Abstract
Necropsy of gastrointestinal tract of 125 free-range chickens from a subtropical and humid zone of northwestern India revealed four nematode spp. (Ascaridia galli, Heterakis gallinarum, Capillaria spp. and Cheilospirurahamulosa) and four cestode spp. (Raillietina cesticillus, Raillietina echinobothrida, Raillietina tetragona and Amoebotaenia cuneata) The overall prevalence of the helminth parasites was 72.0%. Amongst various helminth species encountered in the region, A. galli emerged out as the most prevalent, followed by H. gallinarum, R. cesticillus and R. echinobothrida. The impact of helminthic infections on body weight gain in growing chickens was investigated. One hundred growing chickens, aged 40 days were randomly assigned to two groups (treated and untreated controls) of 50 birds each. The birds in treated group were given fenbendazole at 7.5 mg per kg body weight in drinking water, while the birds in other group served as untreated controls. At the end of the 90 days of the field trial, the mean body weight gain of untreated controls was 1232.2 ± 7.28 g (13.7 g/day) compared with 1617.6 ± 5.43 g (18.0 g/day) in the treated group. It was associated with a significantly (P < 0.05) higher mean worm burden (32.92 ± 6.12) in untreated controls than the treated group (2.46 ± 1.14). The prevalences of helminthic species and their impact on body weight gain in growing backyard chickens have been discussed.
Keywords: Backyard chickens, Helminths, Prevalence, Northwestern India, Subtropical and humid, Weight gain
Introduction
Amongst food animals, poultry ranks high in their ability to convert feed into high energy food products (meat and eggs) for human consumption. In the Indian subcontinent, this efficiency has been greatly exploited as a revenue provider and consequently, poultry has been one of the most intensively reared domesticated species. India has 498 million poultry population with an average growth rate of 8–10% per annum (Singh et al. 2009). Despite above rosy popularity of poultry industry, traditional backyard poultry keeping with a flock size of 5–20 birds, with almost zero financial input is quite popular amongst rural population comprising of farm women, landless labours and marginal farmers. It contributes to nearly 30% of national egg production (Singh et al. 2009). Helminthiasis is considered as one of the most significant constraints on poultry production in humid tropical climatic conditions of India which are favourable for faster propagation and development of the larval stages of helminth parasites (Matta and Ahluwalia 1981; Malhotra 1983; Kulkarni et al. 2001). Although, these helminthic infections are rarely fatal and are often neglected but they cause heavy economical loss to poultry farmers due to reduced productivity (meat and eggs), beside some helminths also act as carriers of pathogenic agents. Despite this, the impact of helminthic infections on production traits in poultry, in general, and in backyard chickens in particular has not been precisely assessed. Keeping in view above facts, the present study was planned to determine the prevalence of gastrointestinal (GI) helminths and their impact on the growth of backyard chickens at R.S. Pura, Jammu, India.
Materials and methods
The study area
The study was carried out at R.S. Pura of Jammu district during July–December 2009. The study area is located 332 m above sea level and has a subtropical and humid climate, with an annual rainfall of about 1,069 mm. The mean annual minimum and maximum temperature is about 16.36 and 30.18°C, respectively. The socio-economic status of the families in the region is poor and their livelihood mainly depends on sheep and goat husbandry; beside they have 1–2 dairy animals.
Examination of chickens to determine the prevalence of helminthic infections
In the study area, indigenous chickens are reared in open backyards of houses. Shelter with inadequate space and minimal inputs by the owner are to provide protection against thefts and predators. One hundred and 25 growing and adult indigenous backyard birds, irrespective of age and sex, were randomly selected from villages around R.S. Pura (Jammu), brought to the laboratory and examined as follows:
After decapitation, the trachea was examined directly and the entire GI tract including oesophagus was collected from each bird. GI tract was opened in a longitudinal section and its contents were carefully washed into a petri dish. Larger helminths were collected directly and smaller ones were isolated under the stereomicroscope. The mucosa was scraped in order to collect the helminths embedded in the mucosal layer. In addition, keratinised layer of the gizzard was peeled off in order to look for the nematodes which are embedded in the muscular layer. Nematodes were collected by the help of curved needle and were preserved in glycerine alcohol, whereas cestodes were fixed under slight cover glass pressure in 10% formalin and stained with aqueous borax carmine. The helminth species were identified according to the description given by Soulsby (1982).
