search for natural anthelmintics, in vitro test are used for preliminary studies of plants (Akthar et al. 2000; Kushwaha et al. 2004; Jiraungkoorskul 2005). O. ficus indica extract at 2.5 and 5% concentration caused death in flukes. Microscopically rupture of uterus and caeca were noticed. It also caused complete desquamation of gut epithelial cells. Perusal of available literature did not show study of anthelmintic effect of O. ficus indica. But antiulcer activity was reported (Galati et al. 2001).
Allium sativum extract was effective in inhibition of motility and killing of F. gigantica at 5% concentration only. Alcoholic extracts of A. sativum inhibited the motility of F. gigantica at 100 μg per ml (Singh et al. 2007). Microscopically rupture of intestinal caeca, blebbing of tegument was noticed. Microscopical changes of flukes treated with A. sativum was not reported. This extract was evaluated against Haemonchus contortus (Iqbal et al. 2001; Veerakumari and Navaneethakumari 2006), cercariae of flukes (Nama and Bhatnagar 1990) and Ascaris lumbricoides (Bastidas 1969; Kalesraj 1975). Allium sativum was also used for treating Ascaris suum and Giardia in pigs (Lans et al. 2007). Lawsonia inermis extract caused paralysis followed by death at 5% concentration. Microscopically detachment of spines noticed at same concentration. It was reported that L. inermis could be tried for treatment of fasciolosis in ruminants (Nwude and Ibrahim 1980).
Thus it is evident from the results that the extracts of O. ficus indica at 2.5 and 5% concentration and A. sativum, L. inermis at 5% concentration were effective. It is concluded that, the results of this study may be a base for developing herbal-based anthelmintics for the control of F. gigantica in livestock.
Acknowledgments
The authors wish to thank the Dean, Madras Veterinary College, Chennai-600007, India for providing necessary facilities. The research was funded by Tamilnadu state council for science and technology under the project “Evaluation of efficacy of extracts of ethno medicinal plants against Fasciola gigantica, the liver fluke of ruminants” (Code No. VS. 05).
References
- Akthar MS, Iqbal Z, Khan MN, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin Res. 2000;38:99–107. doi: 10.1016/S0921-4488(00)00163-2. [DOI] [Google Scholar]
- Bastidas GJ. Effect of ingested garlic on Necator americanus and Ancylostoma caninum. Am J Trop Med Hyg. 1969;18:920–923. doi: 10.4269/ajtmh.1969.18.920. [DOI] [PubMed] [Google Scholar]
- Galati EM, Monforte MT, Tripodo MM, d'Aquino A, Mondello MR. Antiulcer activity of Opuntia ficus indica (L.) Mill. (Cactaceae): ultrastructural study. J Ethnopharmacol. 2001;76:1–9. doi: 10.1016/S0378-8741(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Githiori B, Athanasiadou S, Thamsborg M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet Parasitol. 2006;139:308–320. doi: 10.1016/j.vetpar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Nadeem QK, Khan MN, Akthar MS, Waraich FN. In vitro anthelmintic activity of Allium sativum, Zingiber officinale, Cucurbita mexicana and Ficus religosa. Int J Agric Biol. 2001;3:454–457. [Google Scholar]
- Jiraungkoorskul W, Sahaphong S, Tansatit Eurytrema pancreaticum: the in vitro effect of praziquantel and triclabendazole on the adult fluke. Exp Parasitol. 2005;111:172–177. doi: 10.1016/j.exppara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kalesraj R. Screening of some indigenous plants for anthelmintic action against Ascaris lumbricoides. Part 2. Indian J physiol Pharmacol. 1975;19:47–49. [PubMed] [Google Scholar]
- Kushwaha DS, Kumar D, Tripathi HC, Tandan SK. Effect of some indigenous medicinal plants on Fasciola gigantica in vitro. Indian J Ani Sci. 2004;74:143–146. [Google Scholar]
- Lans C, Turner W, Khan T, Braner G. Ethnoveterinary medicines used to treat endoparasites and stomach problems in pigs and pets in British, Columbia, Canada. Vet Parasitol. 2007;148:325–340. doi: 10.1016/j.vetpar.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Nama HS, Bhatnagar B. Laboratory evaluation of cercaricidal properties of certain plant extracts. Ind J Parasitol. 1990;14:79–82. [Google Scholar]
- Nwude N, Ibrahim MA. Plants used in traditional veterinary practice in Nigeria. J Vet Pharmacol Ther. 1980;3:261–273. doi: 10.1111/j.1365-2885.1980.tb00491.x. [DOI] [Google Scholar]
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. A handboook of medicinal plants. Jodhpur: Agrobios (India) publisher; 2007. pp. 172–186. [Google Scholar]
- Singh TU, Kumar D, Guptha PK, Tandan SK. Inhibitory effect of alcoholic extracts of Alliumsativum and Piperlongum on gross visual motility and glucose uptake of Fasciolagigantica and Gigantocotyleexplanatum. J Vet Parasitol. 2007;21:121–124. [Google Scholar]
- Schillhorn TW. Sense or non sense? Traditional methods of animal parasitic control. Vet Parasitol. 1997;71:177–194. doi: 10.1016/S0304-4017(97)00031-9. [DOI] [PubMed] [Google Scholar]
- Veerakumari L, Navaneethakumari K. In vitro effect of Alliumsativum on lactate dehydrogenase activity of Haemonchuscontortus. J Vet Parasitol. 2006;20:93–96. [Google Scholar]