Examination of backyard chickens to investigate impact of helminthiases on weight gain
Forty days old 100 birds which had been vaccinated against new castle disease (NCD attenuated vaccine, Ventri Biologicals, Pune), infectious bursal disease (IBD attenuated vaccine, Ventri Biologicals, Pune) and Marek’s disease (MD live vaccine, Ventri Biologicals, Pune), were purchased from Government Poultry Farm, Belicharana, Jammu and were distributed amongst ten families (ten birds each) of village Chak-siyan, R.S. Pura, Jammu. These birds were kept in small poultry houses (size varies from 4 × 3′ to 6.5 × 5′) made of indigenous material (bamboo, mud, brick, straw, wooden, etc.) and rice husk was used as litter. The drinking water was provided ad libitum in earthen and plastic utensils. The birds were placed in these houses in the night and were allowed to roam freely in families backyards during the day time.
These birds were divided into two groups (50 birds each). Fifty birds of five families were treated with fenbendazole (Fenbezol, Ranbaxy) at 7.5 mg/kg body weight in drinking water at monthly intervals, while other 50 birds of five families were kept as untreated control. To nullify the effect of coccidiosis, the birds in both groups were given prophylactic anticoccidial treatment using sulphadimidine (Pabadine, Intas Pharmaceuticals Ltd.) at a dose rate of 0.2% in the drinking water, two treatments each for 2 days with 3 days interval. Prophylactic antibiotic treatment was also provided with tetracycline hydrochloride (Intervet India Pvt. Ltd.) at 500 mg/litre of water for 3 days. The birds were weighed at monthly intervals and on spot weight of each bird were recorded. The mean body weight (±SEM) of the birds in untreated and treated groups was 212.6 ± 1.79 and 210.3 ± 0.48 g, respectively at the start of the experiment. At the end of the field trial, all the survived chickens were purchased, brought to the laboratory, euthanised, necropsied and in situ worm population was recorded as described above.
Statistical analysis
The experimental data generated during the study were analysed using descriptive statistics (SPSS 16.0 versions). A two-tailed test was used to compare the means. Simple linear correlation method (Pearson r) was used to calculate the correlation between weight gain and worm burden in the treated and untreated groups. A P value <0.05 was considered significant.
Results and discussion
Prevalences and parasite species
The prevalences, parasite species and mean worm burden (±SEM) recorded in 125 free-range chickens during the present investigation are summarised in Table 1. The overall prevalence of GI helminths parasitising backyard chickens was 72.0%. Out of total infected birds, 56.66% were infected with nematodes, while 43.33% were found positive for cestode infections. Mixed infections accounted for 32% cases, while 68% birds had single infection. A total of eight species, four nematodes and four cestodes were identified. No parasite was recovered from trachea, oesophagus and crop in any carcass. The results are comparable to those reported by Saad et al. (1989) and Puttalakshmamma et al. (2008), who reported 77.3 and 71% helminthic infections in local chickens, respectively. The number of helminthic species observed in the present study was lower than those reported earlier by Kulkarni et al. (2001) that might be attributed to the short period of the study as well the study was conducted in a limited area.
Table 1.
Prevalence and mean worm burden (±SEM) of helminths in backyard chickens at Jammu
| Helminth spp. | Total no. infected | Prevalence (%) | Mean ± SEM |
|---|---|---|---|
| N = 125 | |||
| Nematodes | |||
| Ascaridia galli | 37 | 29.6 | 14.2 ± 34.04 |
| Heterakis gallinarum | 30 | 24.0 | 11.0 ± 37.32 |
| Capillaria spp. | 3 | 2.4 | 0.7 ± 0.62 |
| Cheilospirura hamulosa | 2 | 1.6 | 0.4 ± 0.57 |
| Cestodes | |||
| Raillietina cesticillus | 24 | 19.2 | 7.9 ± 22.31 |
| Raillietina echinobothrida | 17 | 13.6 | 2.0 ± 0.91 |
| Raillietina tetragona | 12 | 9.6 | 3.5 ± 4.72 |
| Amoebotaenia cuneata | 5 | 4.0 | 1.8 ± 1.1 |
| Total | 90a | 72.0 | |
a40 birds had mixed infection
Ascaridia galli emerged as the most prevalent helminth spp. (29.6%) in the region, with Heterakis gallinarum (24.0%), Raillietina cesticillus (19.2%) and Raillietina echinobothrida (13.6%) also being common, while the least prevalent species were Raillietina tetragona (9.6%), Amoebotaeniacuneata (4.0%), Capillaria spp. (2.4%) and Cheilospirurahamulosa (1.6%). The prevalence of nematodes (A. galli, H. gallinarum and C. hamulosa) in this study are high than that of documented by Kulkarni et al. (2001) from Maharashtra region and could be attributed to the difference in the ecology of the study areas. The ambient temperature and high rainfall in Jammu region offer favourable epizootiological determinants for faster development and propagation of the different stages of nematodes.
Backyard chickens satisfy their food requirements by scavenging habits and they usually seek their food in the superficial layers of the soil, drains etc. which contain various insects that may act as intermediate hosts/vectors for cestodes (Pandey 1992; Permin et al. 1997). Moreover, the development of these insects is also favoured by epizootiological determinants discussed above and was responsible for a relatively higher prevalence of Raillietina spp. infection in comparison to earlier findings (Puttalakshmamma et al. 2008).
Worm burden and its impact on live weight gain in growing chickens
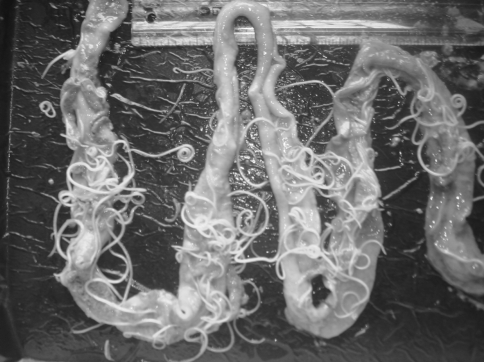
At the end of the field trial, only 34 birds in untreated group (32% mortalities) and 41 birds in treated group (18% mortalities) were examined. High mortality in untreated group could be attributed to helminthic infection as observed during necropsy of the birds (Fig. 1). In untreated group, the prevalence of helminthic infections was 79.41%. Ascaridia galli was the predominant species (prevalence 35.29%, mean worm burden ± SEM 19.3 ± 26.1), followed by H. gallinarum (29.41%, 13.0 ± 41.1), R. tetragona (23.52%, 5.08 ± 11.3), R. cesticillus (17.64%, 6.9 ± 17.3), R. echinobothrida (11.76%, 2.6 ± 1.4), A. cuneata (8.82%, 0.6 ± 0.7), Capillaria spp. (5.88%, 1.2 ± 0.9) and C. hamulosa (2.94%, 0.8 ± 0.72). In treated group, 14.63% of the examined chickens were found to be positive for helminthic infections. Only A. galli (9.75%, 1.75 ± 6.87), H. gallinarum (7.31%, 1.22 ± 4.19), R. cesticillus (2.43%, 0.91 ± 1.05) and R. tetragona (2.43%, 0.64 ± 1.2) were recorded. The total worm counts in untreated group was 1,119 (mean 32.92 ± 6.12) which was significantly higher (P < 0.05) than the total worm counts of 101 (mean 2.46 ± 1.14) in the treated group.
Fig. 1.
Ascaridia galli infection in intestine of poultry
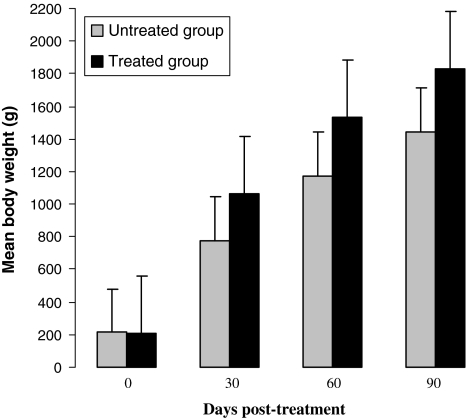
The monthly body weights of the untreated and treated groups are shown in Fig. 2. The treated birds continued to gain weight steadily until the end of the field trial. However, the untreated birds did not gain weight appreciably and their mean body weights remained lower than the mean body weights of treated group. The differences in body weight gain between the groups were significant (P < 0.05) from the day 30 after treatment. The untreated birds gained only 1232.2 ± 7.28 g live weight (13.7 g/day), whereas treated birds gained 1617.6 ± 5.43 g live weight (18.0 g/day) at the end of the 90 days period of the field trial. A strong negative correlation (r = −0.296) was observed between the weight gain and the total worm count in untreated group, whereas a weak negative correlation (r = −0.044) was noticed in treated group.
Fig. 2.
Mean body weight gain (±SEM) in the respective groups
Regular deworming improved the growth and subsequently the mean body weight gain in treated group, while the untreated birds were smaller in size, weak and emaciated in comparison to treated birds. These findings are in broad agreement with earlier reports (Malhotra 1983; Negesse 1991; Phiri et al. 2007). The results of the present study have also shown that in favourable environmental conditions, helminthic infections can be minimised by regular deworming so that the negative effects on the weight gain are reduced.
The various helminth spp. encountered in the present study are serious pathogens of poultry. They not only affect live body weight gain but also cause interference with host metabolism, influencing in vivo feed conversion efficiency, onset of puberty and production losses in terms of egg and meat. In order to curtail these losses in backyard poultry farming the strategic deworming schedule has to be followed, with due consideration for development of anthelmintic resistant strains of the prevalent parasites, so as to ensure better productivity and financial gains to the poultry owners. Help of extension agencies is needed to implement these programmes, as the farmers are totally ignorant, especially about poultry deworming.
Acknowledgment
We thankfully acknowledged financial support from Department of Biotechnology, New Delhi, India, under the project titled ‘Empowerment of rural women through backyard poultry farming in Jammu region’ (No. BT/PR 9600/SPD/11/1076/2007).
References
- Kulkarni GM, Narladkar BW, Deshpande PD. Helminthic infections in desi fowl (Gallus gallus domesticus) in Marathwada region. J Vet Parasitol. 2001;15:137–139. [Google Scholar]
- Malhotra KS. Population distribution of Heterakis pusilla in Gallus gallus L. from India. J Helminthol. 1983;57:117–126. doi: 10.1017/S0022149X00009378. [DOI] [PubMed] [Google Scholar]
- Matta SC, Ahluwalia SS. Note on the survey of gastrointestinal helminths of domestic fowls in UP. Indian J Anim Sci. 1981;51:1013–1015. [Google Scholar]
- Negesse T. Survey of internal parasites of local chickens of southern Ethiopia. Indian J Poult Sci. 1991;26:128–129. [Google Scholar]
- Pandey VS (1992) Epidemiology and economics of village poultry production in Africa: overview. In: Pandey VS, Demey S (eds) Village poultry production in Africa, Rabat, Morocco, pp 124–128
- Permin A, Magwisha H, Kassuku AA, Nansen P, Biggaard M, Frandsen F, Gibbons L. A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. J Helminthol. 1997;71:233–240. doi: 10.1017/S0022149X00015972. [DOI] [PubMed] [Google Scholar]
- Phiri IK, Phiri AM, Ziela M, Chota A, Masuku M, Monrad J. Prevalence and distribution of gastrointestinal helminths and their effects on weight gain in free-range chickens in central Zambia. Trop Anim Health Prod. 2007;39:309–315. doi: 10.1007/s11250-007-9021-5. [DOI] [PubMed] [Google Scholar]
- Puttalakshmamma GC, Ananda KJ, Prathiush PR, Mamatha GS, Rao S. Prevalence of gastrointestinal parasites of poultry in and around Bangalore. Vet World. 2008;1:201–202. [Google Scholar]
- Saad MB, El Sadig AA, Shammat AM. Helminth parasites of the local breed of poultry in Kordofan region, Sudanese. J Vet Sci Anim Husb. 1989;28:54–55. [Google Scholar]
- Singh A, Anish Y, Khajuria JK, Borkataki S, Pande N, Konwar D, Katoch R. Comparative evaluation of different breeds of backyard poultry under field conditions. Vet Pract. 2009;10:181–182. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]